A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series
Abstract
1. Introduction
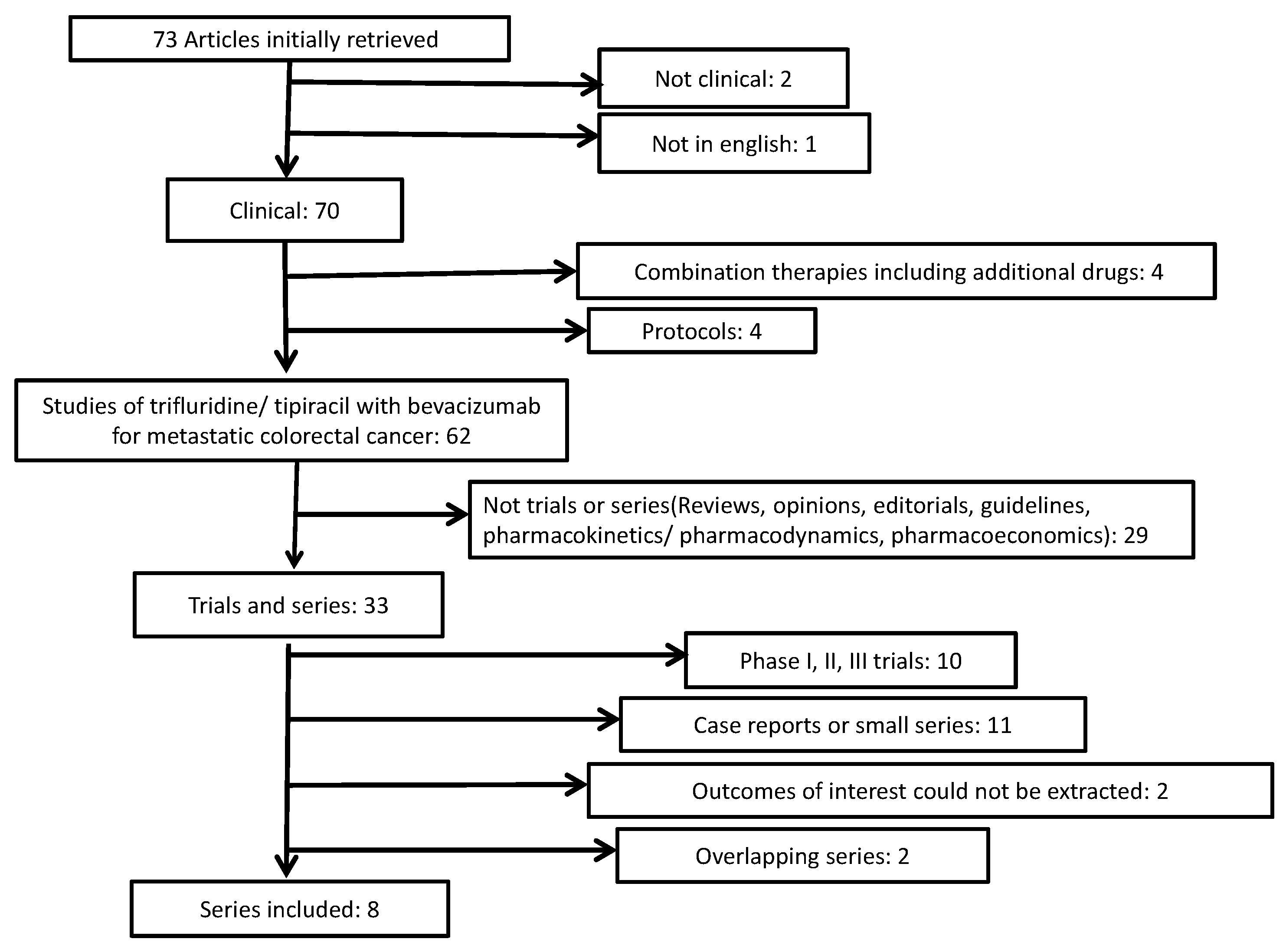
2. Methods
3. Results
4. Discussion
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Sauer, A.G.; Fedewa, S.A.; Butterly, L.F.; Anderson, J.C.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 145–164. [Google Scholar] [CrossRef]
- Riedesser, J.E.; Ebert, M.P.; Betge, J. Precision medicine for metastatic colorectal cancer in clinical practice. Ther. Adv. Med. Oncol. 2022, 14, 17588359211072703. [Google Scholar] [CrossRef]
- Rankin, A.; Klempner, S.J.; Erlich, R.; Sun, J.X.; Grothey, A.; Fakih, M.; George, T.J., Jr.; Lee, J.; Ross, J.S.; Stephens, P.J.; et al. Broad Detection of Alterations Predicted to Confer Lack of Benefit from EGFR Antibodies or Sensitivity to Targeted Therapy in Advanced Colorectal Cancer. Oncologist 2016, 21, 1306–1314. [Google Scholar] [CrossRef]
- Tabernero, J.; Grothey, A.; Van Cutsem, E.; Yaeger, R.; Wasan, H.; Yoshino, T.; Desai, J.; Ciardiello, F.; Loupakis, F.; Hong, Y.S.; et al. Encorafenib Plus Cetuximab as a New Standard of Care for Previously Treated BRAF V600E-Mutant Metastatic Colorectal Cancer: Updated Survival Results and Subgroup Analyses from the BEACON Study. J. Clin. Oncol. 2021, 39, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Siena, S.; Di Bartolomeo, M.; Raghav, K.; Masuishi, T.; Loupakis, F.; Kawakami, H.; Yamaguchi, K.; Nishina, T.; Fakih, M.; Elez, E.; et al. Trastuzumab deruxtecan (DS-8201) in patients with HER2-expressing metastatic colorectal cancer (DESTINY-CRC01): A multicentre, open-label, phase 2 trial. Lancet Oncol. 2021, 22, 779–789. [Google Scholar] [CrossRef]
- André, T.; Shiu, K.K.; Kim, T.W.; Jensen, B.V.; Jensen, L.H.; Punt, C.; Smith, D.; Garcia-Carbonero, R.; Benavides, M.; Gibbs, P.; et al. Pembrolizumab in Microsatellite-Instability-High Advanced Colorectal Cancer. N. Engl. J. Med. 2020, 383, 2207–2218. [Google Scholar] [CrossRef]
- Hurwitz, H.; Kabbinavar, F. Bevacizumab combined with standard fluoropyrimidine-based chemotherapy regimens to treat colorectal cancer. Oncology 2005, 69 (Suppl. 3), 17–24. [Google Scholar] [CrossRef]
