Young Myeloma Patients: A Systematic Review of Manifestations and Outcomes
Abstract
1. Introduction
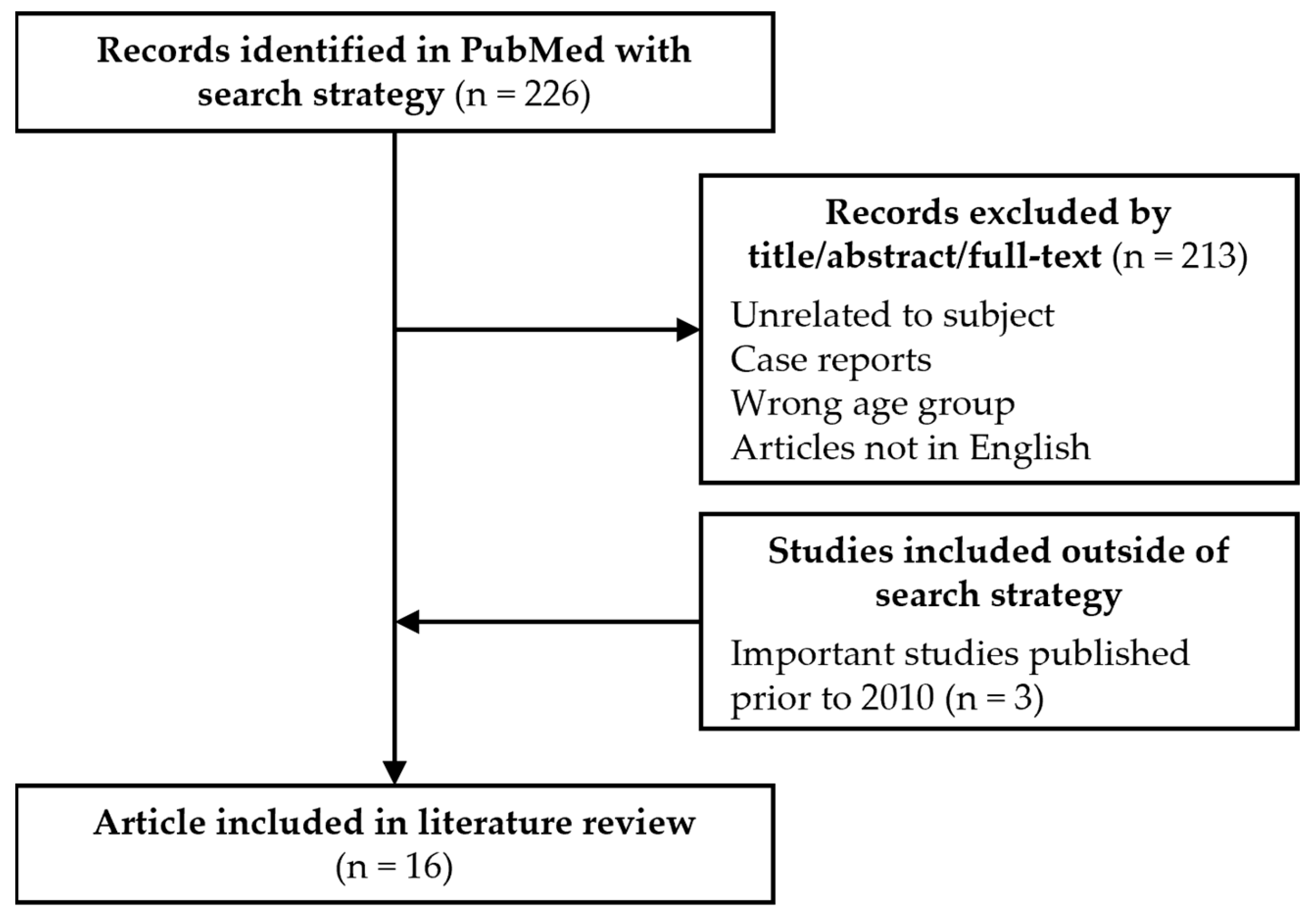
2. Methods
3. Results
3.1. Disease Characteristics at Diagnosis
3.2. Cytogenetics
3.3. Treatments and Outcomes
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Blimark, C.H.; Turesson, I.; Genell, A.; Ahlberg, L.; Björkstrand, B.; Carlson, K.; Forsberg, K.; Juliusson, G.; Linder, O.; Mellqvist, U.-H.; et al. Outcome and survival of myeloma patients diagnosed 2008–2015. Real-world data on 4904 patients from the Swedish Myeloma Registry. Haematologica 2018, 103, 506–513. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute. SEER Cancer Statistics Factsheets: Multiple Myeloma. Available online: https://seer.cancer.gov/statfacts/html/mulmy.html (accessed on 3 January 2023).
