Abstract
Adverse drug reactions (ADRs) are responsible for almost 5% of hospital admissions, making it necessary to implement different pharmacovigilance strategies. The additional monitoring (AM) concept has been highlighted and intended to increase the number of suspected ADRs reported, namely in medicines with limited safety data. A prospective, descriptive study of active pharmacovigilance (AP) was conducted between 2019 and 2021 in the Local Health Unit of Matosinhos (LHUM) (Porto, Portugal). A model of AP for medicines under AM, namely oral antineoplastic agents, was designed. Follow-up consultations were performed, and adverse events (AEs) data were collected. The overall response to the treatment was evaluated through the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria. A total of 52 patients were included in the study, and 14 antineoplastic drugs under AM were analyzed. Of the total number of patients included, only 29 developed at least one type of toxicity. Hematological disorders were the most reported suspected ADR. However, only four patients interrupted their treatment due to toxicity. After 12 months of treatment, most patients had disease progression, which was the main reason for therapy discontinuation. This AP model played an important role in the early detection of AEs and, consequently, contributed to better management of them. Increasing the number of suspected ADR reports is crucial for drugs with limited safety data.
1. Introduction
Adverse drug reactions (ADRs) are the fifth leading cause of death in hospital settings [1]. According to data from the European Union (EU), ADRs are responsible for almost 5% of hospital admissions [1]. In 2005, results showed that ADRs were responsible for 197,000 deaths annually [2]. Moreover, the impact and management of ADRs also constitute significant economic burdens [3,4,5]. ADRs are almost always associated with antineoplastic therapy and are widely accepted as unavoidable by both patients and healthcare providers. In several studies, all patients (100%) receiving anticancer drugs had at least one ADR, and a normal range of 2–7 ADRs per cancer patient was referred [6,7,8]. Currently, in daily oncology clinical practice, many side effects can be attributed to antineoplastic targeted drugs. Although different from the well-known side effects of chemotherapy, these adverse effects can seriously compromise patients’ quality of life and may even lead patients to discontinue or request a change in treatment. It is important to study the toxicity of antineoplastic targeted therapies because most of the ADRs included in the summary of product characteristics (SmPC) come from pivotal clinical trials: the list of ADRs identified in these trials is unlikely to be comprehensive because, in clinical trials, drugs are tested under controlled conditions in selected patients [9].
Several authorities, such as the European Medicines Agency (EMA), the Member States of the EU, and the European Commission have contributed to the development of pharmacovigilance activities, such as the introduction of Regulation (EU) No 520/2012 of 19 June 2012 [10]. In this context, among other measures implemented through this legislation, the additional monitoring (AM) concept is highlighted [1,10]. This concept intends to increase the number of suspected ADR notifications reported, namely in medicines that have limited safety data, thus requiring closer monitoring by regulatory authorities. Despite additional safety monitoring, these medicines are considered safe since only medicines with benefits that outweigh the risks are introduced into the European market [11]. AM status includes medicines in the following cases: (i) medicines that contain a new active substance authorized in the EU after 1 January 2011; (ii) biological medicines, such as vaccines or plasma-derived medicines, authorized in the UE after 1 January 2011; (iii) medicines with a conditional approval (cases in which the company that markets the medicine must provide more data about it) or medicines authorized under exceptional circumstances (when there are specific reasons why the company cannot provide a comprehensive set of data); (iv) medicines for which further studies are needed (e.g., to provide more data on the long-term use of the medicine or a rare side effect seen during clinical trials); (v) medicines authorized with specific obligations on the recording of suspected ADRs. Other medicines can be included in the list of medicines under AM based on advice from the EMA’s Pharmacovigilance Risk Assessment Committee (PRAC) [11]. The PRAC is the European Medicines Agency committee responsible for assessing and monitoring the safety of human medicines. This committee is responsible for reviewing the list of medicines under AM every month. Medicines can be included on the list when approved for the first time or at any time during their life cycle and remain on it for 5 years or until being removed under the decision of the PRAC [5]. Medicines under AM are labeled with a black inverted triangle (▼) in their SmPC and package leaflet, accompanied by a brief explanatory statement, which allows for the quick identification of these medicines by patients and healthcare professionals [12,13].
