Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study
Abstract
1. Introduction
2. Patients and Methods
- Patients who have progressed following exposure to at least two prior regimens of standard chemotherapy using fluoropyrimidine, irinotecan, oxaliplatin, anti-vascular endothelial growth factor (VEGF) antibodies (bevacizumab and aflibercept), or anti-EGFR antibodies (cetuximab or panitumumab);
- Age > 18 years;
- Eastern Cooperative Oncology Group performance status (ECOG PS) 0–2;
- Known RAS mutation status;
- Adequate organ function at the start of treatment;
- Histologically confirmed stage IV adenocarcinoma of the colon or rectum with unresectable metastatic disease.
Statistical Analysis
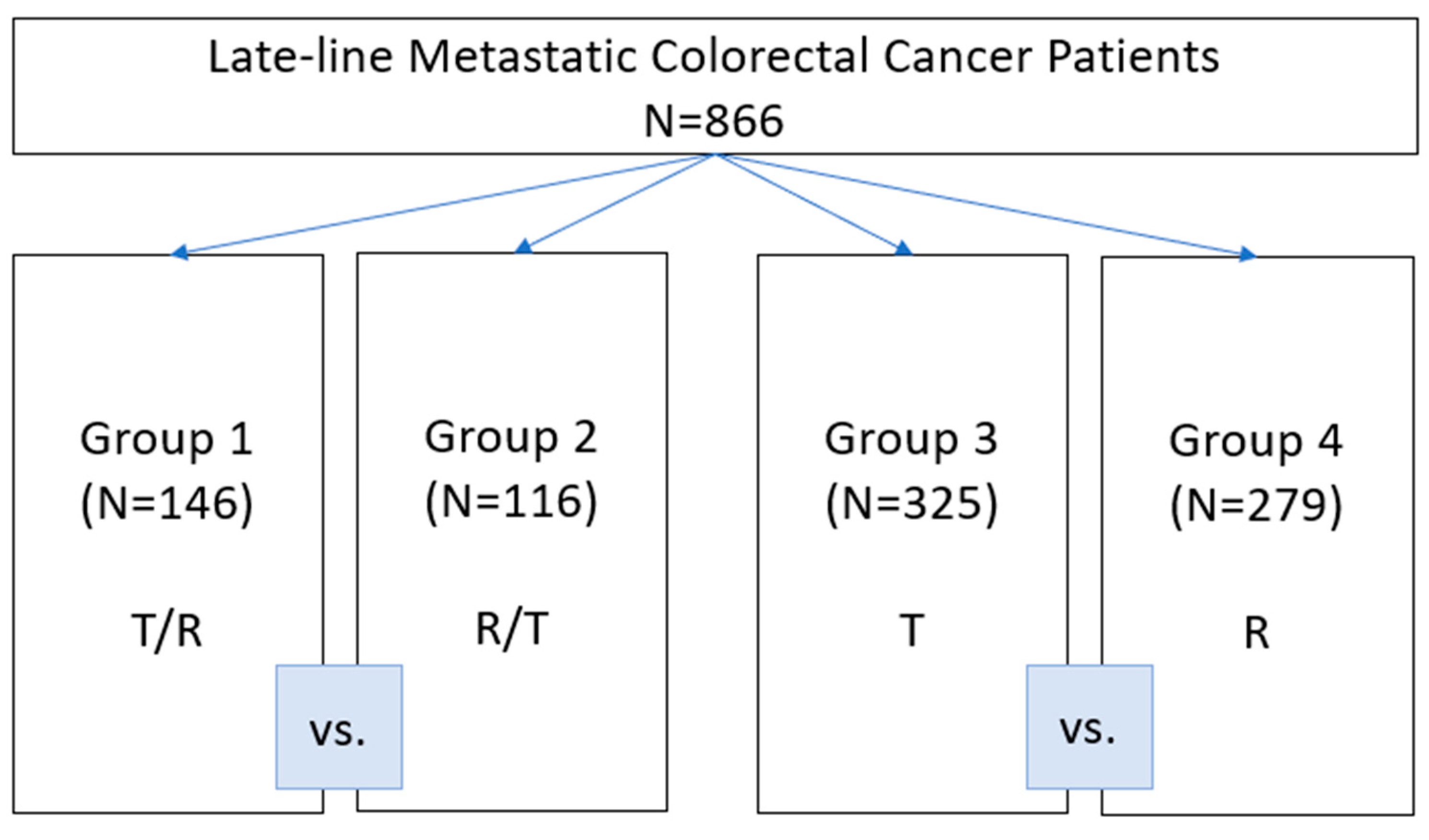
3. Results
3.1. Patient Characteristics
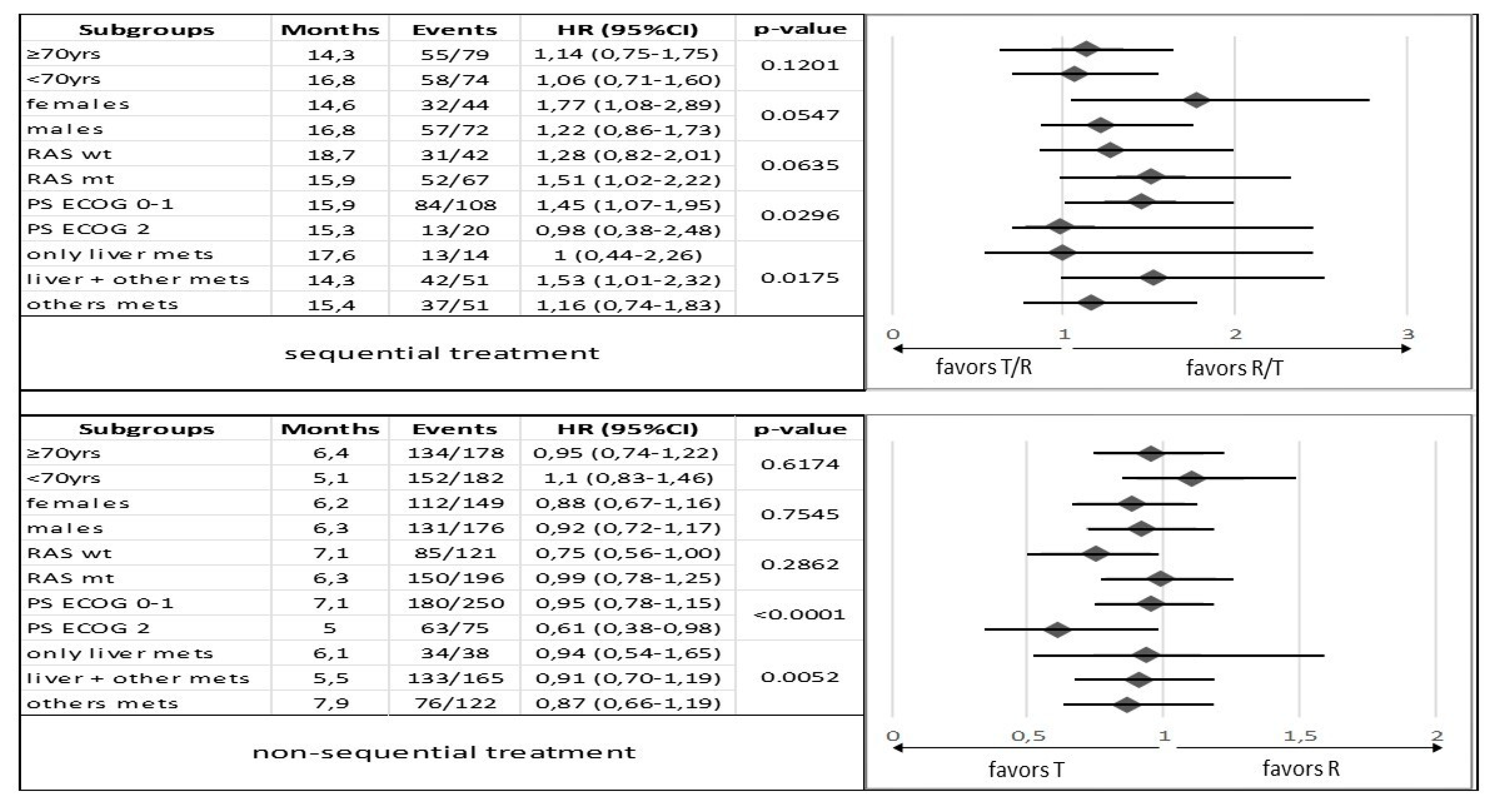
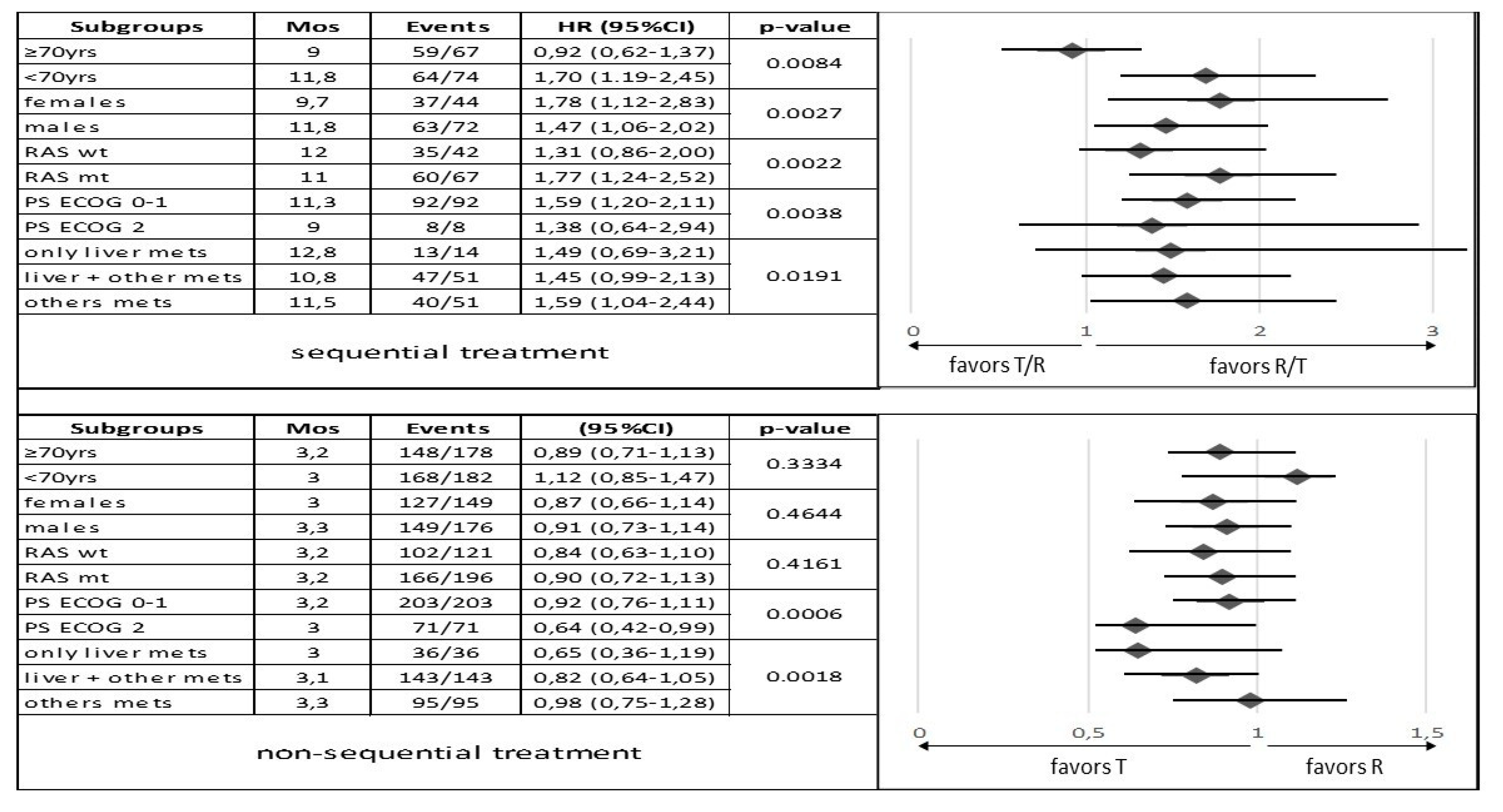
3.2. Survival and Responses in Sequential Treatment Groups
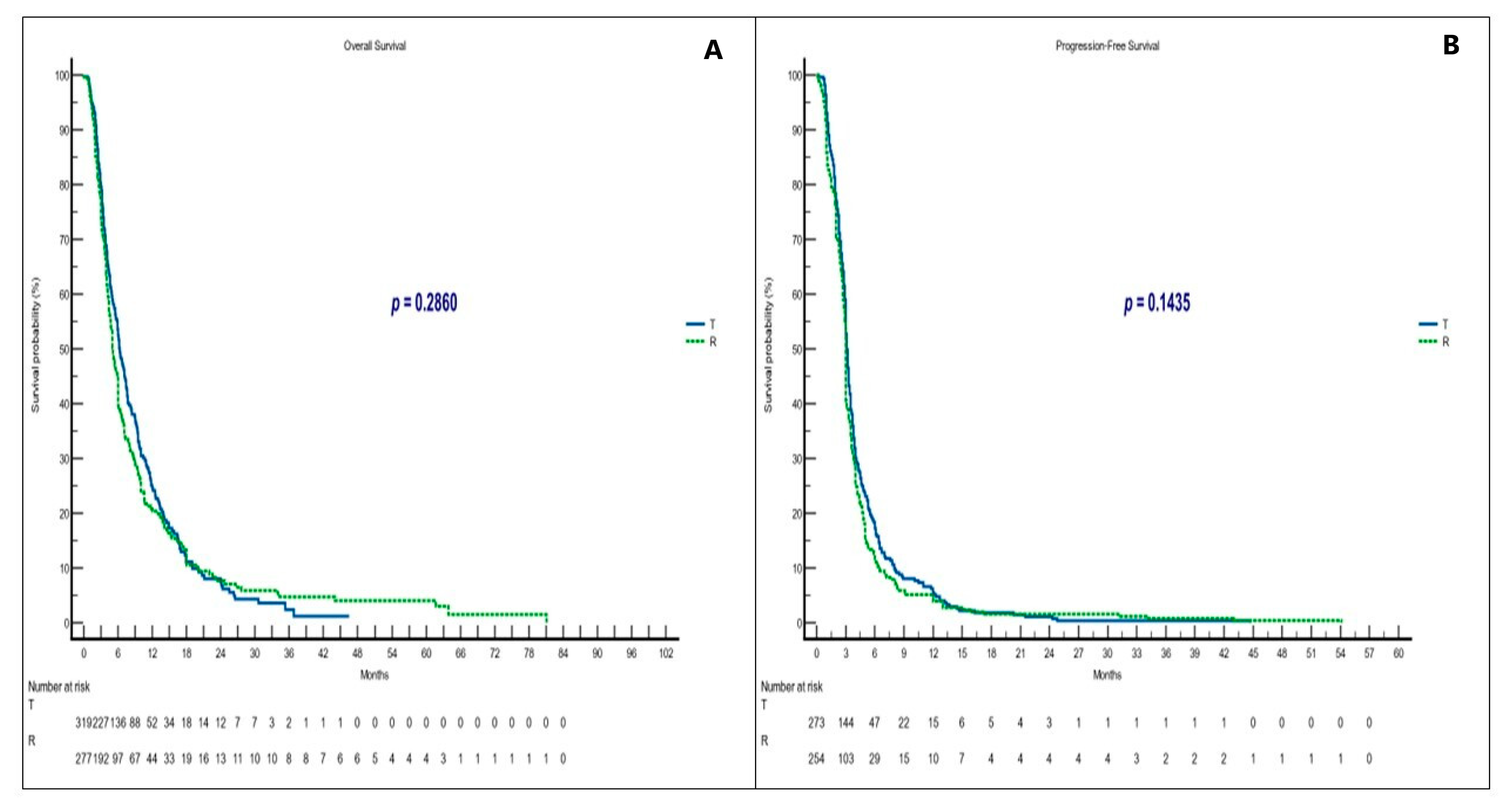
3.3. Survival and Responses in Non-Sequential Treatment Groups
3.4. Safety
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Morris, V.K.; Kennedy, E.B.; Baxter, N.N.; Benson, A.B., 3rd; Cercek, A.; Cho, M.; Ciombor, K.K.; Cremolini, C.; Davis, A.; Deming, D.A.; et al. Treatment of Metastatic Colorectal Cancer: ASCO Guideline. J. Clin. Oncol. 2023, 41, 678–700. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®®) Colon Cancer Version 3.2022–January 25, 2023. Available online: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (accessed on 25 January 2023).