- Kyle, R.A.; Gertz, M.A.; Witzig, T.E.; Lust, J.A.; Lacy, M.Q.; Dispenzieri, A.; Fonseca, R.; Rajkumar, S.V.; Offord, J.R.; Larson, D.R.; et al. Review of 1027 patients with newly diagnosed multiple myeloma. Mayo Clin. Proc. 2003, 78, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Bolejack, V.; Crowley, J.; Bladé, J.; Miguel, J.S.; Kyle, R.A.; Rajkumar, S.V.; Shimizu, K.; Turesson, I.; Westin, J.; et al. Survival and Years of Life Lost in Different Age Cohorts of Patients with Multiple Myeloma. J. Clin. Oncol. 2010, 28, 1599–1605. [Google Scholar] [CrossRef] [PubMed]
- Van der Poel, M.W.M.; Oerlemans, S.; Schouten, H.C.; van de Poll-Franse, L.V. Elderly multiple myeloma patients experience less deterioration in health-related quality of life than younger patients compared to a normative population: A study from the population-based PROFILES registry. Ann. Hematol. 2015, 94, 651–661. [Google Scholar] [CrossRef]
- Lenhoff, S.; Hjorth, M.; Westin, J.; Brinch, L.; Bäckström, B.; Carlson, K.; Christiansen, I.; Dahl, I.M.; Gimsing, P.; Hammerström, J.; et al. Impact of age on survival after intensive therapy for multiple myeloma: A population-based study by the Nordic Myeloma Study Group. Br. J. Haematol. 2006, 133, 389–396. [Google Scholar] [CrossRef]
- Bladé, J.; Kyle, R.A.; Greipp, P.R. Presenting features and prognosis in 72 patients with multiple myeloma who were younger than 40 years. Br. J. Haematol. 1996, 93, 345–351. [Google Scholar] [CrossRef]
- Corso, A.; Klersy, C.; Lazzarino, M.; Bernasconi, C. Multiple myeloma in younger patients: The role of age as prognostic factor. Ann. Hematol. 1998, 76, 67–72. [Google Scholar] [CrossRef]
- Ludwig, H.; Durie, B.G.; Bolejack, V.; Turesson, I.; Kyle, R.A.; Blade, J.; Fonseca, R.; Dimopoulos, M.; Shimizu, K.; San Miguel, J.; et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: An analysis of 10 549 patients from the International Myeloma Working Group. Blood 2008, 111, 4039–4047. [Google Scholar] [CrossRef]
- Shin, J.; Koh, Y.; Youk, J.; Kim, M.; Kim, B.S.; Choi, C.W.; Sung, H.J.; Park, Y.; Yoon, S.S.; Kim, I. Clinicopathological characteristics of extremely young Korean multiple myeloma patients: Therapeutic implications. Korean J. Intern. Med. 2017, 32, 722–730. [Google Scholar] [CrossRef]
- Ravi, P.; Kumar, S.K.; Cerhan, J.R.; Maurer, M.J.; Dingli, D.; Ansell, S.M.; Rajkumar, S.V. Defining cure in multiple myeloma: A comparative study of outcomes of young individuals with myeloma and curable hematologic malignancies. Blood Cancer J. 2018, 8, 26. [Google Scholar] [CrossRef]
- Yanamandra, U.; Saini, N.; Chauhan, P.; Sharma, T.; Khadwal, A.; Prakash, G.; Varma, N.; Lad, D.; Varma, S.; Malhotra, P. AYA-Myeloma: Real-World, Single-Center Experience Over Last 5 Years. J. Adolesc. Young Adult Oncol. 2018, 7, 120–124. [Google Scholar] [CrossRef]
- Jurczyszyn, A.; Davila, J.; Kortüm, K.M.; Jayabalan, D.S.; Vij, R.; Fiala, M.; Milunovic, V.; Chim, C.S.; Wiśniewska-Piąty, K.; Waszczuk-Gajda, A.; et al. Multiple myeloma in patients up to 30 years of age: A multicenter retrospective study of 52 cases. Leuk. Lymphoma 2019, 60, 471–476. [Google Scholar] [CrossRef]
- Pál, I.; Illés, Á.; Váróczy, L. Multiple Myeloma of the Young—A Single Center Experience Highlights Future Directions. Pathol. Oncol. Res. 2020, 26, 419–424. [Google Scholar] [CrossRef]
- Duek, A.; Trakhtenbrot, L.; Avigdor, A.; Nagler, A.; Leiba, M. Multiple Myeloma Presenting in Patients Younger than 50 Years of Age: A Single Institution Experience. Acta Haematol. 2021, 144, 58–65. [Google Scholar] [CrossRef]