Since a substantial part of these medicines is provided by hospital pharmacists, in an outpatient regime, the role of these healthcare professionals is extremely relevant along with the collaboration of patients and other healthcare professionals, such as doctors and nurses [14]. In the literature, there is an evident lack of studies about the real-world safety, efficacy/effectiveness, and cooperation between healthcare professionals in the management of the ADRs of medicines under AM. Although these medicines are already on the market and almost all their adverse effects, identified by clinical trials, are already described in the respective SmPC, the fact that they have the status of drugs under AM justifies the realization of real-world pharmacovigilance studies. Real-world studies are performed in real clinical practice settings and are better able to assess the efficacy/effectiveness and safety of drugs when used in real life by patients and physicians.
The under-reporting of ADRs in oncology can be addressed through proactive forms of pharmacovigilance and multidisciplinary collaborations [9]. This study intends to develop, implement, and evaluate the impact of an active pharmacovigilance (AP) model for oral antineoplastic agents labeled with the black inverted triangle, through an internal procedure involving the pharmaceutical services, the oncology service, and the clinical hematology service. Using a multidisciplinary approach, we want to improve the early identification of adverse events (AEs) associated with these medicines and assess these AEs. We also intend to educate patients or caregivers on the importance of being aware of the signs/symptoms of possible AEs that occur during treatment and to encourage their reporting. By improving knowledge of the AEs of these drugs, better risk management is expected.
2. Materials and Methods
2.1. Study Design
A prospective, descriptive study of AP was conducted between 2019 and 2021 in the Local Health Unit of Matosinhos (LHUM) (Porto, Portugal). The study was supported by the collaboration of 3 services, namely: the pharmaceutical services that integrate the hospital pharmacy, the oncology service, and the clinical hematology service. This study was approved by the ethics committee and authorized by the Board of Directors of the Hospital Unit (NS 64/CE/JAS-07/06/2019). All subjects provided written informed consent for participation. The eligible patients were those 18 years or older whose therapy included at least one oral anti-cancer drug submitted to AM. The active substances of medicines under AM considered in the study were alectinib, cabozantinib, entrectinib, ixazomib, lenalidomide, lorlatinib, niraparib, osimertinib, palbociclib, ribociclib, trametinib in association with dabrafenib, vandetanib, venetoclax, and trifluridine plus tipiracil.
2.2. Risk Minimization Measures
The initial phase of this study was performed through the implementation of risk minimization measures for AEs caused by the selected medicines. Risk minimization emerged from the need to inform patients and raise awareness among healthcare professionals [15]. According to the guidelines on good pharmacovigilance practices, the goal of risk minimization measures is to facilitate informed decision-making to support risk minimization when prescribing, dispensing, and/or using a medicinal product [16]. The successful implementation of risk minimization measures guarantees the principles of risk management where the benefits of a medicinal product exceed the risks by a large margin [17].
In this context, a list of the medicines under AM prescribed in the hospital was created. This list is periodically updated and published together with a poster that contains information about the medicines under the AM concept and about the meaning of the black inverted triangle, and it is shown in a place visible to patients and healthcare professionals. In the hospital pharmacy, these medicines are identified with an orange label in their storage facility. An information leaflet that contained several details about these identified medicines—mainly the most frequent AEs reported in their SmPC, recommendations for prescribing, and preventive measures as well as signs/symptoms to be monitored—was also written for healthcare professionals.
2.3. Adverse Events Monitoring
After the prescription of the drugs under AM, the patients had their first pharmaceutical consultation with a hospital pharmacist. Aspects related to the dosage, drug–drug and drug–food interactions, storage conditions, and most relevant excipients (e.g., lactose and sodium), which could potentially result in some interactions, were explained. In this first consultation, the pharmacist also clarified the concept of medicines under AM and the main purpose of this study. The signs and symptoms of the AEs and recommendations for their management were also highlighted to the patients. In the following visits to the hospital, after their report in medical and pharmaceutical follow-up consultations, the AEs were monitored by the hospital pharmacists. More details are discussed below. The next follow-up consultations were carried out according to the evaluation cycle of each drug. For instance, palbociclib and ribociclib are evaluated every 15 days during the first two cycles. If no toxicity is detected, the frequency of the evaluation becomes monthly.