- Benson, A.B.; Venook, A.P.; Al-Hawary, M.M.; Arain, M.A.; Chen, Y.J.; Ciombor, K.K.; Cohen, S.; Cooper, H.S.; Deming, D.; Farkas, L.; et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2021, 19, 329–359. [Google Scholar] [CrossRef] [PubMed]
- Marcus, L.; Lemery, S.J.; Khasar, S.; Wearne, E.; Helms, W.S.; Yuan, W.; He, K.; Cao, X.; Yu, J.; Zhao, H.; et al. FDA Approval Summary: TAS-102. Clin. Cancer Res. 2017, 23, 2924–2927. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Voutsadakis, I.A. A Systematic Review and Meta-analysis of Retrospective Series of Regorafenib for Treatment of Metastatic Colorectal Cancer. Anticancer Res. 2017, 37, 5925–5934. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Biomarkers of Trifluridine-Tipiracil Efficacy. J. Clin. Med. 2021, 10, 5568. [Google Scholar] [CrossRef] [PubMed]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Tabernero, J.; Prager, G.W.; Fakih, M.; Ciardiello, F.; Van Cutsem, E.; Elez, E.; Cruz, F.M.; Wyrwicz, L.; Stroyakovskiy, D.; Papai, Z.; et al. Trifluridine/tipiracil plus bevacizumab for third-line treatment of refractory metastatic colorectal cancer: The phase 3 randomized SUNLIGHT study. J. Clin. Oncol. 2023, 41 (Suppl. 4), 4. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef]
- Neyeloff, J.L.; Fuchs, S.C.; Moreira, L.B. Meta-analyses and Forest plots using a microsoft excel spreadsheet: Step-by-step guide focusing on descriptive data analysis. BMC Res. Notes 2012, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Xu, W.; Chen, B.; Lv, H.; Wang, J.; Liu, Y.; He, Y.; Wang, S.; Zhao, J.; Chen, X. An Exploration of Trifluridine/Tipiracil Monotherapy and in Combination with Bevacizumab or Immune Checkpoint Inhibitors for Patients with Metastatic Colorectal Cancer: A Real-World Study. Clin. Color. Cancer 2023, 22, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Arrichiello, G.; Perrone, A.; Napolitano, S.; Martini, G.; De Falco, V.; Incoronato, P.; Laterza, M.M.; Facchini, G.; Famiglietti, V.; Nacca, V.; et al. Real-World Activity and Safety of Trifluridine-Tipiracil Plus Bevacizumab Therapy in Patients with Refractory Metastatic Colorectal Cancer. Target Oncol. 