- Caulier, A.; Roussel, M.; Morel, P.; Lombion, N.; Branco, B.; Galtier, J.; Hulin, C.; Perrot, A.; Richez, V.; Michaud-Robert, A.V.; et al. Epidemiological landscape of young multiple myeloma patients diagnosed earlier than 40 years: The french experience. Blood 2021, 138, 2686–2695. [Google Scholar] [CrossRef]
- Bao, A.; Zhao, Q.; Merritt, E.; Bumma, N.; Devarakonda, S.; Khan, A.M.; Umyarova, E.; Rosko, A.E.; Benson, D.M.; Cottini, F. Racial differences as predictors of outcomes in young patients with multiple myeloma. Blood Cancer J. 2022, 12, 114. [Google Scholar] [CrossRef]
- Lu, J.; Lu, J.; Chen, W.; Wang, J.; Huo, Y.; Hou, J.; Huang, X. More frequent IgD and reduced CD200 expression in Chinese patients younger than 50 years old with multiple myeloma: A multicenter analysis. Drug Des. Dev. 2016, 10, 3673–3679. [Google Scholar] [CrossRef]
- Jurczyszyn, A.; Nahi, H.; Avivi, I.; Gozzetti, A.; Niesvizky, R.; Yadlapati, S.; Jayabalan, D.S.; Robak, P.; Pika, T.; Andersen, K.T.; et al. Characteristics and outcomes of patients with multiple myeloma aged 21–40 years versus 41–60 years: A multi-institutional case-control study. Br. J. Haematol. 2016, 175, 884–891. [Google Scholar] [CrossRef]
- Dhakal, B.; Nelson, A.; Murthy, G.S.G.; Fraser, R.; Eastwood, D.; Hamadani, M.; Pasquini, M.; D’Souza, A.; Hari, P. Autologous Hematopoietic Cell Transplantation in Patients with Multiple Myeloma: Effect of Age. Clin. Lymphoma Myeloma Leuk. 2017, 17, 165–172. [Google Scholar] [CrossRef]
- Nakaya, A.; Kohara, T.; Shibayama, H.; Onda, Y.; Kanda, J.; Kaneko, H.; Imada, K.; Kida, T.; Kosugi, S.; Ishikawa, J.; et al. Retrospective multi-center study of Adolescent and Young Adult (AYA) Multiple Myeloma in Kansai Myeloma Forum registry. Int. J. Hematol. 2020, 112, 435–438. [Google Scholar] [CrossRef]
- Pydi, V.R.; Bala, S.C.; Kuruva, S.P.; Chennamaneni, R.; Konatam, M.L.; Gundeti, S. Multiple Myeloma in Young Adults: A Single Centre Real World Experience. Indian J. Hematol. Blood Transfus. Off. J. Indian Soc. Hematol. Blood Transfus. 2021, 37, 679–683. [Google Scholar] [CrossRef]
- Rafae, A.; Malik, M.N.; Abu Zar, M.; Durer, S.; Durer, C. An Overview of Light Chain Multiple Myeloma: Clinical Characteristics and Rarities, Management Strategies, and Disease Monitoring. Cureus 2018, 10, e3148. [Google Scholar] [CrossRef] [PubMed]
- Kastritis, E.; Terpos, E.; Roussou, M.; Gavriatopoulou, M.; Migkou, M.; Eleutherakis-Papaiakovou, E.; Fotiou, D.; Ziogas, D.; Panagiotidis, I.; Kafantari, E.; et al. Evaluation of the Revised International Staging System in an independent cohort of unselected patients with multiple myeloma. Haematologica 2017, 102, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Altieri, A.; Chen, B.; Bermejo, J.L.; Castro, F.; Hemminki, K. Familial risks and temporal incidence trends of multiple myeloma. Eur. J. Cancer 2006, 42, 1661–1670. [Google Scholar] [CrossRef] [PubMed]
- Vachon, C.M.; Kyle, R.A.; Therneau, T.M.; Foreman, B.J.; Larson, D.R.; Colby, C.L.; Phelps, T.K.; Dispenzieri, A.; Kumar, S.K.; Katzmann, J.A.; et al. Increased risk of monoclonal gammopathy in first-degree relatives of patients with multiple myeloma or monoclonal gammopathy of undetermined significance. Blood 2009, 114, 785–790. [Google Scholar] [CrossRef]
- Clay-Gilmour, A.I.; Kumar, S.; Rajkumar, S.V.; Rishi, A.; Kyle, R.A.; Katzmann, J.A.; Murray, D.L.; Norman, A.D.; Greenberg, A.J.; Larson, D.R.; et al. Risk of MGUS in relatives of multiple myeloma cases by clinical and tumor characteristics. Leukemia 2019, 33, 499–507. [Google Scholar] [CrossRef]