The 4 minimum criteria to report an adverse drug reaction were: an identifiable patient, a suspected medicine, a suspected reaction, and an identifiable reporter. According to the guideline on good pharmacovigilance practices (GVP) published by the European Medicines Agency and the Heads of Medicines Agencies, an adverse reaction is characterized by the fact that a causal relationship between a medicinal product and an occurrence is suspected [18]. For regulatory reporting purposes, if an event is spontaneously reported—even if the relationship is unknown or unstated—it meets the definition of an adverse reaction. Therefore, all spontaneous reports submitted by healthcare professionals or consumers are considered suspected adverse reactions, since they convey the suspicions of the primary sources, unless the reporters specifically state that they believe the events to be unrelated or that a causal relationship can be excluded [18].
In this active pharmacovigilance study, alongside the spontaneous reports of ADRs made by health professionals and patients (passive pharmacovigilance), data on ADRs obtained using the active pharmacovigilance model developed for this purpose were predominantly collected. In this model of active pharmacovigilance, healthcare professionals themselves (physicians and pharmacists, in our study) deliberately question the patients and other healthcare professionals (e.g., other physicians and nurses) about the occurrence of ADRs and obligatorily register them in a database created for this purpose. The use of active pharmacovigilance models to identify ADRs has been highlighted in pharmacovigilance activities, as they are able to detect a greater number of patients (and with more diverse characteristics) with ADRs and, consequently, reduce the chronic underreporting of adverse drug events [19].
The collection of AEs—and, consequently, their monitoring—was supported by several tools developed by the hospital pharmacists in collaboration with the oncology service, namely:
An AE monitoring card was a card delivered to the patient during the first pharmaceutical consultation. This card contained the medicine’s name, highlighting the fact that the patient was taking a medicine under AM and the most frequent AEs requiring monitoring. This card allowed the patient to register several points, mainly: the exact start and the end of the AE occurrence, whether the physician was contacted by the patient during the occurrence, and what action was taken after the contact. In addition, the card included telephone numbers, such as the hospital unit, pharmaceutical services, oncology and clinical hematology services numbers, and other emergency contacts. The electronic addresses of INFARMED and Porto’s Pharmacovigilance Unit were also mentioned to encourage an increase in suspected ADR reports by patients.
The patient information leaflet (PIL) was a supporting document that explained, in more detail, the information mentioned in the AE monitoring card related to medicine under AM.
A webpage discussing the active pharmacovigilance of medicines under AM was also available on the hospital intranet. A page on the LHUM intranet was created with all the information and documents related to the study (informed consent form, PIL, AE monitoring card, and SmPC of the medicines included). It was accessible to all hospital pharmacists. It also included a simplified database of the AEs reported during the study period and direct access to the platforms used for reporting suspected ADRs, such as Porto’s Pharmacovigilance Unit.
The Medicines under AM Monitoring platform was a platform restricted to the study investigators that contained all data collected from AE monitoring, namely: dose reductions, the temporary or permanent suspension of treatment, and the causes that originated it. This record was compiled by drug and by patient. Other data, such as the information reported by the patient in medical and pharmaceutical follow-up consultations (even over the telephone) and AE monitoring card records, were mentioned. These results were used for the next phase.
2.4. Effectiveness and Safety Monitoring Database
The data obtained on the safety and effectiveness of drugs was discussed among healthcare professionals following the study. After data analysis, an effectiveness and safety monitoring database was created, allowing for the generation of clinical outcomes experienced by the patients. The Common Terminology Criteria for Adverse Events (CTCAE, also called common toxicity criteria (CTC)) was used to classify the AE of antineoplastic drugs according to the severity grades (1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening, and 5 = death) [20]. In the CTCAE, an adverse event is defined as any abnormal clinical finding temporally associated with the use of a therapy for cancer; causality is not required. These criteria are used for the management of chemotherapy administration and dosing and to provide standardization and consistency in the definition of treatment-related toxicity. In addition, the overall response to the treatment was evaluated through the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 criteria [21].
A scheme of the methods section is displayed in Figure 1.
