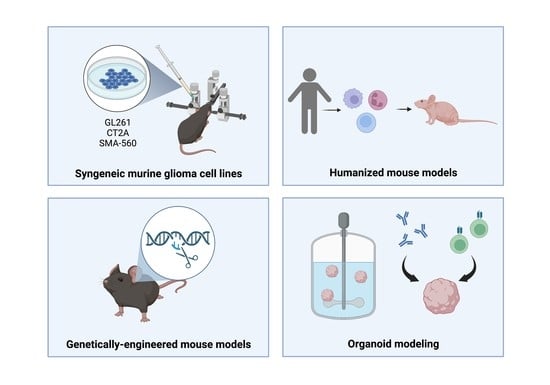
Translational Models in Glioma Immunotherapy Research
Abstract
1. Introduction
2. Syngeneic Murine Glioma Cell Lines
2.1. GL261
2.2. CT-2A
2.3. SMA-560
3. Genetically Engineered Mouse Models
4. Humanized Mouse Models
5. Organoids
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ostrom, Q.T.; Gittleman, H.; Liao, P.; Vecchione-Koval, T.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2010–2014. Neuro -Oncol. 2017, 19, v1–v88. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; O’Day, S.J.; McDermott, D.F.; Weber, R.W.; Sosman, J.A.; Haanen, J.B.; Gonzalez, R.; Robert, C.; Schadendorf, D.; Hassel, J.C.; et al. Improved Survival with Ipilimumab in Patients with Metastatic Melanoma. N. Engl. J. Med. 2010, 363, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef] [PubMed]
- Burtness, B.; Harrington, K.J.; Greil, R.; Soulières, D.; Tahara, M.; Castro, G.d.; Psyrri, A.; Basté, N.; Neupane, P.; Bratland, Å.; et al. Pembrolizumab Alone or with Chemotherapy versus Cetuximab with Chemotherapy for Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (KEYNOTE-048): A Randomised, Open-Label, Phase 3 Study. Lancet 2019, 394, 1915–1928. [Google Scholar] [CrossRef]
- Chen, Z.; Hambardzumyan, D. Immune Microenvironment in Glioblastoma Subtypes. Front. Immunol. 2018, 9, 1004. [Google Scholar] [CrossRef]
- Mirabelli, P.; Coppola, L.; Salvatore, M. Cancer Cell Lines Are Useful Model Systems for Medical Research. Cancers 2019, 11, 1098. [Google Scholar] [CrossRef]
- Oh, T.; Fakurnejad, S.; Sayegh, E.T.; Clark, A.J.; Ivan, M.E.; Sun, M.Z.; Safaee, M.; Bloch, O.; James, C.D.; Parsa, A.T. Immunocompetent Murine Models for the Study of Glioblastoma Immunotherapy. J. Transl. Med. 2014, 12, 107. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Amara, D.; Berger, M.S.; Raleigh, D.R.; Aghi, M.K.; Butowski, N.A. Mouse Models of Glioblastoma for the Evaluation of Novel Therapeutic Strategies. Neuro -Oncol. Adv. 2021, 3, vdab100. [Google Scholar] [CrossRef]
- Letchuman, V.; Ampie, L.; Shah, A.H.; Brown, D.A.; Heiss, J.D.; Chittiboina, P. Syngeneic Murine Glioblastoma Models: Reactionary Immune Changes and Immunotherapy Intervention Outcomes. Neurosurg. Focus 2022, 52, E5. [Google Scholar] [CrossRef]
- Seligman, A.M.; Shear, M.J.; Alexander, L. Studies in Carcinogenesis: VIII. Experimental Production of Brain Tumors in Mice with Methylcholanthrene1. Am. J. Cancer 1939, 37, 364–395. [Google Scholar] [CrossRef]
- Perese, D.M.; Moore, G.E. Methods of Induction and Histogenesis of Experimental Brain Tumors. J. Neurosurg. 1960, 17, 677–699. [Google Scholar] [CrossRef]
- Ausman, J.I.; Shapiro, W.R.; Rall, D.P. Studies on the Chemotherapy of Experimental Brain Tumors: Development of an Experimental Model. Cancer Res. 1970, 30, 2394–2400. [Google Scholar]
- Akbasak, A.; Oldfield, E.H.; Saris, S.C. Expression and Modulation of Major Histocompatibility Antigens on Murine Primary Brain Tumor in Vitro. J. Neurosurg. 1991, 75, 922–929. [Google Scholar] [CrossRef]
- Szatmári, T.; Lumniczky, K.; Désaknai, S.; Trajcevski, S.; Hídvégi, E.J.; Hamada, H.; Sáfrány, G. Detailed Characterization of the Mouse Glioma 261 Tumor Model for Experimental Glioblastoma Therapy. Cancer Sci. 2006, 97, 546–553. [Google Scholar] [CrossRef]