- Walker, B.A.; Wardell, C.P.; Murison, A.; Boyle, E.M.; Begum, D.B.; Dahir, N.M.; Proszek, P.Z.; Melchor, L.; Pawlyn, C.; Kaiser, M.F.; et al. APOBEC family mutational signatures are associated with poor prognosis translocations in multiple myeloma. Nat. Commun. 2015, 6, 6997. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Fonseca, R.; Blood, E.; Rue, M.; Harrington, D.; Oken, M.M.; Kyle, R.A.; Dewald, G.W.; Van Ness, B.; Van Wier, S.A.; Henderson, K.J.; et al. Clinical and biologic implications of recurrent genomic aberrations in myeloma. Blood 2003, 101, 4569–4575. [Google Scholar] [CrossRef]
- Fonseca, R.; Debes-Marun, C.S.; Picken, E.B.; Dewald, G.W.; Bryant, S.C.; Winkler, J.M.; Blood, E.; Oken, M.M.; Santana-Dávila, R.; González-Paz, N.; et al. The recurrent IgH translocations are highly associated with nonhyperdiploid variant multiple myeloma. Blood 2003, 102, 2562–2567. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2020 update on diagnosis, risk-stratification and management. Am. J. Hematol. 2020, 95, 548–567. [Google Scholar] [CrossRef]
- Manier, S.; Salem, K.Z.; Park, J.; Landau, D.A.; Getz, G.; Ghobrial, I.M. Genomic complexity of multiple myeloma and its clinical implications. Nat. Rev. Clin. Oncol. 2017, 14, 100–113. [Google Scholar] [CrossRef]
- Singh, C.; Panakkal, V.; Sreedharanunni, S.; Jandial, A.; Jain, A.; Lad, D.; Prakash, G.; Khadwal, A.; Malhotra, P. Presentation and Impact of Double and Triple hit Cytogenetics in Patients With Multiple Myeloma in the Real World. Clin. Lymphoma Myeloma Leuk. 2022, 22, e685–e690. [Google Scholar] [CrossRef] [PubMed]
- Marcon, C.; Simeon, V.; Deias, P.; Facchin, G.; Corso, A.; Derudas, D.; Montefusco, V.; Offidani, M.; Petrucci, M.T.; Zambello, R.; et al. Experts’ consensus on the definition and management of high risk multiple myeloma. Front. Oncol. 2022, 12, 1096852. [Google Scholar] [CrossRef]
- Walker, B.A.; Mavrommatis, K.; Wardell, C.P.; Ashby, T.C.; Bauer, M.; Davies, F.; Rosenthal, A.; Wang, H.; Qu, P.; Hoering, A.; et al. A high-risk, Double-Hit, group of newly diagnosed myeloma identified by genomic analysis. Leukemia 2019, 33, 159–170. [Google Scholar] [CrossRef]
- Langseth, Ø.O.; Myklebust, T.; Johannesen, T.B.; Hjertner, Ø.; Waage, A. Incidence and survival of multiple myeloma: A population-based study of 10 524 patients diagnosed 1982–2017. Br. J. Haematol. 2020, 191, 418–425. [Google Scholar] [CrossRef]
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Rosiñol, L.; Oriol, A.; Rios, R.; Sureda, A.; Blanchard, M.J.; Hernández, M.T.; Martínez-Martínez, R.; Moraleda, J.M.; Jarque, I.; Bargay, J.; et al. Bortezomib, lenalidomide, and dexamethasone as induction therapy prior to autologous transplant in multiple myeloma. Blood 2019, 134, 1337–1345. [Google Scholar] [CrossRef]
- Roussel, M.; Lauwers-Cances, V.; Robillard, N.; Hulin, C.; Leleu, X.; Benboubker, L.; Marit, G.; Moreau, P.; Pegourie, B.; Caillot, D.; et al. Front-Line Transplantation Program With Lenalidomide, Bortezomib, and Dexamethasone Combination As Induction and Consolidation Followed by Lenalidomide Maintenance in Patients With Multiple Myeloma: A Phase II Study by the Intergroupe Francophone du Myélome. J. Clin. Oncol. 2014, 32, 2712–2717. [Google Scholar] [CrossRef]
- Richardson, P.G.; Weller, E.; Lonial, S.; Jakubowiak, A.J.; Jagannath, S.; Raje, N.S.; Avigan, D.E.; Xie, W.; Ghobrial, I.M.; Schlossman, R.L.; et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood 2010, 116, 679–686. [Google Scholar] [CrossRef]