- Araghi, M.; Soerjomataram, I.; Jenkins, M.; Brierley, J.; Morris, E.; Bray, F.; Arnold, M. Global trends in colorectal cancer mortality: Projections to the year 2035. Int. J. Cancer 2019, 144, 2992–3000. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Adam, R.; Sobrero, A.; Van Krieken, J.H.; Aderka, D.; Aranda Aguilar, E.; Bardelli, A.; Benson, A.; Bodoky, G.; et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016, 27, 1386–1422. [Google Scholar] [CrossRef]
- Sonbol, M.B.; Benkhadra, R.; Wang, Z.; Firwana, B.; Walden, D.J.; Mody, K.; Hubbard, J.M.; Murad, M.H.; Ahn, D.H.; Bekaii-Saab, T. A Systematic Review and Network Meta-Analysis of Regorafenib and TAS-102 in Refractory Metastatic Colorectal Cancer. Oncologist 2019, 24, 1174–1179. [Google Scholar] [CrossRef]
- Puthiamadathil, J.M.; Weinberg, B.A. Emerging combination therapies for metastatic colorectal cancer-the impact of trifluridine/tipiracil. Cancer Manag. Res. 2017, 9, 461–469. [Google Scholar] [CrossRef]
- Chiang, C.L.; Choi, H.C.; Lam, K.O.; Chan, B.Y.; Lee, S.F.; Yeung, S.Y.; Lau, K.S.; Chan, S.Y.; Choy, T.S.; Yuen, K.K. Real-world treatment patterns and outcomes in refractory metastatic colorectal cancer. Asia Pac. J. Clin. Oncol. 2019, 15 (Suppl. S2), 5–13. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomized, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Xu, J.; Kim, T.W.; Shen, L.; Sriuranpong, V.; Pan, H.; Xu, R.; Guo, W.; Han, S.W.; Liu, T.; Park, Y.S.; et al. Results of a Randomized, Double-Blind, Placebo-Controlled, Phase III Trial of Trifluridine/Tipiracil (TAS-102) Monotherapy in Asian Patients with Previously Treated Metastatic Colorectal Cancer: The TERRA Study. J. Clin. Oncol. 2018, 36, 350–358. [Google Scholar] [CrossRef]
- Fondevila, F.; Mendez-Blanco, C.; Fernandez-Palanca, P.; Gonzalez-Gallego, J.; Mauriz, J.L. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp. Mol. Med. 2019, 51, 1–15. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. CONCUR Investigators. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomized, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. RECOURSE Study Group. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Arita, S.; Shirakawa, T.; Matsushita, Y.; Shimokawa, H.K.; Hirano, G.; Makiyama, A.; Shibata, Y.; Tamura, S.; Esaki, T.; Mitsugi, K.; et al. Efficacy and safety of TAS-102 in clinical practice of salvage chemotherapy for metastatic colorectal cancer. Anticancer Res. 2016, 36, 1959–1966. [Google Scholar]
- Fernandez Montes, A.; Vazquez Rivera, F.; Martinez Lago, N.; Covela Rúa, M.; Cousillas Castiñeiras, A.; Gonzalez Villarroel, P.; de la Cámara Gómez, J.; Méndez Méndez, J.C.; Salgado Fernández, M.; Candamio Folgar, S.; et al. Efficacy and safety of trifluridine/tipiracil in third-line and beyond for the treatment of patients with metastatic colorectal cancer in routine clinical practice: Patterns of use and prognostic nomogram. Clin. Transl. Oncol. 2020, 22, 351–359. [Google Scholar] [CrossRef]
- Moriwaki, T.; Fukuoka, S.; Taniguchi, H.; Takashima, A.; Kumekawa, Y.; Kajiwara, T.; Yamazaki, K.; Esaki, T.; Makiyama, C.; Denda, T.; et al. Propensity Score Analysis of Regorafenib versus Trifluridine/Tipiracil in Patients with Metastatic Colorectal Cancer Refractory to Standard Chemotherapy (REGOTAS): A Japanese Society for Cancer of the Colon and Rectum Multicenter Observational Study. Oncologist 2018, 23, 7–15. [Google Scholar] [CrossRef]
- Tabernero, J.; Argiles, G.; Sobrero, A.F.; Borg, C.; Ohtsu, A.; Mayer, R.J.; Vidot, L.; Moreno Vera, S.R.; Van Cutsem, E. Effect of trifluridine/tipiracil in patients treated in RECOURSE by prognostic factors at baseline: An exploratory analysis. ESMO Open 2020, 5, e000752. [Google Scholar] [CrossRef]
- Tabernero, J.; Prager, G.W.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Papai, Z.; et al. Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: The phase 3 randomized SUNLIGHT study. J. Clin. Oncol. 2023, 41, 4. [Google Scholar] [CrossRef]