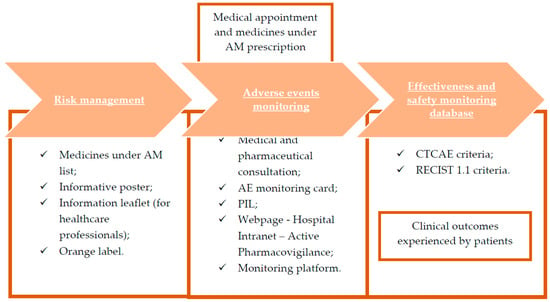
Figure 1.
Methodology representation. AE, adverse events; AM, additional monitoring; CTCAE, Common Terminology Criteria for Adverse Events; PIL, patient information leaflet; RECIST, Response Evaluation Criteria in Solid Tumors.
3. Results
A total of 52 patients were included, and 14 drugs under AM were studied according to their base pathology. Table 1 describes the number of patients involved according to their diagnosis and the medicine under AM that was prescribed. Except for lenalidomide and trifluridine plus tipiracil, all other oral anticancer drugs are classified as targeted drugs consisting of small molecule inhibitors (e.g., tyrosine kinase inhibitors). Of the total number of drugs under AM analyzed, trifluridine plus tipiracil was the most prescribed drug. A total of 9 patients were monitored in terms of AEs in the treatment of colon adenocarcinoma, rectal adenocarcinoma, and gastric adenocarcinoma. Osimertinib and venetoclax were prescribed to 7 patients each for the treatment of lung adenocarcinoma and chronic lymphocytic leukemia, respectively. Cabozantinib, entrectinib, and trametinib, in association with dabrafenib and vandetanib, were prescribed to only one patient.

Table 1.
Medicines under AM included in the study according to their base pathology.
Of the 52 patients, 31 were female and 21 were male. Palbociclib and ribociclib were prescribed to female patients, considering their base pathology. Other drugs, such as cabozantinib, lorlatinib, and niraparib, were also prescribed to females only, while entrectinib, vandetanib, and the association trametinib plus dabrafenib were prescribed to males only. The predominant age groups were 50–60 and 60–70 years, with 15 patients in each group. The results are presented in Table 2 and Table 3.

Table 2.
Patients categorized by gender and the medicine under AM that was taken.

Table 3.
Patients categorized by gender according to their age group.
A total of 29 (55.8%) patients developed at least one type of suspected toxicity to the drugs prescribed. In patients who reported only one toxicity, most patients developed grade 2 toxicity. Overall, 5 patients developed grade 1 toxicity, 15 patients developed grade 2 toxicity, and 9 patients developed grade 3 toxicity. Grade 3 toxicity was mostly related to the development of hematological disorders in patients taking ixazomib, palbociclib, or ribociclib. Patients taking alectinib, lenalidomide, osimertinib, or trifluridine plus tipiracil developed more than one type of toxicity. Indeed, gastrointestinal disorders are common in patients taking alectinib and trifluridine plus tipiracil [22,23], as also reported in our study. Only four patients had more than one toxicity. For instance, the patient that was taking cabozantinib developed mucositis grade 3 toxicity and hypertension grade 4 toxicity. The other three patients within whom more than one type of toxicity was reported were taking alectinib, entrectinib, and vandetanib. Table 4 describes the type of toxicity associated with the medicines under study as well as their grade (for additional information about the molecular structure of the studied oral antineoplastic drugs, see Supplementary Material).

Table 4.
Type and grade of suspected toxicity associated with the medicines in this study.
A total of 33 (63.5%) of the 52 patients included in the study discontinued their treatment. The causes of discontinuation included four reasons, namely: the therapeutic scheme was completed, hyperbilirubinemia, disease progression (DP), and other types of toxicity. DP was the main discontinuation cause, involving 26 patients. In this context, all patients taking the most prescribed drug in the study, trifluridine plus tipiracil, stopped their treatment. The same was verified in patients taking cabozantinib, entrectinib, and ixazomib.