- Moreau, P.; Attal, M.; Hulin, C.; Arnulf, B.; Belhadj, K.; Benboubker, L.; Béné, M.C.; Broijl, A.; Caillon, H.; Caillot, D.; et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): A randomised, open-label, phase 3 study. Lancet 2019, 394, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Voorhees, P.M.; Kaufman, J.L.; Laubach, J.P.; Sborov, D.W.; Reeves, B.; Rodriguez, C.; Chari, A.; Silbermann, R.W.; Costa, L.J.; Anderson, L.D.; et al. Depth of Response to Daratumumab (DARA), Lenalidomide, Bortezomib, and Dexamethasone (RVd) Improves over Time in Patients (pts) with Transplant-Eligible Newly Diagnosed Multiple Myeloma (NDMM): Griffin Study Update. Blood 2019, 134, 691. [Google Scholar] [CrossRef]
- Landgren, O.; Hultcrantz, M.; Diamond, B.; Lesokhin, A.M.; Mailankody, S.; Hassoun, H.; Tan, C.; Shah, U.A.; Lu, S.X.; Salcedo, M.; et al. Safety and Effectiveness of Weekly Carfilzomib, Lenalidomide, Dexamethasone, and Daratumumab Combination Therapy for Patients With Newly Diagnosed Multiple Myeloma: The MANHATTAN Nonrandomized Clinical Trial. JAMA Oncol. 2021, 7, 862–868. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Multiple myeloma: 2022 update on diagnosis, risk stratification, and management. Am. J. Hematol. 2022, 97, 1086–1107. [Google Scholar] [CrossRef] [PubMed]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Facon, T.; Kumar, S.; Plesner, T.; Orlowski, R.Z.; Moreau, P.; Bahlis, N.; Basu, S.; Nahi, H.; Hulin, C.; Quach, H.; et al. Daratumumab plus Lenalidomide and Dexamethasone for Untreated Myeloma. N. Engl. J. Med. 2019, 380, 2104–2115. [Google Scholar] [CrossRef]
- Claveau, J.-S.; Buadi, F.K.; Kumar, S. Current Role of Allogeneic Stem Cell Transplantation in Multiple Myeloma. Oncol. Ther. 2022, 10, 105–122. [Google Scholar] [CrossRef]
- LeBlanc, R.; Ahmad, I.; Terra, R.; Boudreault, J.-S.; Ogez, D.; Lamore, K.; Delisle, J.-S.; Bambace, N.; Bernard, L.; Cohen, S.; et al. Outcomes in newly diagnosed young or high-risk myeloma patients receiving tandem autologous/allogeneic transplant followed by bortezomib maintenance: A phase II study. Bone Marrow Transplant. 2022, 57, 252–260. [Google Scholar] [CrossRef]
- Munshi, N.C.; Avet-Loiseau, H.; Anderson, K.C.; Neri, P.; Paiva, B.; Samur, M.; Dimopoulos, M.; Kulakova, M.; Lam, A.; Hashim, M.; et al. A large meta-analysis establishes the role of MRD negativity in long-term survival outcomes in patients with multiple myeloma. Blood Adv. 2020, 4, 5988–5999. [Google Scholar] [CrossRef]
- Goicoechea, I.; Puig, N.; Cedena, M.-T.; Burgos, L.; Cordón, L.; Vidriales, M.-B.; Flores-Montero, J.; Gutierrez, N.C.; Calasanz, M.-J.; Ramos, M.-L.M.; et al. Deep MRD profiling defines outcome and unveils different modes of treatment resistance in standard- and high-risk myeloma. Blood 2021, 137, 49–60. [Google Scholar] [CrossRef]

| Bladé 1996 [7] | Shin 2017 [10] | Ravi 2018 [11] | Yanamandra 2018 [12] | Jurczyszyn 2019 [13] | Pál 2020 [14] | Duek 2021 [15] | Caulier 2021 [16] | Bao 2022 [17] | |
|---|---|---|---|---|---|---|---|---|---|
| n | 72 | 32 | 212 | 40 | 52 | 16 | 23 | 214 | 258 |
| Country | USA | South Korea | USA | India | Europe, USA, Brazil, Hong Kong | Hungary | Israel | France, Belgium | USA |
| Years of diagnosis | 1956–1992 | 2000–2015 | 2005–2015 | 2010–2015 | 1989–2016 | 2006–2015 | 2009–2014 | 2000–2015 | 1992–2019 |
| Patients’ age (years) | <40 | ≤40 | ≤50 | <40 | ≤30 | ≤40 | <50 | ≤40 | <50 |