2022, 17, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Lago, N.; Chucla, T.C.; De Castro, B.A.; Ponte, R.V.; Rendo, C.R.; Rodriguez, M.I.G.; Diaz, S.S.; Suarez, B.G.; de la Cámara Gomez, J.; Fernández, F.B.; et al. Efficacy, safety and prognostic factors in patients with refractory metastatic colorectal cancer treated with trifluridine/tipiracil plus bevacizumab in a real-world setting. Sci. Rep. 2022, 12, 14612. [Google Scholar] [CrossRef]
- Tamaki, S.; Ishikawa, H.; Suzuki, K.; Kimura, Y.; Maemoto, R.; Abe, I.; Endo, Y.; Kakizawa, N.; Watanabe, F.; Futsuhara, K.; et al. Prophylactic use of pegfilgrastim enables the management of severe neutropenia without dose delays in patients with metastatic colorectal cancer treated with TAS-102 plus bevacizumab. Mol. Clin. Oncol. 2022, 16, 103. [Google Scholar] [CrossRef] [PubMed]
- Kamiimabeppu, D.; Osumi, H.; Shinozaki, E.; Ooki, A.; Wakatsuki, T.; Yoshino, K.; Sato, T.; Nakayama, I.; Ogura, M.; Takahari, D.; et al. Effect of neutropenia on survival outcomes of patients with metastatic colorectal cancer receiving trifluridine/tipiracil plus bevacizumab. Oncol. Lett. 2021, 22, 783. [Google Scholar] [CrossRef]
- Chida, K.; Kotani, D.; Nakamura, Y.; Kawazoe, A.; Kuboki, Y.; Shitara, K.; Kojima, T.; Taniguchi, H.; Watanabe, J.; Endo, I.; et al. Efficacy and safety of trifluridine/tipiracil plus bevacizumab and trifluridine/tipiracil or regorafenib monotherapy for chemorefractory metastatic colorectal cancer: A retrospective study. Ther. Adv. Med. Oncol. 2021, 13, 17588359211009143. [Google Scholar] [CrossRef]
- Nose, Y.; Kagawa, Y.; Hata, T.; Mori, R.; Kawai, K.; Naito, A.; Sakamoto, T.; Murakami, K.; Katsura, Y.; Ohmura, Y.; et al. Neutropenia is an indicator of outcomes in metastatic colorectal cancer patients treated with FTD/TPI plus bevacizumab: A retrospective study. Cancer Chemother. Pharmacol. 2020, 86, 427–433. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Matsuhashi, N.; Kitahora, M.; Takahashi, T.; Hirose, C.; Iihara, H.; Yamada, Y.; Watanabe, D.; Ishihara, T.; Suzuki, A.; et al. Bevacizumab in Combination with TAS-102 Improves Clinical Outcomes in Patients with Refractory Metastatic Colorectal Cancer: A Retrospective Study. Oncologist 2020, 25, e469–e476. [Google Scholar] [CrossRef] [PubMed]