- Zagzag, D.; Amirnovin, R.; Greco, M.A.; Yee, H.; Holash, J.; Wiegand, S.J.; Zabski, S.; Yancopoulos, G.D.; Grumet, M. Vascular Apoptosis and Involution in Gliomas Precede Neovascularization: A Novel Concept for Glioma Growth and Angiogenesis. Lab. Invest. 2000, 80, 837–849. [Google Scholar] [CrossRef]
- Newcomb, E.W.; Zagzag, D. The Murine GL261 Glioma Experimental Model to Assess Novel Brain Tumor Treatments. In CNS Cancer: Models, Markers, Prognostic Factors, Targets, and Therapeutic Approaches; Meir, E.G., Ed.; Cancer Drug Discovery and Development; Humana Press: Totowa, NJ, USA, 2009; pp. 227–241. ISBN 978-1-60327-553-8. [Google Scholar]
- Plautz, G.E.; Touhalisky, J.E.; Shu, S. Treatment of Murine Gliomas by Adoptive Transfer Ofex VivoActivated Tumor-Draining Lymph Node Cells. Cell. Immunol. 1997, 178, 101–107. [Google Scholar] [CrossRef]
- Ni, H.-T.; Spellman, S.R.; Jean, W.C.; Hall, W.A.; Low, W.C. Immunization with Dendritic Cells Pulsed with Tumor Extract Increases Survival of Mice Bearing Intracranial Gliomas. J. Neurooncol. 2001, 51, 1–9. [Google Scholar] [CrossRef]
- Pellegatta, S.; Poliani, P.L.; Corno, D.; Grisoli, M.; Cusimano, M.; Ubiali, F.; Baggi, F.; Bruzzone, M.G.; Finocchiaro, G. Dendritic Cells Pulsed with Glioma Lysates Induce Immunity against Syngeneic Intracranial Gliomas and Increase Survival of Tumor-Bearing Mice. Neurol. Res. 2006, 28, 527–531. [Google Scholar] [CrossRef]
- Bagley, S.J.; Desai, A.S.; Linette, G.P.; June, C.H.; O’Rourke, D.M. CAR T-Cell Therapy for Glioblastoma: Recent Clinical Advances and Future Challenges. Neuro -Oncol. 2018, 20, 1429–1438. [Google Scholar] [CrossRef]
- Chen, M.; Sun, R.; Shi, B.; Wang, Y.; Di, S.; Luo, H.; Sun, Y.; Li, Z.; Zhou, M.; Jiang, H. Antitumor Efficacy of Chimeric Antigen Receptor T Cells against EGFRvIII-Expressing Glioblastoma in C57BL/6 Mice. Biomed. Pharmacother. 2019, 113, 108734. [Google Scholar] [CrossRef] [PubMed]
- Agliardi, G.; Liuzzi, A.R.; Hotblack, A.; De Feo, D.; Núñez, N.; Stowe, C.L.; Friebel, E.; Nannini, F.; Rindlisbacher, L.; Roberts, T.A.; et al. Intratumoral IL-12 Delivery Empowers CAR-T Cell Immunotherapy in a Pre-Clinical Model of Glioblastoma. Nat. Commun. 2021, 12, 444. [Google Scholar] [CrossRef]
- Zhang, C.; Burger, M.C.; Jennewein, L.; Genßler, S.; Schönfeld, K.; Zeiner, P.; Hattingen, E.; Harter, P.N.; Mittelbronn, M.; Tonn, T.; et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. JNCI J. Natl. Cancer Inst. 2016, 108, djv375. [Google Scholar] [CrossRef]
- Reardon, D.A.; Gokhale, P.C.; Klein, S.R.; Ligon, K.L.; Rodig, S.J.; Ramkissoon, S.H.; Jones, K.L.; Conway, A.S.; Liao, X.; Zhou, J.; et al. Glioblastoma Eradication Following Immune Checkpoint Blockade in an Orthotopic, Immunocompetent Model. Cancer Immunol. Res. 2016, 4, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Patel, M.A.; Mangraviti, A.; Kim, E.S.; Theodros, D.; Velarde, E.; Liu, A.; Sankey, E.W.; Tam, A.; Xu, H.; et al. Combination Therapy with Anti-PD-1, Anti-TIM-3, and Focal Radiation Results in Regression of Murine Gliomas. Clin. Cancer Res. 2017, 23, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Ladomersky, E.; Zhai, L.; Lenzen, A.; Lauing, K.L.; Qian, J.; Scholtens, D.M.; Gritsina, G.; Sun, X.; Liu, Y.; Yu, F.; et al. IDO1 Inhibition Synergizes with Radiation and PD-1 Blockade to Durably Increase Survival Against Advanced Glioblastoma. Clin. Cancer Res. 2018, 24, 2559–2573. [Google Scholar] [CrossRef]