| Median age (range) | 36 (19–39) | 37 (17–40) | 45 (22–49) | 38 (18–39) | 28 (8–30) | 39 (31–40) | 41.5 (27–49) | 37.2 (18.6–40.9) | 46 (17–50) |
| Male/female | 50/22 | 19/13 | 129/83 | 26/14 | 35/17 | 10/6 | 17/6 | 137/77 | 165/93 |
| ISS | |||||||||
| I | NA | 10/31 (32) | 74/212 (35) b | 5/40 (13) | 32/47 (68) | 7/16 (44) | 5/14 (36) | 99/189 (52) | 89/212 (42) |
| II | NA | 15/31 (48) | 46/212 (22) b | 7/40 (18) | 7/47 (15) | 5/16 (31) | 6/14 (43) | 52/189 (28) | 66/212 (31) |
| III | NA | 6/31 (19) | 48/212 (23) b | 28/40 (70) | 8/47 (17) | 4/16 (25) | 3/14 (21) | 38/189 (20) | 57/212 (27) |
| Disease features at diagnosis | |||||||||
| Anemia (<100 g/L) | NA | 9/31 (29) | NA | 21/40 (53) | 13/43 (30) | 2/16 (13) | 6/18 (33) | 71/202 (35) | NA |
| Kidney disease a | 15/52 (29) | 4/32 (13) | NA | 12/40 (30) | 4/22 (18) | 2/16 (13) | 3/18 (17) | 34/200 (17) | NA |
| Low albumin a | 8/49 (16) | 9/32 (28) | NA | NA | 11/41 (27) | NA | 8/18 (44) | NA | NA |
| Hypercalcemia a | 16/53 (30) | 9/32 (28) | NA | 9/37 (24) | 6/42 (14) | 3/16 (19) | 1/18 (6) | 25/195 (13) | NA |
| Lytic bone lesions | 44/65 (68) | 27/31 (87) | NA | 16/37 (59) | 36/44 (82) | 14/16 (88) | 16/18 (89) | 149/200 (75) | NA |
| Elevated ß2MG a | 18/33 (55) | 14/29 (48) | NA | NA | 11/41 (27) | NA | NA | NA | NA |
| Protein isotype | |||||||||
| Heavy chain | NA | ||||||||
| IgG | 34/66 (51) | 14/30 (47) | NA | (76) | 27/49 (55) | 8/16 (50) | 11/22 (50) | 130/162 (80) | 121/258 (47) |
| IgA | 7/66 (11) | 5/30 (17) | NA | (11) | 9/49 (18) | 3/16 (19) | 2/22 (9) | 28/162 (17) | 53/258 (21) |
| IgD | 4/66 (6) | 2/30 (7) | NA | NA | NA | 0/16 (0) | NA | 3/162 (2) | NA |
| IgM | NA | NA | NA | (3) | NA | 0/16 (0) | NA | 1/162 (0.6) | NA |
| Light chain only | 21/66 (32) | 9/30 (30) | NA | (11) | 11/49 (22) | 3/16 (19) | 10/22 (45) c | 51/213 (24) | 72/258 (28) |
| Corso 1998 [8] | Ludwig 2008 [9] | Lu 2016 [18] | Jurczyszyn 2016 [19] | Dhakal 2017 [20] | Nakaya 2020 [21] | Pydi 2021 [22] | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | 356 | 10,549 | 940 | 1089 | 191 | 2303 | 280 | |||||||
| Country | Italy | North America, Europe, Japan | China | Europe, USA | USA | Japan | India | |||||||
| Years of diagnosis | 1973–1994 | 1981–2002 | 2008–2011 | 2000–2015 | 2000 to 2015 | 1998–2018 | 2013–2018 | |||||||
| Age studied (years) | <50 | ≥50 | <50 | ≥50 | <50 | ≥50 | 21–40 | 41–60 | ≤50 | >70 | <40 | All | ≤40 | All patients |
| n (by age group) | 61 | 295 | 1689 | 8860 | 194 | 746 | 173 | 916 | 86 | 105 | 26 | 2277 | 22 | 258 |
| Median age (range) | 45 (33–49) | 63 (50–87) | 36 (20–49) | 62 (50–93) | 46 (20–49) | 62 (50–88) | 37 (21–40) | 55 (41–60) | 46 (32–50) | 73 (71–79) | 36 (20–39) | 74 (20–96) | 33.5 (18–40) | 56 (18–84) |
| Male/female | 32/29 | 169/126 | 1023/666 | 5014/3846 | 113/81 | 457/289 | 104/69 | 510/406 | 70/16 | 58/47 | 13/13 | 1116/1161 | 14:8 | NA |
| ISS | ||||||||||||||
| I | NA | NA | 492/1267 (39) | 1790/6776 (26) p < 0.001 | (30) | (17) p < 0.001 | 71/151 (47) | 303/729 (42) p = 0.40 | 15/86 (17) d | 29/105 (27) p = 0.31 d | (43) | (23) p = 0.019 | (18) | (17) |
| II | NA | NA | 438/1267 (35) | 2675/6776 (39) p < 0.001 | (31) | (32) p = 0.774 | 50/151 (33) | 280/729 (38) | 22/86 (26) d | 22/105 (21) d | (38) | (40) p = 0.910 | (32) | (33) |
| III | NA | NA | 337/1267 (27) | 2311/6776 (34) p < 0.001 | (39) | (51) p 0.007 | 30/151 (20) | 149/729 (20) | 20/86 (23) d | 28/105 (27) d | (19) | (37) p = 0.022 | (50) | (50) |
| Disease features at diagnosis | ||||||||||||||