- Kotani, D.; Kuboki, Y.; Horasawa, S.; Kaneko, A.; Nakamura, Y.; Kawazoe, A.; Bando, H.; Taniguchi, H.; Shitara, K.; Kojima, T.; et al. Retrospective cohort study of trifluridine/tipiracil (TAS-102) plus bevacizumab versus trifluridine/tipiracil monotherapy for metastatic colorectal cancer. BMC Cancer 2019, 19, 1253. [Google Scholar] [CrossRef] [PubMed]
- Matsuhashi, N.; Takahashi, T.; Fujii, H.; Suetsugu, T.; Fukada, M.; Iwata, Y.; Tokumaru, Y.; Imai, T.; Mori, R.; Tanahashi, T.; et al. Combination chemotherapy with TAS-102 plus bevacizumab in salvage-line treatment of metastatic colorectal cancer: A single-center, retrospective study examining the prognostic value of the modified Glasgow Prognostic Score in salvage-line therapy of metastatic colorectal cancer. Mol. Clin. Oncol. 2019, 11, 390–396. [Google Scholar] [CrossRef]
- Shahda, S.; Saif, M.W. Regorafenib: From bench to bedside in colorectal cancer. Expert Rev. Clin. Pharmacol. 2013, 6, 243–248. [Google Scholar] [CrossRef]
- Grothey, A.; Van Cutsem, E.; Sobrero, A.; Siena, S.; Falcone, A.; Ychou, M.; Humblet, Y.; Bouché, O.; Mineur, L.; Barone, C.; et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): An international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013, 381, 303–312. [Google Scholar] [CrossRef]
- Li, J.; Qin, S.; Xu, R.; Yau, T.C.; Ma, B.; Pan, H.; Xu, J.; Bai, Y.; Chi, Y.; Wang, L.; et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2015, 16, 619–629. [Google Scholar] [CrossRef]
- Tabernero, J.; Taieb, J.; Prager, G.W.; Ciardiello, F.; Fakih, M.; Leger, C.; Fougeray, R.; Amellal, N.; van Cutsem, E. Trifluridine/tipiracil plus bevacizumab for third-line management of metastatic colorectal cancer: SUNLIGHT study design. Future Oncol. 2021, 17, 1977–1985. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Nishina, T.; Shinozaki, E.; Yamazaki, K.; Shitara, K.; Okamoto, W.; Kajiwara, T.; Matsumoto, T.; Tsushima, T.; Mochizuki, N.; et al. TAS-102 plus bevacizumab for patients with metastatic colorectal cancer refractory to standard therapies (C-TASK FORCE): An investigator-initiated, open-label, single-arm, multicentre, phase 1/2 study. Lancet Oncol. 