- Adenis, A.; de la Fouchardiere, C.; Paule, B.; Burtin, P.; Tougeron, D.; Wallet, J.; Dourthe, L.M.; Etienne, P.L.; Mineur, L.; Clisant, S.; et al. Survival, safety, and prognostic factors for outcome with Regorafenib in patients with metastatic colorectal cancer refractory to standard therapies: Results from a multicenter study (REBECCA) nested within a compassionate use program. BMC Cancer 2016, 16, 412, Erratum in BMC Cancer 2016, 16, 518. [Google Scholar] [CrossRef]
- Ducreux, M.; Petersen, L.N.; Öhler, L.; Bergamo, F.; Metges, J.P.; de Groot, J.W.; Wang, J.Y.; García Paredes, B.; Dochy, E.; Fiala-Buskies, S.; et al. CORRELATE Investigators. Safety and effectiveness of regorafenib in patients with metastatic colorectal cancer in routine clinical practice in the prospective, observational CORRELATE study. Eur. J. Cancer. 2019, 123, 146–154. [Google Scholar] [CrossRef]
- García-Alfonso, P.; Feliú, J.; García-Carbonero, R.; Grávalos, C.; Guillén-Ponce, C.; Sastre, J.; García-Foncillas, J. Is regorafenib providing clinically meaningful benefits to pretreated patients with metastatic colorectal cancer? Clin. Transl. Oncol. 2016, 18, 1072–1081. [Google Scholar] [CrossRef]
- Arrieta Loitegui, M.; Lázaro Cebas, A.; Rodríguez Quesada, P.; García Muñoz, C.; Rosas Espinoza, C.; Ferrari Piquero, J.M. Efectividad y seguridad de regorafenib y trifluridina/tipiracilo en cáncer colorrectal metastático. Rev. De La OFIL 2020, 30, 99–104. [Google Scholar] [CrossRef]
- Patel, A.K.; Duh, M.S.; Barghout, V.; Yenikomshian, M.A.; Xiao, Y.; Wynant, W.; Tabesh, M.; Fuchs, C.S. Real-world Treatment Patterns among Patients with Colorectal Cancer Treated with Trifluridine/Tipiracil and Regorafenib. Clin. Color. Cancer 2018, 17, e531–e539. [Google Scholar] [CrossRef]
- Abrahao, A.B.K.; Ko, Y.J.; Berry, S.; Chan, K.K.W. A Comparison of Regorafenib and TAS-102 for Metastatic Colorectal Cancer: A Systematic Review and Network Meta-analysis. Clin. Color. Cancer 2018, 17, 113–120. [Google Scholar] [CrossRef]
- Ogata, M.; Kotaka, M.; Ogata, T.; Hatachi, Y.; Yasui, H.; Kato, T.; Tsuji, A.; Satake, H. Regorafenib vs trifluridine/tipiracil for metastatic colorectal cancer refractory to standard chemotherapies: A multicenter retrospective comparison study in Japan. PLoS ONE 2020, 15, e0234314. [Google Scholar] [CrossRef]
- Sueda, T.; Sakai, D.; Kudo, T.; Sugiura, T.; Takahashi, H.; Haraguchi, N.; Nishimura, J.; Hata, T.; Hayashi, T.; Mizushima, T.; et al. Efficacy and Safety of Regorafenib or TAS-102 in Patients with Metastatic Colorectal Cancer Refractory to Standard Therapies. Anticancer Res. 2016, 36, 4299–4306. [Google Scholar]
- Masuishi, T.; Taniguchi, H.; Hamauchi, S.; Komori, A.; Kito, Y.; Narita, Y.; Tsushima, T.; Ishihara, M.; Todaka, A.; Tanaka, T.; et al. Regorafenib versus trifluridine/tipiracil for refractory metastatic colorectal cancer: A retrospective comparison. Clin. Color. Cancer 2017, 16, e15–e22. [Google Scholar] [CrossRef]
- Masuishi, T.; Taniguchi, H.; Kawakami, T.; Kawamoto, Y.; Kadowaki, S.; Onozawa, Y.; Muranaka, T.; Tajika, M.; Yasui, H.; Nakatsumi, H.; et al. Impact of tumour growth rate during preceding treatment on tumour response to regorafenib or trifluridine/tipiracil in refractory metastatic colorectal cancer. ESMO Open 2019, 4, e000584. [Google Scholar] [CrossRef]
- Nakashima, M.; Takeuchi, M.; Kawakami, K. Effectiveness and Safety of Regorafenib vs. Trifluridine/Tipiracil in Unresectable Colorectal Cancer: A Retrospective Cohort Study. Clin. Color. Cancer 2020, 19, e208–e225. [Google Scholar] [CrossRef]
- Weinberg, B.A.; Marshall, J.L.; Salem, M.E. Trifluridine/tipiracil and regorafenib: New weapons in the war against metastatic colorectal cancer. Clin. Adv. Hematol. Oncol. 2016, 14, 630–638. [Google Scholar]