| Anemia (<100 g/L) | NA | NA | 596/1614 (37) | 3465/8539 (41) p = 0.006 | (56) | (61) p = 0.265 | 53/173 (31) | 247/925 (27) p = 0.29 | NA | NA | 6/26 (23) | NA | (68) | (63) |
| Kidney disease a | 5/61 (8) | 41/295 (14) | 240/1594 (15) | 1484/8573 (17) p = 0.028 | (21) | (25) p = 0.300 | 40/160 (25) | 265/855 (31) p = 0.13 | NA | NA | 11/26 (42) | NA | (50) | (36) |
| Low albumin a | NA | NA | 458/1396 (33) | 3276/7912 (41) p < 0.001 | (37) | (58) p < 0.001 | NA | NA | NA | NA | NA | NA | NA | NA |
| Hypercalcemia a | 4/61 (6) | 17/295 (6) | 481/1445 (33) | 2652/7870 (34) p = 0.762 | NA | NA | 26/160 (16) | 86/668 (13) p = 0.26 | NA | NA | 1/26 (4) | NA | (9) | (26) |
| Lytic bone lesions | 26/61 (43) b | 100/295 (34) b | 617/1292 (48) c | 3457/7423 (47) c p = 0.431 | 82/109 (75) c | 334/403 (83) p = 0.569 c | 139/170 (82) | 644/868 (74) p = 0.04 | NA | NA | 18/26 (69) | NA | (59) | (76) |
| Elevated ß2MG a | NA | NA | 613/1377 (45) | 4141/7061 (59) p < 0.001 | (46) | (62) p < 0.001 | NA | NA | NA | NA | NA | NA | NA | NA |
| Protein isotype | ||||||||||||||
| Heavy chain | ||||||||||||||
| IgG | 40/61 (65) | 197/295 (67) | 924/1538 (60) | 4853/8091 (60) p = 0.943 | 75/194 (39) | 341/746 (46) p = 0.078 | 107/156 (69) | 375/632 (59) p = 0.10 | 34/86 (40) | 56/105 (53) p = 0.06 | (45) | (58) p = 0.237 | (50) | (55) |
| IgA | 11/61 (18) | 59/295 (20) | 318/1538 (21) | 2009/8091 (25) p < 0.001 | 28/194 (14) | 141/746 (19) p = 0.149 | 26/156 (17) | 127/632 (20) | 9/86 (10) | 21/105 (20) | (11) | (22) p = 0.080 | (5) | (12) |
| IgD | 1/61 (1) | 3/295 (1) | 43/1538 (3) | 251/8091 (3) p = 0.522 | 20/194 (10) | 41/746 (5.5) p = 0.015 | 1/156 (0.6) | 16/632 (3) | NA | NA | (4) | (1) p = 0.375 | NA | NA |
| IgM | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Light chain only | 8/61 (12) | 26/295 (8) | 197/1538 (13) | 824/8091 (10) p = 0.002 | 64/194 (33) | 195/746 (26) p = 0.057 | NA | NA | 26/86 (30) | 19/105 (18) | (33) | (16) p = 0.021 | (41) | (33) |
| Studies without a Comparator | Studies with a Comparator | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Shin 2017 [10] | Jurczyszyn 2019 [13] | Pál 2020 [14,15] | Duek 2021 [15] | Caulier 2021 [16] | Pydi 2021 [22] | Bao 2022 [17] h | Ludwig 2008 [9] | Jurczyszyn 2016 [19] | Lu 2016 [18] | Nakaya 2020 [21] | |||||
| Age studied (years) | ≤40 | ≤30 | ≤40 | <50 | ≤40 | ≤40 | <50 | <50 | ≥50 | 21–40 | 41–60 | <50 | ≥50 | <40 years | All patients Median 74 |
| Hyperdiploid | NA | NA | 6/11 (55) | 1/22 (4.5) | NA | NA | NA | NA | NA | NA | NA | 3/33 (9) | 8/120 (7) | NA | NA |
| Non-hyperdiploid | NA | 19/21 (90) | NA | NA | NA | NA | Hypodiploid 5/210 (2) | NA | NA | NA | NA | 4/33 (12) | 24/120 (20) | NA | NA |
| t(11;14) | NA | 1/20 (5) | NA | 15/22 (68) a,b | 9/35 (26) | 2/7 (29) | 42/210 (20) | NA | NA | NA | NA | NA | NA | 1/5 (20) | 83/316 (26) p = 0.461 |
| t(14;16) | 0/11 (0) | NA | NA | 0/22 (0) | 1/39 (2.5) | 1/7 (14) | NA | NA | NA | NA | NA | NA | NA | 0/7 (0) | 27/532 (5) p = 0.063 |
| t(14;20) | 0/6 (0) | NA | NA | 0/22 (0) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| t(8;14) | NA | NA | NA | NA | NA | NA | 4/210 (2) | NA | NA | NA | NA | NA | NA | NA | NA |
| t(4;14) | 1/10 (10) | 0/20 (0) | 3/11 (27) | 0/22 (0) | 19/156 (12) e | 1/7 (14) | 15/210 (7) | NA | NA | 26/81 (32) j | 31/181 (17) j p = 0.007 | NA | NA | 2/8 (25) | 168/802 (21) p = 0.659 |
| del (17p)/17 delp53 | 1/9 (11) | 2/21 (10) | 2/11 (18) | 1/22 (4.5) | 17/141 (12) e | 1/7 (14) | 15/210 (7) | NA | NA | 17/91 (19) i | 61/351 (17) p 0.771 i | 3/9 (33) | 86/606 (14) p = 0.008 | ||