2017, 18, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, P.; Yilmaz, M.; Möller, S.; Zitnjak, D.; Krogh, M.; Petersen, L.N.; Poulsen, L.Ø.; Winther, S.B.; Thomsen, K.G.; Qvortrup, C. TAS-102 with or without bevacizumab in patients with chemorefractory metastatic colorectal cancer: An investigator-initiated, open-label, randomised, phase 2 trial. Lancet Oncol. 2020, 21, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.J.; Hu, T.; Shao, P.; Chu, W.Y.; Cao, Y.; Zhang, F. TAS-102 Monotherapy and Combination Therapy with Bevacizumab for Metastatic Colorectal Cancer. Gastroenterol. Res. Pract. 2021, 2021, 4014601. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, T.; Taieb, J.; Kuboki, Y.; Pfeiffer, P.; Kumar, A.; Hochster, H.S. Trifluridine/tipiracil with or without bevacizumab in metastatic colorectal cancer: Results of a systematic review and meta-analysis. Ther. Adv. Med. Oncol. 2023, 15, 17588359221146137. [Google Scholar] [CrossRef]
- Andersen, S.E.; Andersen, I.B.; Jensen, B.V.; Pfeiffer, P.; Ota, T.; Larsen, J.S. A systematic review of observational studies of trifluridine/tipiracil (TAS-102) for metastatic colorectal cancer. Acta Oncol. 2019, 58, 1149–1157. [Google Scholar] [CrossRef] [PubMed]
- Sonbol, M.B.; Benkhadra, R.; Wang, Z.; Firwana, B.; Walden, D.J.; Mody, K.; Hubbard, J.M.; Murad, M.H.; Ahn, D.H.; Bekaii-Saab, T. A Systematic Review and Network Meta-Analysis of Regorafenib and TAS-102 in Refractory Metastatic Colorectal Cancer. Oncologist 2019, 24, 1174–1179. [Google Scholar] [CrossRef]
- Nevala-Plagemann, C.; Sama, S.; Ying, J.; Shen, J.; Haaland, B.; Florou, V.; Garrido-Laguna, I. A Real-World Comparison of Regorafenib and Trifluridine/Tipiracil in Refractory Metastatic Colorectal Cancer in the United States. J. Natl. Compr. Cancer Netw. 2023, 21, 257–264. [Google Scholar] [CrossRef]
- Yoshino, T.; Cleary, J.M.; Van Cutsem, E.; Mayer, R.J.; Ohtsu, A.; Shinozaki, E.; Falcone, A.; Yamazaki, K.; Nishina, T.; Garcia-Carbonero, R.; et al. Neutropenia and survival outcomes in metastatic colorectal cancer patients treated with trifluridine/tipiracil in the RECOURSE and J003 trials. Ann. Oncol. 2020, 31, 88–95. [Google Scholar] [CrossRef]