- Matsumoto, T.; Ikoma, T.; Yamamura, S.; Miura, K.; Tsuduki, T.; Watanabe, T.; Nagai, H.; Takatani, M.; Yasui, H. Regorafenib is suitable for advanced colorectal cancer pts who have previously received trifluridine/tipiracil plus bevacizumab. Sci. Rep. 2023, 13, 2433. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Ou, F.S.; Ahn, D.H.; Boland, P.M.; Ciombor, K.K.; Heying, E.N.; Dockter, T.J.; Jacobs, N.L.; Pasche, B.C.; Cleary, J.M.; et al. Regorafenib dose-optimization in pts with refractory metastatic colorectal cancer (ReDOS): A randomized, multicentre, open-label, phase 2 study. Lancet Oncol. 2019, 20, 1070–1082. [Google Scholar] [CrossRef]
- Patel, A.K.; Abhyankar, R.; Brais, L.K.; Duh, M.S.; Barghout, V.E.; Huynh, L.; Yenikomshian, M.A.; Ng, K.; Fuchs, C.S. Trifluridine/Tipiracil and Regorafenib in Pts with Metastatic Colorectal Cancer: A Retrospective Study at a Tertiary Oncology Center. Oncologist 2021, 26, e2161–e2169. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, A.; Sadahiro, S.; Suzuki, T.; Okada, K.; Saito, G.; Miyakita, H. Retrospective study of regorafenib and trifluridine/tipiracil efficacy as a third-line or later chemotherapy regimen for refractory metastatic colorectal cancer. Oncol. Lett. 2018, 16, 6589–6597. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Fukuoka, S.; Masuishi, T.; Takashima, A.; Kumekawa, Y.; Kajiwara, T.; Yamazaki, K.; Negoro, Y.; Komoda, M.; Makiyama, A.; et al. Clinical Impact of Primary Tumor Location in Metastatic Colorectal Cancer Patients under Later-Line Regorafenib or Trifluridine/Tipiracil Treatment. Front. Oncol. 2021, 11, 688709. [Google Scholar] [CrossRef] [PubMed]
- Signorelli, C.; Gemma, D.; Grande, R.; De Marco, S.; Saltarelli, R.; Morandi, M.G.; Spinelli, G.P.; Zoratto, F.; Sperduti, I.; Chilelli, M.G.; et al. Regorafenib-to-trifluridine/tipiracil versus the Reverse Sequence for Refractory Metastatic Colorectal Cancer Pts: A Multicenter Retrospective Real-life Experience. Anticancer Res. 2021, 41, 2553–2561. [Google Scholar] [CrossRef]
- Unseld, M.; Drimmel, M.; Siebenhüner, A.; Gleiss, A.; Bianconi, D.; Kieler, M.; Scheithauer, W.; Winder, T.; Prager, G.W. Optimizing Treatment Sequence for Late-line Metastatic Colorectal Cancer Patients Using Trifluridine/Tipiracil and Regorafenib. Clin. Color. Cancer 2018, 17, 274–279. [Google Scholar] [CrossRef]
- Modest, D.P.; Pant, S.; Sartore-Bianchi, A. Treatment sequencing in metastatic colorectal cancer. Eur. J. Cancer 2019, 109, 70–83. [Google Scholar] [CrossRef]
- Signorelli, C.; Schirripa, M.; Chilelli, M.G.; Calegari, M.A.; Basso, M.; Anghelone, A.; Lucchetti, J.; Minelli, A.; Angotti, A.; Gemma, D.; et al. Sequential treatment with regorafenib and trifluridine/tipiracil in later-line refractory metastatic colorectal cancer: A real-world multicenter retrospective study. J. Clin. Oncol. 2023, 41, 45. [Google Scholar] [CrossRef]
- Coutzac, C.; Trouilloud, I.; Artru, P.; Henriques, J.; Masson, T.; Doat, S.; Bouché, O.; Coriat, R.; Saint, A.; Moulin, V.; et al. Sequential Treatment with Trifluridine/Tipiracil and Regorafenib in Refractory Metastatic Colorectal Cancer Pts: An AGEO Prospective “Real-World Study”. Clin. Color. Cancer 2022, 21, 132–140. [Google Scholar] [CrossRef]
- Oshima, K.; Hirano, H.; Shoji, H.; Iwasa, S.; Okita, N.; Takashima, A.; Boku, N. Influence of precedent drug on the subsequent therapy in the sequence of trifluridine/tipiracil with/out bevacizumab and regorafenib for unresectable or recurrent colorectal cancer. PLoS ONE 2022, 17, e0269115. [Google Scholar] [CrossRef]
- Christopher Nevala-Plagemann, C.; Sama, S.; Ying, J.; Shen, J.; Haaland, B.; Florou, V.; Garrido-Laguna, I. A Real-World Comparison of Regorafenib and Trifluridine/Tipiracil in Refractory Metastatic Colorectal Cancer in the United States. J. Natl. Compr. Cancer Netw. 2023, 21, 257–264. [Google Scholar] [CrossRef]