| + or amp 1q21/1q gain | 4/15 (27) | 2/17 (12) | NA | NA | 17/56 (30) f | NA | 48/210 (23) | NA | NA | NA | NA | 49/87 (56) i | 139/313 (44) p = 0.064 i | NA | NA |
| del (1p32) | NA | NA | NA | 1/22 (4.5) c | 8/46 (17) g | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| del (13q)/ del 13 | 4/17 (24) | 8/26 (31) | NA | 9/22 (40) d | NA | 3/7 (43) | 72/210 (34) | 32/53 (60) i | 150/320 (47) p = 0.069 i | NA | NA | 13/37 (35) i | 58/141 (41) p = 0.507 i | 4/8 (50) | 211/435 (49) p = 1.000 |
| 17/109 (16) ii | 45/345 (13) p = 0.499 ii | 3/33 (9) ii | 9/120 (8) p = 0.767 ii | ||||||||||||
| del (9) | 1/16 (6) | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| Studies without a Comparator | Studies with a Comparator | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Shin 2017 [10] | Ravi 2018 [11] | Jurczyszyn 2019 [13] | Caulier 2021 [16] | Bao 2022 [17] | Jurczyszyn 2016 [19] | Nakaya 2020 [21] | Pál 2020 [14] | ||||
| n | 32 | 212 | 52 | 214 | 258 | 173 | 916 | 26 | 2277 | 16 | 296 |
| Age studied (years) | ≤40 | ≤50 | ≤30 | ≤40 | <50 | 21–40 | 41–60 | <40 | All | ≤40 | >40 |
| Year of diagnosis | 2000–2015 | 2005–2015 | 2006–2016 | 2000–2015 | 1992–2019 | 2000–2015 | 1998–2018 | 2006–2015 | |||
| Induction treatments (%) | Novel agents, unspecified | ||||||||||
| PI based | 10 | 45 b | 41 | 30 | 22 | NA | NA | NA | NA | 6 | NA |
| IMID based | 37 | 32 b | 24 | 1 | 10 | NA | NA | NA | NA | 13 | NA |
| PI + IMIDs | 40 a | 15 b | 21 | 37 | 27 | NA | NA | NA | NA | 69 | NA |
| Other (chemotherapy, melphalan, dexamethasone only) | 13 | 6 b | 15 | 26 | 41 | NA | NA | NA | NA | 13 | NA |
| Radiotherapy only | NA | NA | NA | 6 | NA | NA | NA | NA | NA | NA | NA |
| Transplant | |||||||||||
| ASCT (%) 1st line | 62 | NA | 62 | NA | NA | NA | NA | NA | NA | 88 | NA |
| ASCT (%)—at any stage | 79 | 52 | NA | 93 | 87 | 11 | 89 | 39 | NA | NA | NA |
| Allo-SCT (%) | 0 | NA | 3 | 25 | 5 c | NA | NA | 42 d | NA | NA | NA |
| Survival data | |||||||||||
| Median follow-up (months) | 64 | 69.6 | 86 | 76 | 93.6 | 51 | 78 | NA | NA | ||
| Median OS (months) | 61 | NA | 166 | 175 | 112.8 | NA | NA | NA | NA | NA | NA |
| 5-years OS (%) | 54 | 70 | 77 | 84 | 86 (NHBP) 66 (NHWP) | 83 | 67 p < 0.001 | 71 | 56 | 83 | 53 |
| Median PFS (months) | 16 | NA | NA | 41 | 38.4 (NHWP), 70.8 (NHPB) | NA | NA | NA | NA | NA | NA |
| 5-years PFS (%) | 14 | 28 | NA | NA | NA | NA | NA | NA | NA | 48 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanguay, M.; Dagenais, C.; LeBlanc, R.; Ahmad, I.; Claveau, J.-S.; Roy, J. Young Myeloma Patients: A Systematic Review of Manifestations and Outcomes. Curr. Oncol. 2023, 30, 5214-5226. https://doi.org/10.3390/curroncol30060396
Tanguay M, Dagenais C, LeBlanc R, Ahmad I, Claveau J-S, Roy J. Young Myeloma Patients: A Systematic Review of Manifestations and Outcomes. Current Oncology. 2023; 30(6):5214-5226. https://doi.org/10.3390/curroncol30060396
Chicago/Turabian StyleTanguay, Mégane, Christophe Dagenais, Richard LeBlanc, Imran Ahmad, Jean-Sébastien Claveau, and Jean Roy. 2023. "Young Myeloma Patients: A Systematic Review of Manifestations and Outcomes" Current Oncology 30, no. 6: 5214-5226. https://doi.org/10.3390/curroncol30060396
APA StyleTanguay, M., Dagenais, C., LeBlanc, R., Ahmad, I., Claveau, J.-S., & Roy, J. (2023). Young Myeloma Patients: A Systematic Review of Manifestations and Outcomes. Current Oncology, 30(6), 5214-5226. https://doi.org/10.3390/curroncol30060396