- Kuramochi, H.; Yamada, T.; Yoshida, Y.; Matsuda, A.; Kamiyama, H.; Kosugi, C.; Ishibashi, K.; Fukazawa, A.; Ihara, K.; Sonoda, H.; et al. The Pre-treatment Lymphocyte-to-Monocyte Ratio Predicts Efficacy in Metastatic Colorectal Cancer Treated With TAS-102 and Bevacizumab. Anticancer Res. 2021, 41, 3131–3137. [Google Scholar] [CrossRef]
- Policicchio, A.; Mercier, J.; Digklia, A.; Voutsadakis, I.A. Platelet and Neutrophil Counts as Predictive Markers of Neoadjuvant Therapy Efficacy in Rectal Cancer. J. Gastrointest. Cancer 2019, 50, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Mercier, J.; Voutsadakis, I.A. The platelets-neutrophils to lymphocytes ratio: A new prognostic marker in metastatic colorectal cancer. J. Gastrointest. Oncol. 2018, 9, 478–486. [Google Scholar] [CrossRef]
- Mercier, J.; Voutsadakis, I.A. Comparison of Hematologic and Other Prognostic Markers in Metastatic Colorectal Cancer. J. Gastrointest. Cancer 2019, 50, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Steele, M.; Voutsadakis, I.A. Pre-treatment platelet counts as a prognostic and predictive factor in stage II and III rectal adenocarcinoma. World J. Gastrointest. Oncol. 2017, 9, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, E.; Narita, Y.; Obuchi, K.; Ueda, A.; Saito, S.; Tadaka, S.; Kinoshita, K.; Maekawa, M.; Mano, N.; Hirasawa, N.; et al. Importance of Rare DPYD Genetic Polymorphisms for 5-Fluorouracil Therapy in the Japanese Population. Front. Pharmacol. 2022, 13, 930470. [Google Scholar] [CrossRef] [PubMed]
- Rocca, A.; Brunese, M.C.; Santone, A.; Avella, P.; Bianco, P.; Scacchi, A.; Scaglione, M.; Bellifemine, F.; Danzi, R.; Varriano, G.; et al. Early Diagnosis of Liver Metastases from Colorectal Cancer through CT Radiomics and Formal Methods: A Pilot Study. J. Clin. Med. 2021, 11, 31. [Google Scholar] [CrossRef]
- Rocca, A.; Scacchi, A.; Cappuccio, M.; Avella, P.; Bugiantella, W.; De Rosa, M.; Costa, G.; Polistena, A.; Codacci-Pisanelli, M.; Amato, B.; et al. Robotic surgery for colorectal liver metastases resection: A systematic review. Int. J. Med. Robot. 2021, 17, e2330. [Google Scholar] [CrossRef]