- Walter, T.; Hawkins, N.S.; Pollock, R.F.; Colaone, F.; Shergill, S.; Ross, P.J. Systematic review and network meta-analyses of third-line treatments for metastatic colorectal cancer. J. Cancer Res. Clin. Oncol. 2020, 146, 2575–2587. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Wang, J.; Lin, H.; Peng, Y. Comparison of Regorafenib, Fruquintinib, and TAS-102 in Previously Treated Patients with Metastatic Colorectal Cancer: A Systematic Review and Network Meta-Analysis of Five Clinical Trials. Med. Sci. Monit. 2019, 25, 9179–9191. [Google Scholar] [CrossRef]
- Casadei-Gardini, A.; Vagheggini, A.; Gelsomino, F.; Spallanzani, A.; Ulivi, P.; Orsi, G.; Rovesti, G.; Andrikou, K.; Tamburini, E.; Scartozzi, M.; et al. Is There an Optimal Choice in Refractory Colorectal Cancer? A Network Meta-Analysis. Clin. Color. Cancer 2020, 19, 82–90.e9. [Google Scholar] [CrossRef] [PubMed]
- Vitale, P.; Zanaletti, N.; Famiglietti, V.; De Falco, V.; Cervantes, A.; Rosellò, S.; Fenocchio, E.; Milanesio, M.; Lombardi, P.; Ciardiello, D.; et al. Retrospective Study of Regorafenib versus TAS-102 Efficacy and Safety in Chemorefractory Metastatic Colorectal Cancer (mCRC) Patients: A Multi-institution Real Life Clinical Data. Clin. Color. Cancer 2021, 20, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Giuliani, J.; Fiorica, F.; Ponturo, G.; Azzurro, M.; Ruzzenente, A.; Bonetti, A. Dose-escalation strategy in refractory metastatic colorectal cancer: A change in terms of cost-effectiveness. J. Oncol. Pharm. Pract. 2021, 27, 974–977. [Google Scholar] [CrossRef]

| T/R | R/T | p-Value | T | R | p-Value | |
|---|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |||
| Total | 146 (16.8) | 116 (13.3) | 325 (37.5) | 279 (32.2) | ||
| Median age (years) | 69 (-) | 66 (-) | 71 (-) | 65 (-) | ||
| Patients ≥ 70yrs | 0.1306 | <0.00001 | ||||
| yes | 67 (45.8) | 42 (36.2) | 178 (54.7) | 97 (34.7) | ||
| no | 79 (54.1) | 74 (63.7) | 147 (45.2) | 182 (65.2) | ||
| Gender | 0.6981 | 0.4115 | ||||
| Female | 51 (34.9) | 44 (37.9) | 149 (45.8) | 118 (42.2) | ||
| Male | 95 (65.0) | 72 (62.0) | 176 (54.1) | 161 (57.7) | ||
| RAS status | 0.5986 | 0.1807 | ||||
| Wild type | 49 (33.5) | 42 (36.2) | 122 (37.5) | 122 (43.7) | ||
| Mutant type | 90 (61.6) | 67 (57.7) | 195 (60.0) | 154 (55.1) | ||
| Tumor location | 0.2926 | 0.6611 | ||||
| Right side | 52 (35.6) | 34 (29.3) | 100 (30.7) | 91 (32.6) | ||
| Left side | 94 (64.3) | 82 (70.6) | 225 (69.2) | 188 (67.3) | ||
| MSI | 0.4594 | 0.675 | ||||
| yes | 3 (2.0) | 4 (3.4) | 3 (0.9) | 3 (1.0) | ||
| no | 89 (60.9) | 64 (55.1) | 177 (54.4) | 105 (37.6) | ||
| ECOG PS | 0.2113 | 0.3228 | ||||
| 0–1 | 128 (87.6) | 108 (93.1) | 250 (76.9) | 224 (80.2) | ||
| 2 | 18 (12.3) | 8 (6.8) | 75 (23.0) | 55 (19.7) | ||
| Prior adjuvant therapy | 0.232 | 0.0094 | ||||
| yes | 46 (31.5) | 43 (37.0) | 90 (27.6) | 52 (18.6) | ||
| no | 100 (68.4) | 66 (56.8) | 235 (72.3) | 227 (81.3) | ||
| Metastatic disease sites | 0.4418 | 0.4508 | ||||
| Liver only | 23 (15.7) | 14 (12.0) | 38 (11.6) | 31 (11.1) | ||
| Liver + other | 80 (54.7) | 51 (43.9) | 133 (40.9) | 156 (55.9) | ||
| Others | 43 (29.4) | 51 (43.9) | 154 (47.3) | 92 (32.9) | ||
| CT 1°line regimen | 0.4008 | 0.6262 | ||||
| Doublet chemotherapy | 112 (76.7) | 94 (81.0) | 265 (81.5) | 140 (50.1) | ||
| Triplet chemotherapy | 16 (10.9) | 9 (7.7) | 33 (10.1) | 14 (5.0) | ||
| CT 2°line regimen | 1 | 0.3015 | ||||
| Doublet chemotherapy | 119 (81.5) | 94 (81.0) | 258 (79.3) | 119 (42.6) | ||
| Triplet chemotherapy | 2 (1.3) | 2 (1.7) | 5 (1.5) | 5 (1.7) | ||
| CT 3°line regimen | 0.6221 | 0.3334 | ||||
| Fluoropyrimidine alone | 3 (2.0) | 2 (1.7) | 4 (1.2) | 8 (2.8) | ||
| Doublet chemotherapy | 9 (6.1) | 15 (12.9) | 19 (5.8) | 18 (6.4) | ||
| Biological agents 1°line | 0.3873 | 0.378 | ||||
| Anti-EGFR use | 43 (29.4) | 30 (25.8) | 86 (26.4) | 52 (18.6) | ||
| Anti-VEGF use | 72 (49.3) | 65 (56.0) | 176 (54.1) | 87 (31.1) | ||
| Biological agents 2°line | 1 | 0.5379 | ||||
| Anti-EGFR use | 9 (6.1) | 7 (6.0) | 17 (5.2) | 11 (3.9) | ||