- Desai, K.; Hubben, A.; Ahluwalia, M. The Role of Checkpoint Inhibitors in Glioblastoma. Targ. Oncol. 2019, 14, 375–394. [Google Scholar] [CrossRef]
- Reardon, D.A.; Brandes, A.A.; Omuro, A.; Mulholland, P.; Lim, M.; Wick, A.; Baehring, J.; Ahluwalia, M.S.; Roth, P.; Bähr, O.; et al. Effect of Nivolumab vs Bevacizumab in Patients With Recurrent Glioblastoma: The CheckMate 143 Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 1003–1010. [Google Scholar] [CrossRef]
- Lim, M.; Weller, M.; Idbaih, A.; Steinbach, J.; Finocchiaro, G.; Raval, R.R.; Ansstas, G.; Baehring, J.; Taylor, J.W.; Honnorat, J.; et al. Phase III Trial of Chemoradiotherapy with Temozolomide plus Nivolumab or Placebo for Newly Diagnosed Glioblastoma with Methylated MGMT Promoter. Neuro -Oncol. 2022, 24, 1935–1949. [Google Scholar] [CrossRef]
- Omuro, A.; Brandes, A.A.; Carpentier, A.F.; Idbaih, A.; Reardon, D.A.; Cloughesy, T.; Sumrall, A.; Baehring, J.; van den Bent, M.; Bähr, O.; et al. Radiotherapy Combined with Nivolumab or Temozolomide for Newly Diagnosed Glioblastoma with Unmethylated MGMT Promoter: An International Randomized Phase III Trial. Neuro -Oncol. 2023, 25, 123–134. [Google Scholar] [CrossRef]
- Sanchez, V.E.; Lynes, J.P.; Walbridge, S.; Wang, X.; Edwards, N.A.; Nwankwo, A.K.; Sur, H.P.; Dominah, G.A.; Obungu, A.; Adamstein, N.; et al. GL261 Luciferase-Expressing Cells Elicit an Anti-Tumor Immune Response: An Evaluation of Murine Glioma Models. Sci. Rep. 2020, 10, 11003. [Google Scholar] [CrossRef]
- Johanns, T.M.; Ward, J.P.; Miller, C.A.; Wilson, C.; Kobayashi, D.K.; Bender, D.; Fu, Y.; Alexandrov, A.; Mardis, E.R.; Artyomov, M.N.; et al. Endogenous Neoantigen-Specific CD8 T Cells Identified in Two Glioblastoma Models Using a Cancer Immunogenomics Approach. Cancer Immunol. Res. 2016, 4, 1007–1015. [Google Scholar] [CrossRef]
- Zimmerman, H.M.; Arnold, H. Experimental Brain Tumors. I. Tumors Produced with Methylcholanthrene*. Cancer Res. 1941, 1, 919–938. [Google Scholar]
- Seyfried, T.N.; El-Abbadi, M.; Roy, M.L. Ganglioside Distribution in Murine Neural Tumors. Mol. Chem. Neuropathol. 1992, 17, 147–167. [Google Scholar] [CrossRef]
- Martínez-Murillo, R.; Martínez, A. Standardization of an Orthotopic Mouse Brain Tumor Model Following Transplantation of CT-2A Astrocytoma Cells. Histol. Histopathol. 2007, 22, 1309–1326. [Google Scholar] [CrossRef]
- Khalsa, J.K.; Cheng, N.; Keegan, J.; Chaudry, A.; Driver, J.; Bi, W.L.; Lederer, J.; Shah, K. Immune Phenotyping of Diverse Syngeneic Murine Brain Tumors Identifies Immunologically Distinct Types. Nat. Commun. 2020, 11, 3912. [Google Scholar] [CrossRef]
- Binello, E.; Qadeer, Z.A.; Kothari, H.P.; Emdad, L.; Germano, I.M. Stemness of the CT-2A Immunocompetent Mouse Brain Tumor Model: Characterization In Vitro. J. Cancer 2012, 3, 166–174. [Google Scholar] [CrossRef]
- Germano, I.; Swiss, V.; Casaccia, P. Primary Brain Tumors, Neural Stem Cell, and Brain Tumor Cancer Cells: Where Is the Link? Neuropharmacology 2010, 58, 903–910. [Google Scholar] [CrossRef]