Only four patients that were taking lorlatinib, alectinib, vandetanib, and lenalidomide interrupted their treatment due to toxicity. In this context, one of the two patients that were taking lorlatinib interrupted the treatment due to hyperbilirubinemia development. The only patient taking vandetanib for the treatment of medullary thyroid carcinoma discontinued treatment due to toxicity, namely QT prolongation. Table 5 describes the discontinuation causes for the drugs involved in the study.

Table 5.
Causes of discontinuation according to the prescribed drug.
The treatment response was analyzed using RECIST 1.1 criteria every 3 months during the study period. After 3 months of treatment, out of a total of 38 patients, 34 patients did not reveal DP. Most of the patients had a partial response (PR) to the treatment, followed by stable disease (SD). Only four patients had DP. After 12 months of treatment, nine patients did not reveal DP. However, in terms of proportion, the percentage of patients with DP was very similar to that of the patients with no DP. These results are described in Table 6.

Table 6.
Overall treatment response in patients with measurable disease.
4. Discussion
This study intended to investigate, through an AP model, data on the safety and effectiveness of medicines under AM that were prescribed and dispensed in the LHUM from 2019 to 2021.
The first phase of the study included the implementation of risk minimization measures. These measures aimed to share knowledge with patients and healthcare professionals, particularly to clarify the AM concept, the meaning of the black inverted triangle, and the most frequent AEs reported in the SmPC of the drugs included in the study. The collaboration of all, especially the input from patients and healthcare professionals, is essential for effective pharmacovigilance and benefit–risk management [24,25]. After the prescription of medicines under AM, the AEs reported in medical and pharmaceutical consultations were monitored. These data and other relevant information, such as the causes of drug discontinuation and treatment responses, were analyzed. The main results are discussed below.
A total of 52 patients were included in the study; 31 were female and 21 were male. Most patients were between 60 and 80 years old. The most prescribed and dispensed medicine under AM was trifluridine plus tipiracil. This drug association is indicated for the treatment of adult patients with metastatic colorectal and gastric cancer [26]. In total, eight patients were treated with ixazomib and lenalidomide for multiple myeloma [27,28]. For hepatocellular carcinoma and medullary thyroid carcinoma, only one patient for each was enrolled in the study, taking cabozantinib and vandetanib, respectively [29,30]. Cabozantinib is also indicated in renal cell carcinoma and differentiated thyroid carcinoma [29].
Only 29 patients developed at least one type of toxicity to the drugs studied. Of these, 13 patients developed hematological disorders with a toxicity grade ranging between 1 and 3. Although these drugs have differences in the mechanisms of action, hematological disorders were the most reported ADRs in the study. These AEs occurred particularly in patients taking ixazomib, lenalidomide, palbociclib, ribociclib, venetoclax, or trifluridine plus tipiracil. However, only patients taking ixazomib, palbociclib, or ribociclib developed grade 3 toxicity involving hematological disorders. According to data described in the SmPC, neutropenia, leukopenia, anemia, and thrombocytopenia are considered very common AEs in patients taking palbociclib and ribociclib [31,32]. Based on a pooled dataset from 3 randomized studies, grade 3 neutropenia was developed by 500 (57.3%) patients out of a total of 872 taking palbociclib [31]. For patients taking ribociclib, based on phase III studies results, grade 3 or 4 was reported in 62% of patients [32]. These results may be explained by similarities in the mechanism of action. Palbociclib and ribociclib are cyclin-dependent kinase (CDK) 4/6 inhibitors and are indicated as a treatment option for patients with HR+, HER2- advanced breast cancer, either as a first-line therapy combined with an aromatase inhibitor or as second-line therapy in combination with fulvestrant [33].