| Anti-VEGF use | 97 (66.4) | 81 (69.8) | 198 (60.9) | 100 (35.8) | ||
| Biological agents 3°line | 0.7089 | 0.747 | ||||
| Anti-EGFR use | 10 (6.8) | 10 (8.6) | 13 (4.0) | 12 (4.3) | ||
| Anti-VEGF use | 4 (2.7) | 6 (5.1) | 9 (2.7) | 6 (2.1) |
| OS | PFS | ORR | DCR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mOS (mos) | 95% CI | HR | p-Value | mPFS (mos) | 95% CI | HR | p-Value | PR + CR (%) | p-Value | PR + CR + SD (%) | p-Value | |
| T/R | 13.9 | 11.0–15.1 | 0.70 0.52–0.94 | 0.0194 | 8.8 | 8.2–33.8 | 0.61 0.46–0.80 | 0.0005 | 5.8 | 0.5177 | 34.1 | 0.5006 |
| R/T | 15.9 | 13.9–73.0 | 1.41 1.05–1.89 | 11.2 | 9.7–55.0 | 1.62 1.23–2.12 | 3.1 | 47.9 | ||||
| T | 6.3 | 5.6–7.4 | 1.10 0.92–1.32 | 0.2860 | 3.1 | 3.0–44.7 | 0.87 0.73–1.04 | 0.1435 | 2.5 | 1 | 23.3 | 0.6793 |
| R | 5.1 | 4.5–81.1 | 0.90 0.75–1.08 | 3.0 | 2.9–54.1 | 1.14 0.95–1.36 | 2.3 | 21.7 | ||||
| T/R | R/T | T | R | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | p-Value | N | % | N | % | p-Value | |
| All events G3/G4 N = 582 | 155 | 26.6 | 132 | 22.6 | - | 160 | 27.4 | 135 | 23.1 | - |
| All pts who experienced G3/G4 toxicities N = 390 | 89/146 | 60.9 (22.8) | 79/116 | 68.1 (20.2) | 0.24 | 118/325 | 36.3 (30.2) | 104/279 | 37.2 (26.6) | 0.86 |
| All Haematologic events G3/G4 N = 289 | 87 | 56.1 (14.9) | 65 | 49.2 (11.1) | 0.28 | 126 | 78.7 (21.6) | 11 | 8.1 (1.8) | <0.00001 |
| All non Haematologic events G3/G4 N=293 | 68 | 43.8 (11.6) | 67 | 50.7 (11.5) | 34 | 21.2 (5.8) | 124 | 91.8 (21.3) | ||
| Most common Haematologic toxicities G3/G4 | ||||||||||
| Neutropenia | 68 | 78.2 | 43 | 66.2 | 0.13 | 82 | 65.1 | 3 | 27.3 | 0.02 |
| Leucopenia | 2 | 2.3 | 0 | 0 | 0.5 | 6 | 4.8 | 0 | 0 | 1 |
| Thrombocytopenia | 3 | 3.4 | 5 | 7.7 | 0.28 | 3 | 2.4 | 0 | 0 | 1 |
| Anemia | 14 | 16.1 | 17 | 26.2 | 0.15 | 35 | 27.8 | 8 | 72.7 | 0.0042 |
| Most common non Haematologic toxicities G3/G4 | ||||||||||
| Fatigue | 33 | 48.5 | 19 | 28.4 | 0.02 | 21 | 61.8 | 34 | 27.4 | 0.0004 |
| Hand-foot skin reaction | 5 | 7.4 | 25 | 37.3 | 0.01 | 0 | 0 | 42 | 33.9 | 0.01 |
| Liver dysfunctions | 2 | 2.9 | 2 | 3 | 1 | 2 | 5.9 | 1 | 0.8 | 0.11 |
| Diarrhea | 10 | 14.7 | 2 | 3 | 0.03 | 7 | 20.6 | 13 | 10.5 | 1.14 |
| Skin disorders | 2 | 2.9 | 3 | 4.5 | 1 | 0 | 0 | 11 | 8.9 | 0.12 |
| Others | 16 | 23.5 | 16 | 23.9 | 1 | 4 | 11.8 | 23 | 18.5 | 0.44 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Signorelli, C.; Calegari, M.A.; Basso, M.; Anghelone, A.; Lucchetti, J.; Minelli, A.; Angotti, L.; Zurlo, I.V.; Schirripa, M.; Chilelli, M.G.; et al. Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study. Curr. Oncol. 2023, 30, 5456-5469. https://doi.org/10.3390/curroncol30060413
Signorelli C, Calegari MA, Basso M, Anghelone A, Lucchetti J, Minelli A, Angotti L, Zurlo IV, Schirripa M, Chilelli MG, et al. Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study. Current Oncology. 2023; 30(6):5456-5469. https://doi.org/10.3390/curroncol30060413
Chicago/Turabian StyleSignorelli, Carlo, Maria Alessandra Calegari, Michele Basso, Annunziato Anghelone, Jessica Lucchetti, Alessandro Minelli, Lorenzo Angotti, Ina Valeria Zurlo, Marta Schirripa, Mario Giovanni Chilelli, and et al. 2023. "Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study" Current Oncology 30, no. 6: 5456-5469. https://doi.org/10.3390/curroncol30060413
APA StyleSignorelli, C., Calegari, M. A., Basso, M., Anghelone, A., Lucchetti, J., Minelli, A., Angotti, L., Zurlo, I. V., Schirripa, M., Chilelli, M. G., Morelli, C., Dell’Aquila, E., Cosimati, A., Gemma, D., Ribelli, M., Emiliani, A., Corsi, D. C., Arrivi, G., Mazzuca, F., ... Ruggeri, E. M. (2023). Treatment Settings and Outcomes with Regorafenib and Trifluridine/Tipiracil at Third-Line Treatment and beyond in Metastatic Colorectal Cancer: A Real-World Multicenter Retrospective Study. Current Oncology, 30(6), 5456-5469. https://doi.org/10.3390/curroncol30060413