- Barnard, Z.; Wakimoto, H.; Zaupa, C.; Patel, A.P.; Klehm, J.; Martuza, R.L.; Rabkin, S.D.; Curry, W.T.J. Expression of FMS-like Tyrosine Kinase 3 Ligand by Oncolytic Herpes Simplex Virus Type I Prolongs Survival in Mice Bearing Established Syngeneic Intracranial Malignant Glioma. Neurosurgery 2012, 71, 741. [Google Scholar] [CrossRef]
- Ramachandran, M.; Yu, D.; Dyczynski, M.; Baskaran, S.; Zhang, L.; Lulla, A.; Lulla, V.; Saul, S.; Nelander, S.; Dimberg, A.; et al. Safe and Effective Treatment of Experimental Neuroblastoma and Glioblastoma Using Systemically Delivered Triple MicroRNA-Detargeted Oncolytic Semliki Forest Virus. Clin. Cancer Res. 2017, 23, 1519–1530. [Google Scholar] [CrossRef]
- Martikainen, M.; Ramachandran, M.; Lugano, R.; Ma, J.; Martikainen, M.-M.; Dimberg, A.; Yu, D.; Merits, A.; Essand, M. IFN-I-Tolerant Oncolytic Semliki Forest Virus in Combination with Anti-PD1 Enhances T Cell Response against Mouse Glioma. Mol. Ther. -Oncolytics 2021, 21, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, H.; Alayo, Q.A.; Penaloza-MacMaster, P.; Freeman, G.J.; Kuchroo, V.K.; Reardon, D.A.; Fernandez, S.; Caligiuri, M.; Chiocca, E.A. Modeling Tumor Immunity of Mouse Glioblastoma by Exhausted CD8+ T Cells. Sci. Rep. 2018, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Schaettler, M.; Blaha, D.T.; Bowman-Kirigin, J.A.; Kobayashi, D.K.; Livingstone, A.J.; Bender, D.; Miller, C.A.; Kranz, D.M.; Johanns, T.M.; et al. Treatment of an Aggressive Orthotopic Murine Glioblastoma Model with Combination Checkpoint Blockade and a Multivalent Neoantigen Vaccine. Neuro -Oncol. 2020, 22, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Woroniecka, K.I.; Rhodin, K.E.; Dechant, C.; Cui, X.; Chongsathidkiet, P.; Wilkinson, D.; Waibl-Polania, J.; Sanchez-Perez, L.; Fecci, P.E. 4-1BB Agonism Averts TIL Exhaustion and Licenses PD-1 Blockade in Glioblastoma and Other Intracranial Cancers. Clin. Cancer Res. 2020, 26, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.M.; Desai, R.; Coxon, A.; Livingstone, A.; Dunn, G.P.; Petti, A.; Johanns, T.M. Impact of CD4 T Cells on Intratumoral CD8 T-Cell Exhaustion and Responsiveness to PD-1 Blockade Therapy in Mouse Brain Tumors. J. Immunother Cancer 2022, 10, e005293. [Google Scholar] [CrossRef]
- Noffsinger, B.; Witter, A.; Sheybani, N.; Xiao, A.; Manigat, L.; Zhong, Q.; Taori, S.; Harris, T.; Bullock, T.; Price, R.; et al. Technical Choices Significantly Alter the Adaptive Immune Response against Immunocompetent Murine Gliomas in a Model-Dependent Manner. J. Neurooncol. 2021, 154, 145–157. [Google Scholar] [CrossRef]
- Fraser, H. Astrocytomas in an Inbred Mouse Strain. J. Pathol. 1971, 103, 266–270. [Google Scholar] [CrossRef]
- Serano, R.D.; Pegram, C.N.; Bigner, D.D. Tumorigenic Cell Culture Lines from a Spontaneous VM/Dk Murine Astrocytoma (SMA). Acta Neuropathol. 1980, 51, 53–64. [Google Scholar] [CrossRef]
- Sampson, J.H.; Ashley, D.M.; Archer, G.E.; Fuchs, H.E.; Dranoff, G.; Hale, L.P.; Bigner, D.D. Characterization of a Spontaneous Murine Astrocytoma and Abrogation of Its Tumorigenicity by Cytokine Secretion. Neurosurgery 1997, 41, 1365–1373. [Google Scholar] [CrossRef]
- Woroniecka, K.; Chongsathidkiet, P.; Rhodin, K.; Kemeny, H.; Dechant, C.; Farber, S.H.; Elsamadicy, A.A.; Cui, X.; Koyama, S.; Jackson, C.; et al. T-Cell Exhaustion Signatures Vary with Tumor Type and Are Severe in Glioblastoma. Clin. Cancer Res. 2018, 24, 4175–4186. [Google Scholar] [CrossRef]