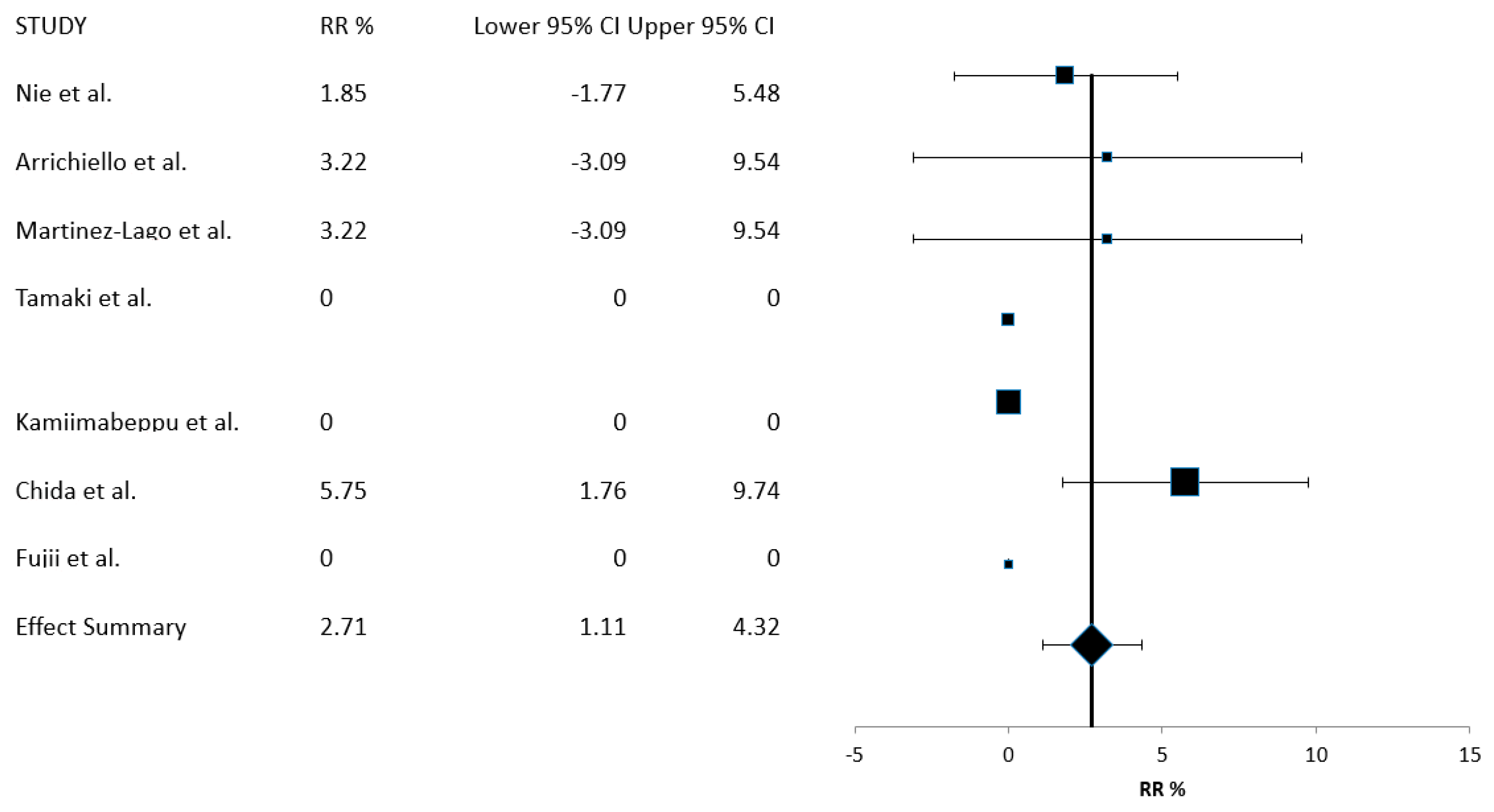
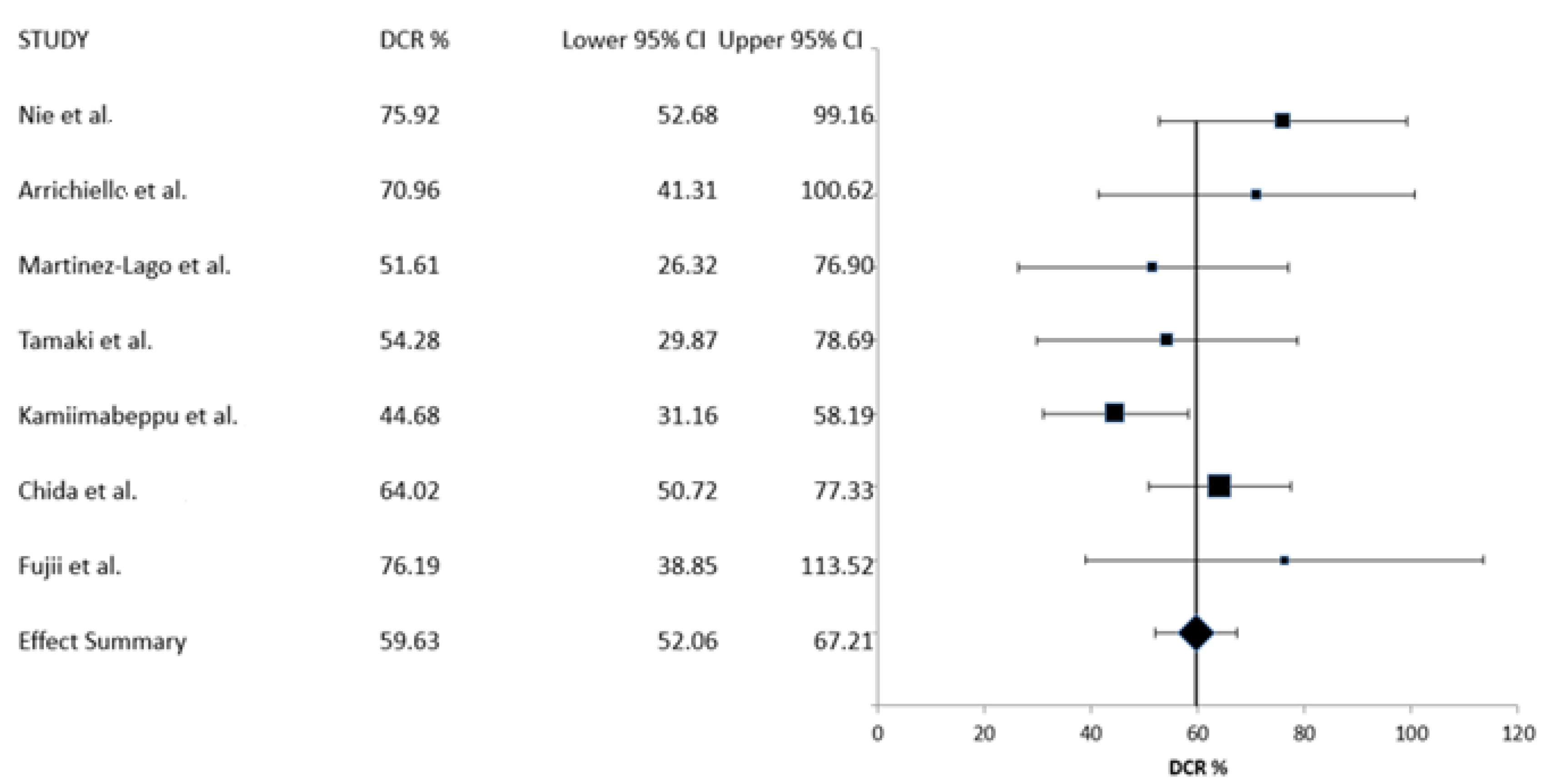
| Study [Reference] | Year of Publication | Country | Number of Patients | RR (%) | DCR (%) |
|---|---|---|---|---|---|
| Nie et al. [18] | 2022 | China | 54 | 1.9 | 75.9 |
| Arrichiello et al. [19] | 2022 | Italy | 31 | 3.2 | 71.0 |
| Martinez-Lago et al. [20] | 2022 | Spain | 35 (31 evaluable for response) | 3.2 | 51.6 |
| Tamaki et al. [21] | 2022 | Japan | 35 | 0 | 54.3 |
| Kamiimabeppu et al. [22] | 2021 | Japan | 94 | 0 | 44.7 |
| Chida et al. [23] | 2021 | Japan | 139 | 5.8 | 64.0 |
| Nose et al. [24] | 2020 | Japan | 32 | NR | NR |
| Fujii et al. [25] | 2020 | Japan | 21 | 0 | 76.2 |
| Patients (%) | Total Patients with Data | Number of Series with Data | |
|---|---|---|---|
| Age (median, range) | 55–73 (26–83) | 441 | 8 |
| SEX | |||
| Male | 233 (52.8%) | 441 | 8 |
| Female | 208 (47.2%) | ||
| ECOG PS | |||
| 0–1 | 388 (92.4%) | 420 | 7 |
| >1 | 32 (7.6%) | ||
| NUMBER OF PRIOR LINES OF CHEMO | |||
| 1–2 | 134 (53.8%) | 249 | 5 |
| >2 | 115 (46.2%) | ||
| Range numbers | 1–>4 | ||
| TYPES OF PRIOR CHEMOTHERAPY | |||
| Fluoropyrimidine | 354 (100%) | 354 | 5 |
| Irinotecan | 338 (95.5%) | 354 | 5 |
| Oxaliplatin | 350 (98.9%) | 354 | 5 |
| Anti-angiogenic | 376 (95.7%) | 393 | 7 |
| Anti-EGFR | 115 (32.5%) | 354 | 5 |
| LOCATION OF PRIMARY | |||
| Colon (side not specified) | 31 (57.4%) | 54 | 1 |
| Right-sided colon | 98 (25.7%) | 382 | 7 |
| Left-sided colon | 24 (27.6%) | 87 | 3 |
| Left-sided colon and rectum | 227 (75.7%) | 300 | 4 |
| Rectum | 41 (37.3%) | 110 | 3 |
| NUMBER OF ORGANS INVOLVED | |||
| 1 | 81 (25.2%) | 321 | 5 |
| 2 | 72 (41.4%) | 174 | 2 |
| 1–2 | 22 (62.9%) | 35 | 1 |
| ≥2 | 118 (80.3%) | 147 | 3 |
| ≥3 | 63 (30.1%) | 209 | 3 |
| SITES INVOLVED | |||
| Lung | 219 (64.6%) | 339 | 5 |
| Liver | 213 (62.8%) | 339 | 5 |
| Peritoneum | 96 (28.3%) | 339 | 5 |
| Lymph nodes | 77 (45.6%) | 169 | 3 |
| SURGERY FOR PRIMARY | |||
| Yes | 187 (72.5%) | 258 | 5 |
| No | 71 (27.5%) | 258 | 5 |
| EFFICACY | |||
| Median OS (months) (95% CI) | 11.17 (10.15–12.19) | 437 | 8 |
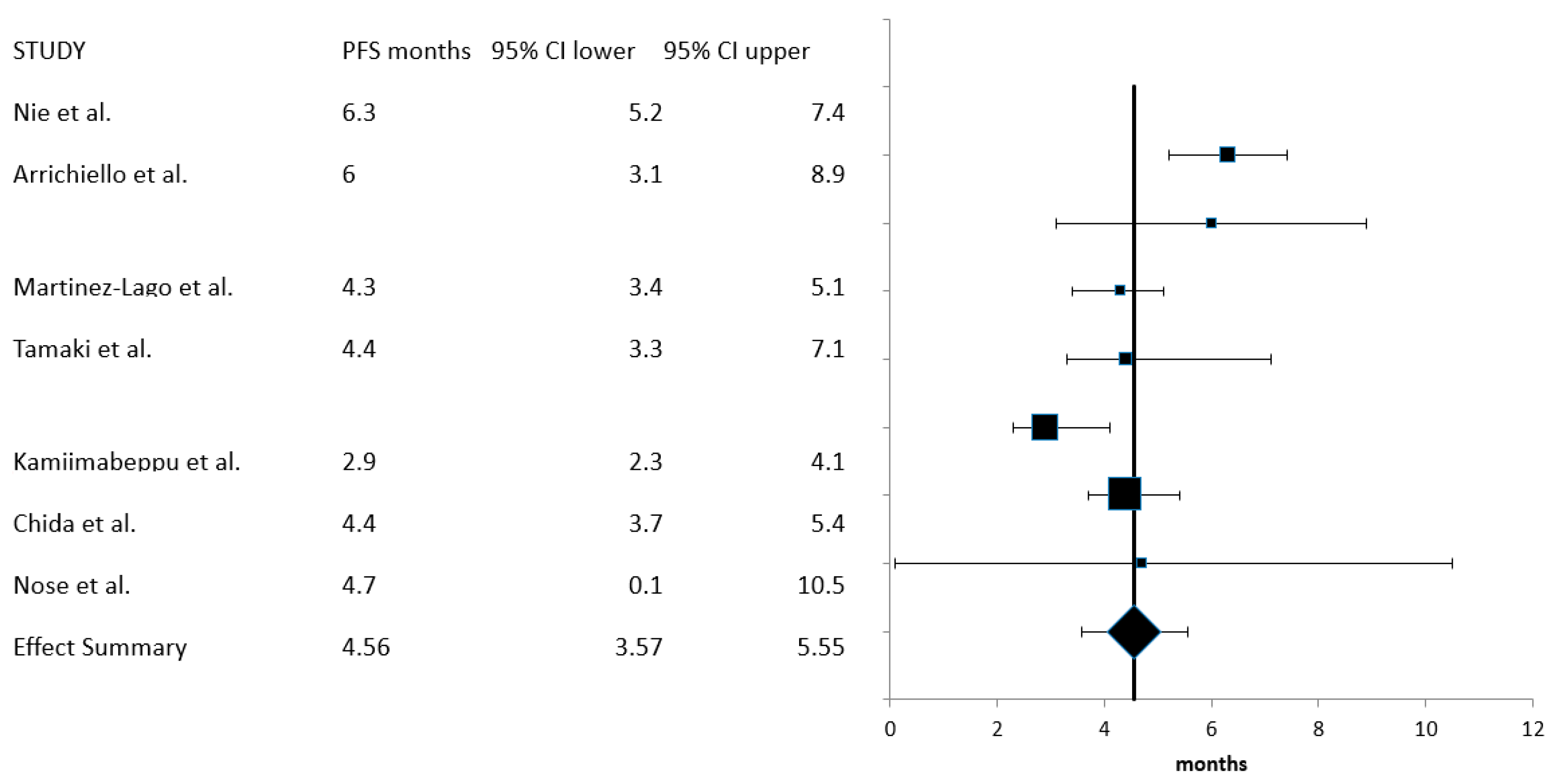