Additionally, four patients reported having more than one toxicity. These patients were taking alectinib, cabozantinib, entrectinib, or vandetanib. In this context, patients that were taking alectinib reported several toxicities, such as gastrointestinal, liver, lung, and kidney disorders. In fact, one of the patients had severe hepatotoxicity with alectinib, similar to what was reported in another real-world study, where hepatic disorders were considered a common ADR with significant identified risk (all grades, 19.8%; grade ≥ 3, 2.0%) [34]. The SmPC describes hepatobiliary disorders as common AEs, namely increased aspartate aminotransferase (AST), increased alanine aminotransferase (ALT), and increased bilirubin [35]. According to the literature, drug-induced hepatotoxicity is more common with crizotinib or ceritinib than alectinib. Approximately 1–5% of patients experienced alectinib-induced liver damage [36]. A case report described a patient diagnosed with advanced lung adenocarcinoma treated with alectinib as a first-line therapy. After the initiation of alectinib, the tumor decreased rapidly. On day 79 of treatment, the serum levels of AST and ALT increased to grade 3, according to the CTCAE criteria. Alectinib was immediately discontinued [37]. In our study, one patient discontinued the treatment due to toxicity. However, for this patient in particular, who developed severe hepatotoxicity, the cause of discontinuation was DP. The patient that was taking vandetanib had treatment discontinuation due to toxicity. QT prolongation is a very common AE in patients taking this drug [30]. In our study, QT prolongation might have been the cause of drug discontinuation. Electrocardiography and serum levels of calcium, potassium, and magnesium should be obtained at baseline and during weeks 2 to 4, weeks 8 to 12, and every 3 months thereafter during the therapy [38].
In addition, the patient taking cabozantinib also developed two types of toxicity, mucositis grade 3 toxicity and hypertension grade 4 toxicity. Oral mucositis with cabozantinib was reported at a frequency of 36% in the CABOSUN trial, and a few patients (5.1%) experienced grade 3 or 4 oral mucositis [39]. Likewise, a total of 81% of patients in the CABOSUN trial reported hypertension, with grade 3/4 hypertension having an incidence of 28% [40]. According to the guidelines, after cabozantinib initiation, blood pressure should be monitored early and regularly, and appropriate antihypertensive therapy should be considered if needed. Cabozantinib should be discontinued if hypertension is severe and persistent despite anti-hypertensive therapy and dose-reduction implementations [29]. Nevertheless, in our study, the cause of cabozantinib discontinuation was DP.
All the patients taking lenalidomide had treatment discontinuation. In a total of five patients that were taking this medicine, two patients interrupted the drug due to the completion of the treatment, and two patients discontinued due to DP. Only one patient interrupted the treatment due to toxicity. Neurological disorders were the suspected ADR that might have caused drug discontinuation. Patients with new or worsening neurological symptoms should be monitored [28]. In the literature, although rare, cases of progressive multifocal leukoencephalopathy have been reported [38,40,41].
Lorlatinib was reported to cause dyslipidemia in one of the two patients treated with this drug. A study of patients (N = 295) treated with lorlatinib at 100 mg once daily revealed hypercholesteremia of any grade in 243 patients [42]. However, this AE was not considered the main cause of treatment discontinuation. In our study, hyperbilirubinemia was the toxicity that led to drug discontinuation. Zhu et al. described a real-world data analysis for the efficacy and safety of lorlatinib. Of a total of 95 patients, only 1 reported an increase in blood bilirubin [43]. Lorlatinib is metabolized by the liver. Any hepatic impairment is likely to increase blood bilirubin concentration [44].
In fact, only four patients had treatment discontinuation due to toxicity, which may demonstrate the safety profile of these drugs. The main cause of drug discontinuation was DP. This result was also highlighted through RECIST 1.1 criteria. After 12 months of treatment, the overall number of patients with measurable disease that had DP (43.75%) was almost the same as the patients that had CR, PR, or SD (56.25%). However, after 3 months of treatment, the overall results were more encouraging.
For this study, some strengths and limitations must be considered. The major strength was the data collected on the safety and effectiveness of drugs that have very few studies described in the literature, particularly in the real-world setting. It is important to note that, for medicines under additional monitoring, even the absence of adverse reactions not described in clinical trials or the SmPC is extremely important postmarketing information for regulatory authorities and healthcare professionals. These real-world data are increasingly being used to evaluate the safety of innovative antineoplastic therapies, such as tumor-targeted therapy. This is particularly useful for assessing drug toxicity profiles in patient populations that are often excluded from randomized clinical trials, such as older patients (such as those included in the present study) who are often patients with comorbidities or poor performance statuses. Furthermore, these antineoplastic treatments can represent a challenge in daily clinical practice, especially in critical and frail subpopulations (such as older patients or polymedicated patients) or in complex socio-health conditions (such as those determined by the recent COVID-19 pandemic that affected the patients included in the present study). We were able to analyze the suspected ADRs and tumor growth in a small, heterogeneous population, which are usually not considered in premarketing clinical trials. In addition, a commitment to pharmacovigilance purposes is notorious among the healthcare professionals and patients who participated in the study.