- Tran, T.-T.; Uhl, M.; Ma, J.Y.; Janssen, L.; Sriram, V.; Aulwurm, S.; Kerr, I.; Lam, A.; Webb, H.K.; Kapoun, A.M.; et al. Inhibiting TGF-β Signaling Restores Immune Surveillance in the SMA-560 Glioma Model. Neuro Oncol. 2007, 9, 259–270. [Google Scholar] [CrossRef]
- Miller, J.; Eisele, G.; Tabatabai, G.; Aulwurm, S.; von Kürthy, G.; Stitz, L.; Roth, P.; Weller, M. Soluble CD70: A Novel Immunotherapeutic Agent for Experimental Glioblastoma: Laboratory Investigation. J. Neurosurg. 2010, 113, 280–285. [Google Scholar] [CrossRef]
- Heimberger, A.B.; Crotty, L.E.; Archer, G.E.; McLendon, R.E.; Friedman, A.; Dranoff, G.; Bigner, D.D.; Sampson, J.H. Bone Marrow-Derived Dendritic Cells Pulsed with Tumor Homogenate Induce Immunity against Syngeneic Intracerebral Glioma. J. Neuroimmunol. 2000, 103, 16–25. [Google Scholar] [CrossRef]
- Sampson, J.H.; Choi, B.D.; Sanchez-Perez, L.; Suryadevara, C.M.; Snyder, D.J.; Flores, C.T.; Schmittling, R.J.; Nair, S.; Reap, E.A.; Norberg, P.K.; et al. EGFRvIII MCAR-Modified T-Cell Therapy Cures Mice with Established Intracerebral Glioma and Generates Host Immunity against Tumor-Antigen Loss. Clin. Cancer Res. 2014, 20, 972–984. [Google Scholar] [CrossRef]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R Inhibition Alters Macrophage Polarization and Blocks Glioma Progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef]
- Przystal, J.M.; Becker, H.; Canjuga, D.; Tsiami, F.; Anderle, N.; Keller, A.-L.; Pohl, A.; Ries, C.H.; Schmittnaegel, M.; Korinetska, N.; et al. Targeting CSF1R Alone or in Combination with PD1 in Experimental Glioma. Cancers 2021, 13, 2400. [Google Scholar] [CrossRef]
- Hambardzumyan, D.; Parada, L.F.; Holland, E.C.; Charest, A. Genetic Modeling of Gliomas in Mice: New Tools to Tackle Old Problems. Glia 2011, 59, 1155–1168. [Google Scholar] [CrossRef]
- Miyai, M.; Tomita, H.; Soeda, A.; Yano, H.; Iwama, T.; Hara, A. Current Trends in Mouse Models of Glioblastoma. J. Neurooncol. 2017, 135, 423–432. [Google Scholar] [CrossRef]
- Noorani, I. Genetically Engineered Mouse Models of Gliomas: Technological Developments for Translational Discoveries. Cancers 2019, 11, 1335. [Google Scholar] [CrossRef]
- Hicks, W.H.; Bird, C.E.; Traylor, J.I.; Shi, D.D.; El Ahmadieh, T.Y.; Richardson, T.E.; McBrayer, S.K.; Abdullah, K.G. Contemporary Mouse Models in Glioma Research. Cells 2021, 10, 712. [Google Scholar] [CrossRef]
- Fomchenko, E.I.; Holland, E.C. Mouse Models of Brain Tumors and Their Applications in Preclinical Trials. Clin. Cancer Res. 2006, 12, 5288–5297. [Google Scholar] [CrossRef] [PubMed]
- Parker-Thornburg, J. Breeding Strategies for Genetically Modified Mice. In Transgenic Mouse: Methods and Protocols; Larson, M.A., Ed.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 163–169. ISBN 978-1-4939-9837-1. [Google Scholar]
- Mou, H.; Kennedy, Z.; Anderson, D.G.; Yin, H.; Xue, W. Precision Cancer Mouse Models through Genome Editing with CRISPR-Cas9. Genome Med. 2015, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Gertsenstein, M.; Mianné, J.; Teboul, L.; Nutter, L.M.J. Targeted Mutations in the Mouse via Embryonic Stem Cells. In Transgenic Mouse: Methods and Protocols; Larson, M.A., Ed.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 59–82. ISBN 978-1-4939-9837-1. [Google Scholar]