| Median PFS (months) (95% CI) | 4.56 (3.57–5.55) | 416 | 7 |
| RR% (95% CI) | 2.71 (1.11–4.32) | 405 | 7 |
| CBR% (95% CI) | 59.63 (52.06–67.21) | 405 | 7 |
| Patients (%) | Total Patients with Data | Number of Series with Data | |
|---|---|---|---|
| MSI | |||
| pMMR/MSS | 98 (81.7%) | 120 | 3 |
| dMMR/MSI | 3 (2.5%) | ||
| Unknown | 19 (15.8%) | ||
| KRAS MUTATION STATUS | |||
| Wild-type | 207 (46.9%) | 441 | 8 |
| Mutated | 228 (51.7%) | ||
| Unknown | 6 (1.4%) | ||
| NRAS MUTATION STATUS | |||
| Wild-type | 44 (81.5%) | 54 | 1 |
| Mutated | 4 (7.4%) | ||
| Unknown | 6 (11.1%) | ||
| BRAF MUTATION STATUS | |||
| Wild-type | 240 (92.7%) | 259 | 4 |
| Mutated | 9 (3.5%) | ||
| Unknown | 10 (3.8%) |
| Toxicity | % All Grades | Total Patients with Data/Series with Data | % Grades 3 and 4 | Total Patients with Data/Series with Data |
|---|---|---|---|---|
| Neutropenia | 68% | 281/6 | 44.9% | 441/8 |
| Anemia | 43% | 302/7 | 12.6% | 420/7 |
| Thrombocytopenia | 28% | 271/6 | 3.9% | 389/6 |
| Asthenia/fatigue | 50.5% | 208/6 | 3.7% | 326/6 |
| Anorexia | 29.3% | 215/4 | 0.3% | 354/5 |
| Diarrhea | 28.4% | 271/6 | 1.8% | 389/6 |
| Hypertension | 15.6% | 270/6 | 1.5% | 388/6 |
| Nausea/GI toxicity | 53.9% | 271/6 | 2.3% | 389/6 |
| Proteinuria | 33.3% | 267/6 | 3.9% | 385/6 |
| Hemorrhage | 12.1% | 124/3 | 0.8% | 124/3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voutsadakis, I.A. A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series. Curr. Oncol. 2023, 30, 5227-5239. https://doi.org/10.3390/curroncol30060397
Voutsadakis IA. A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series. Current Oncology. 2023; 30(6):5227-5239. https://doi.org/10.3390/curroncol30060397
Chicago/Turabian StyleVoutsadakis, Ioannis A. 2023. "A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series" Current Oncology 30, no. 6: 5227-5239. https://doi.org/10.3390/curroncol30060397
APA StyleVoutsadakis, I. A. (2023). A Systematic Review and Meta-Analysis of Trifluridine/Tipiracil plus Bevacizumab for the Treatment of Metastatic Colorectal Cancer: Evidence from Real-World Series. Current Oncology, 30(6), 5227-5239. https://doi.org/10.3390/curroncol30060397