However, some limitations of our study should also be mentioned. The patient sample size was relatively small, since data were collected from 52 patients only, and, in addition, from a single hospital institution. In this way, the results of our case series study cannot be generalized, but must be analyzed together with other similar studies, carried out in other hospital institutions, either in the same country or in other countries and involving other oncologic patients. The follow-up period of treatment was limited to 12 months, which may not be long enough to fully evaluate the safety and effectiveness of the drugs studied. Furthermore, considering the study period, due to the COVID-19 pandemic, some consultations were delayed or, in some cases, did not happen. In this context, data collection, namely the ADR report and overall response, may have been influenced. Similarly, the study did not assess the impact of drug interactions or other factors (e.g., drug-food interactions) that may contribute to ADRs, nor did it control for confounding factors, such as patient characteristics or comorbidities, which may also have an impact on the risk of ADRs.
Despite these limitations, this is an original study with real-world data that may be eligible for inclusion in a systematic review, designated to better determine the frequency of ADRs, as well as the frequency of their severity grades, obtained in the real-life setting and for certain subgroups of patients (e.g., according to age, sex, etc.) with oral antineoplastic drugs under AM.
5. Conclusions
The application of the developed AP model to oral antineoplastic agents under AM has contributed to better management of toxicity and, therefore, to obtain better real-world clinical outcomes for patients. Risk minimization measures were implemented. Patient engagement was crucial to monitor the toxicity in a timely manner. Patients felt better supported, more confident in the treatment instituted, and more encouraged to notify of AEs. The safer use of drugs was promoted, and the quality of services provided by hospital pharmacists was improved. The collaboration and communication between healthcare professionals of the various services involved, enabling teamwork in a multidisciplinary context, are also to be underlined.
We believe that further high-quality clinical studies should be conducted on drugs labeled with the black inverted triangle. This model may be applied and potentially extended to other classes of medicines under AM.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol30040315/s1, Table S1: Chemical structures and suspected toxicities of the studied oral antineoplastics.
Author Contributions
Conceptualization, S.P.C.d.S., M.J. and M.M.; methodology, S.P.C.d.S., M.J. and R.C.e.S.; validation, S.P.C.d.S., M.J., R.C.e.S. and M.M.; formal analysis, S.P.C.d.S., M.J., R.C.e.S., F.R., M.T.H., A.P.D. and M.M.; investigation, S.P.C.d.S., M.J., R.C.e.S. and M.M.; writing—original draft preparation, S.P.C.d.S. and M.J.; writing—review and editing, F.R., M.T.H., R.C.e.S., A.P.D. and M.M.; supervision, A.P.D. and M.M.; project administration, S.P.C.d.S., A.P.D. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by CICS-UBI, which is financed by national funds from Fundação para a Ciência e a Tecnologia (FCT) and by Fundo Europeu de Desenvolvimento Regional (FEDER) under the scope of PORTUGAL 2020 and Programa Operacional do Centro (CENTRO 2020), with the project reference numbers UIDB/00709/2020 and UIDP/00709/2020.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the Local Health Unit of Matosinhos (LHUM) (Porto, Portugal) (protocol code NS 64/CE/JAS-07/06/2019, approval date of 7 June 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data supporting this study consist of irreversibly anonymized clinical data and are included within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strengthening Pharmacovigilance to Reduce Adverse Effects of Medicines. 2008. Available online: https://ec.europa.eu/commission/presscorner/detail/en/MEMO_08_782 (accessed on 2 February 2023).