- Jin, F.; Jin-Lee, H.; Johnson, A. Mouse Models of Experimental Glioblastoma; Exon Publications: Brisbane, Australia, 2021; pp. 15–45. [Google Scholar] [CrossRef]
- Ahronian, L.G.; Lewis, B.C. Using the RCAS-TVA System to Model Human Cancer in Mice. Cold Spring Harb. Protoc. 2014, 2014, pdb.top069831. [Google Scholar] [CrossRef]
- Holmen, S.L.; Williams, B.O. Essential Role for Ras Signaling in Glioblastoma Maintenance. Cancer Res. 2005, 65, 8250–8255. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Singh, R.K.; Fidler, I.J.; Raz, A. Murine Models to Evaluate Novel and Conventional Therapeutic Strategies for Cancer. Am. J. Pathol. 2007, 170, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Kong, L.-Y.; Wu, A.S.; Doucette, T.; Wei, J.; Priebe, W.; Fuller, G.N.; Qiao, W.; Sawaya, R.; Rao, G.; Heimberger, A.B. Intratumoral Mediated Immunosuppression Is Prognostic in Genetically Engineered Murine Models of Glioma and Correlates to Immunotherapeutic Responses. Clin. Cancer Res. 2010, 16, 5722–5733. [Google Scholar] [CrossRef]
- Alghamri, M.S.; McClellan, B.L.; Avvari, R.P.; Thalla, R.; Carney, S.; Hartlage, C.S.; Haase, S.; Ventosa, M.; Taher, A.; Kamran, N.; et al. G-CSF Secreted by Mutant IDH1 Glioma Stem Cells Abolishes Myeloid Cell Immunosuppression and Enhances the Efficacy of Immunotherapy. Sci. Adv. 2021, 7, eabh3243. [Google Scholar] [CrossRef]
- Shingu, T.; Ho, A.L.; Yuan, L.; Zhou, X.; Dai, C.; Zheng, S.; Wang, Q.; Zhong, Y.; Chang, Q.; Horner, J.W.; et al. QKI Deficiency Maintains Stemness of Glioma Stem Cells in Suboptimal Environment by Downregulating Endolysosomal Degradation. Nat. Genet. 2017, 49, 75–86. [Google Scholar] [CrossRef]
- Zamler, D.B.; Shingu, T.; Kahn, L.M.; Huntoon, K.; Kassab, C.; Ott, M.; Tomczak, K.; Liu, J.; Li, Y.; Lai, I.; et al. Immune Landscape of a Genetically Engineered Murine Model of Glioma Compared with Human Glioma. JCI Insight 2022, 7. [Google Scholar] [CrossRef]
- Bender, A.M.; Collier, L.S.; Rodriguez, F.J.; Tieu, C.; Larson, J.D.; Halder, C.; Mahlum, E.; Kollmeyer, T.M.; Akagi, K.; Sarkar, G.; et al. Sleeping Beauty–Mediated Somatic Mutagenesis Implicates CSF1 in the Formation of High-Grade Astrocytomas. Cancer Res. 2010, 70, 3557–3565. [Google Scholar] [CrossRef]
- Huse, J.T.; Holland, E.C. Targeting Brain Cancer: Advances in the Molecular Pathology of Malignant Glioma and Medulloblastoma. Nat. Rev. Cancer 2010, 10, 319–331. [Google Scholar] [CrossRef]
- Chen, Z.; Peng, P.; Zhang, X.; Mania-Farnell, B.; Xi, G.; Wan, F. Advanced Pediatric Diffuse Pontine Glioma Murine Models Pave the Way towards Precision Medicine. Cancers 2021, 13, 1114. [Google Scholar] [CrossRef]
- Huszthy, P.C.; Daphu, I.; Niclou, S.P.; Stieber, D.; Nigro, J.M.; Sakariassen, P.Ø.; Miletic, H.; Thorsen, F.; Bjerkvig, R. In Vivo Models of Primary Brain Tumors: Pitfalls and Perspectives. Neuro -Oncol. 2012, 14, 979–993. [Google Scholar] [CrossRef]
- Kijima, N.; Kanemura, Y. Mouse Models of Glioblastoma. In Glioblastoma; De Vleeschouwer, S., Ed.; Codon Publications: Brisbane, Australia, 2017; ISBN 978-0-9944381-2-6. [Google Scholar]
- Allen, T.M.; Brehm, M.A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L.D.; et al. Humanized Immune System Mouse Models: Progress, Challenges and Opportunities. Nat. Immunol. 2019, 20, 770–774. [Google Scholar] [CrossRef]
- Shultz, L.D.; Ishikawa, F.; Greiner, D.L. Humanized Mice in Translational Biomedical Research. Nat. Rev. Immunol. 2007, 7, 118–130. [Google Scholar] [CrossRef]
- Rongvaux, A.; Takizawa, H.; Strowig, T.; Willinger, T.; Eynon, E.E.; Flavell, R.A.; Manz, M.G. Human Hemato-Lymphoid System Mice: Current Use and Future Potential for Medicine. Annu. Rev. Immunol. 2013, 31, 635–674. [Google Scholar] [CrossRef]
- Wunderlich, M.; Chou, F.-S.; Mizukawa, B.; Link, K.A.; Mulloy, J.C. A New Immunodeficient Mouse Strain, NOD/SCID IL2Rγ−/− SGM3, Promotes Enhanced Human Hematopoietic Cell Xenografts with a Robust T Cell Component. Blood 2009, 114, 3524. [Google Scholar] [CrossRef]
- Rongvaux, A.; Willinger, T.; Martinek, J.; Strowig, T.; Gearty, S.V.; Teichmann, L.L.; Saito, Y.; Marches, F.; Halene, S.; Palucka, A.K.; et al. Development and Function of Human Innate Immune Cells in a Humanized Mouse Model. Nat. Biotechnol. 2014, 32, 364–372. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized Mouse Models for Immuno-Oncology Research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Zhai, L.; Ladomersky, E.; Lauing, K.L.; Wu, M.; Genet, M.; Gritsina, G.; Győrffy, B.; Brastianos, P.K.; Binder, D.C.; Sosman, J.A.; et al. Infiltrating T Cells Increase IDO1 Expression in Glioblastoma and Contribute to Decreased Patient Survival. Clin. Cancer Res. 2017, 23, 6650–6660. [Google Scholar] [CrossRef]
- Ashizawa, T.; Iizuka, A.; Nonomura, C.; Kondou, R.; Maeda, C.; Miyata, H.; Sugino, T.; Mitsuya, K.; Hayashi, N.; Nakasu, Y.; et al. Antitumor Effect of Programmed Death-1 (PD-1) Blockade in Humanized the NOG-MHC Double Knockout Mouse. Clin. Cancer Res. 2017, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Biollaz, G.; Bernasconi, L.; Cretton, C.; Püntener, U.; Frei, K.; Fontana, A.; Suter, T. Site-Specific Anti-Tumor Immunity: Differences in DC Function, TGF-β Production and Numbers of Intratumoral Foxp3+ Treg. Eur. J. Immunol. 2009, 39, 1323–1333. [Google Scholar] [CrossRef]
- Klawitter, M.; El-Ayoubi, A.; Buch, J.; Rüttinger, J.; Ehrenfeld, M.; Lichtenegger, E.; Krüger, M.A.; Mantwill, K.; Koll, F.J.; Kowarik, M.C.; et al. The Oncolytic Adenovirus XVir-N-31, in Combination with the Blockade of the PD-1/PD-L1 Axis, Conveys Abscopal Effects in a Humanized Glioblastoma Mouse Model. Int. J. Mol. Sci. 2022, 23, 9965. [Google Scholar] [CrossRef] [PubMed]
- Guil-Luna, S.; Sedlik, C.; Piaggio, E. Humanized Mouse Models to Evaluate Cancer Immunotherapeutics. Annu. Rev. Cancer Biol. 2021, 5, 119–136. [Google Scholar] [CrossRef]
- Morton, J.J.; Bird, G.; Refaeli, Y.; Jimeno, A. Humanized Mouse Xenograft Models: Narrowing the Tumor–Microenvironment Gap. Cancer Res. 2016, 76, 6153–6158. [Google Scholar] [CrossRef]
- Patton, J.; Vuyyuru, R.; Siglin, A.; Root, M.; Manser, T. Evaluation of the Efficiency of Human Immune System Reconstitution in NSG Mice and NSG Mice Containing a Human HLA.A2 Transgene Using Hematopoietic Stem Cells Purified from Different Sources. J. Immunol. Methods 2015, 422, 13–21. [Google Scholar] [CrossRef]
- Clevers, H.; Tuveson, D.A. Organoid Models for Cancer Research. Annu. Rev. Cancer Biol. 2019, 3, 223–234. [Google Scholar] [CrossRef]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral Organoids Model Human Brain Development and Microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef]
- Ruiz-Garcia, H.; Alvarado-Estrada, K.; Schiapparelli, P.; Quinones-Hinojosa, A.; Trifiletti, D.M. Engineering Three-Dimensional Tumor Models to Study Glioma Cancer Stem Cells and Tumor Microenvironment. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Ogawa, J.; Pao, G.M.; Shokhirev, M.N.; Verma, I.M. Glioblastoma Model Using Human Cerebral Organoids. Cell Rep. 2018, 23, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Linkous, A.; Balamatsias, D.; Snuderl, M.; Edwards, L.; Miyaguchi, K.; Milner, T.; Reich, B.; Cohen-Gould, L.; Storaska, A.; Nakayama, Y.; et al. Modeling Patient-Derived Glioblastoma with Cerebral Organoids. Cell Rep. 2019, 26, 3203–3211.e5. [Google Scholar] [CrossRef] [PubMed]
- Jacob, F.; Salinas, R.D.; Zhang, D.Y.; Nguyen, P.T.T.; Schnoll, J.G.; Wong, S.Z.H.; Thokala, R.; Sheikh, S.; Saxena, D.; Prokop, S.; et al. A Patient-Derived Glioblastoma Organoid Model and Biobank Recapitulates Inter- and Intra-Tumoral Heterogeneity. Cell 2020, 180, 188–204.e22. [Google Scholar] [CrossRef] [PubMed]
- Rybin, M.J.; Ivan, M.E.; Ayad, N.G.; Zeier, Z. Organoid Models of Glioblastoma and Their Role in Drug Discovery. Front. Cell. Neurosci. 2021, 15. [Google Scholar] [CrossRef]
- Raue, K.D.; Duffy, J.T.; Babak, M.V.; Balyasnikova, I.V. Modeling Glioblastoma Complexity with Organoids for Personalized Treatments. Trends Mol. Med. 2023, 29, 282–296. [Google Scholar] [CrossRef]
- Mariappan, A.; Goranci-Buzhala, G.; Ricci-Vitiani, L.; Pallini, R.; Gopalakrishnan, J. Trends and Challenges in Modeling Glioma Using 3D Human Brain Organoids. Cell Death Differ. 2021, 28, 15–23. [Google Scholar] [CrossRef]
- Neal, J.T.; Li, X.; Zhu, J.; Giangarra, V.; Grzeskowiak, C.L.; Ju, J.; Liu, I.H.; Chiou, S.-H.; Salahudeen, A.A.; Smith, A.R.; et al. Organoid Modeling of the Tumor Immune Microenvironment. Cell 2018, 175, 1972–1988.e16. [Google Scholar] [CrossRef]
| Model | Description | Strengths | Limitations |
|---|---|---|---|
| GL261 | Carcinogen-induced glioma cell line derived from C57BL/6 mice |
|
|
| CT-2A | Carcinogen-induced glioma cell line derived from C57BL/6 mice |
|
|
| SMA-560 | Spontaneously forming astrocytoma cell line derived from VM/Dk mice |
|
|
| GEMMs | Mouse models engineered to express mutations in pre-specified genes |
|
|
| Humanized mouse models | Mouse models depleted of native immune system and reconstituted with human immune progenitor cells |
|
|
| Organoids | 3D cultures derived from primary tissue |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, A.L.; Wu, J.Y.; Lee, S.Y.; Lim, M. Translational Models in Glioma Immunotherapy Research. Curr. Oncol. 2023, 30, 5704-5718. https://doi.org/10.3390/curroncol30060428
Ren AL, Wu JY, Lee SY, Lim M. Translational Models in Glioma Immunotherapy Research. Current Oncology. 2023; 30(6):5704-5718. https://doi.org/10.3390/curroncol30060428
Chicago/Turabian StyleRen, Alexander L., Janet Y. Wu, Si Yeon Lee, and Michael Lim. 2023. "Translational Models in Glioma Immunotherapy Research" Current Oncology 30, no. 6: 5704-5718. https://doi.org/10.3390/curroncol30060428
APA StyleRen, A. L., Wu, J. Y., Lee, S. Y., & Lim, M. (2023). Translational Models in Glioma Immunotherapy Research. Current Oncology, 30(6), 5704-5718. https://doi.org/10.3390/curroncol30060428