- Bouvy, J.C.; De Bruin, M.L.; Koopmanschap, M.A. Epidemiology of adverse drug reactions in Europe: A review of recent observational studies. Drug Saf. 2015, 38, 437–453. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.J.; Kedia, M.S.; Bajpai, D.; Mehta, S.S.; Kshirsagar, N.A.; Gogtay, N.J. Evaluation of the prevalence and economic burden of adverse drug reactions presenting to the medical emergency department of a tertiary referral centre: A prospective study. BMC Clin. Pharmacol. 2007, 7, 8. [Google Scholar] [CrossRef] [PubMed]
- Adedapo, A.D.A.; Adedeji, W.A.; Adedapo, I.A.; Adedapo, K.S. Cohort study on adverse drug reactions in adults admitted to the medical wards of a tertiary hospital in Nigeria: Prevalence, incidence, risk factors and fatality. Br. J. Clin. Pharmacol. 2021, 87, 1878–1889. [Google Scholar] [CrossRef] [PubMed]
- Formica, D.; Sultana, J.; Cutroneo, P.M.; Lucchesi, S.; Angelica, R.; Crisafulli, S.; Ingrasciotta, Y.; Salvo, F.; Spina, E.; Trifirò, G. The economic burden of preventable adverse drug reactions: A systematic review of observational studies. Expert Opin. Drug Saf. 2018, 17, 681–695. [Google Scholar] [CrossRef]
- Singh, S.; Dhasmana, D.C.; Bisht, M.; Singh, P.K. Pattern of Adverse Drug Reactions to Anticancer Drugs: A Quantitative and Qualitative Analysis. Indian J. Med. Paediatr. Oncol. 2017, 38, 140–145. [Google Scholar]
- Guo, H.J.; Ren, F.; Zhang, D.; Ji, M. Monitoring report on 341 cases of adverse reactions caused by antitumor drugs. Afr. J. Microbiol. Res. 2012, 6, 3774–3777. [Google Scholar]
- Vijayalakshmi, M.K.; Palatty, P.L.; Bhat, P.; Dinesh, M. A comparative assessment of the ADR profile in various anti-cancer regimens excluding gastro-intestinal and haematological toxicity at a tertiary care centre. J. Clin. Diagn. Res. 2011, 5, 1209–1213. [Google Scholar]
- Baldo, P.; Fornasier, G.; Ciolfi, L.; Sartor, I.; Francescon, S. Pharmacovigilance in oncology. Int. J. Clin. Pharm. 2018, 40, 832–841. [Google Scholar] [CrossRef]
- Legal Framework: Pharmacovigilance. Available online: https://www.ema.europa.eu/en/human-regulatory/overview/pharmacovigilance/legal-framework-pharmacovigilance (accessed on 2 February 2023).
- Medicines under Additional Monitoring. Available online: https://www.ema.europa.eu/en/human-regulatory/post-authorisation/pharmacovigilance/medicines-under-additional-monitoring (accessed on 2 February 2023).
- Guideline on Good Pharmacovigilance Practices Module X—Additional Monitoring. 2013. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-x-additional-monitoring_en.pdf (accessed on 2 February 2023).
- Regulation (EC) No 726/2004 of the European Parliament and of the Council of 31 March 2004 laying down Community Procedures for the Authorisation and Supervision of Medicinal Products for Human and Veterinary Use and Establishing a European Medicines Agency. Official Journal of the European Union, L–136, pp. 1–33. 2004. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2004:136:0001:0033:en:PDF (accessed on 3 February 2023).
- Lively, A.; Minard, L.V.; Scott, S.; Deal, H.; Lambourne, T.; Giffin, J. Exploring the perspectives of healthcare professionals in delivering optimal oncology medication education. PLoS ONE 2020, 15, e0228571. [Google Scholar] [CrossRef]
- Reumerman, M.; Tichelaar, J.; Piersma, B.; Richir, M.C.; van Agtmael, M.A. Urgent need to modernize pharmacovigilance education in healthcare curricula: Review of the literature. Eur. J. Clin. Pharmacol. 2018, 74, 1235–1248. [Google Scholar] [CrossRef]
- Guideline on Good Pharmacovigilance Practices (GVP). Module XVI—Risk Minimisation Measures: Selection of Tools and Effectiveness Indicators (Rev 2). European Medicines Agency. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-xvi-risk-minimisation-measures-selection-tools_en-3.pdf (accessed on 3 February 2023).
- Guideline on Good Pharmacovigilance Practices (GVP). Module V—Risk Management Systems (Rev 2). European Medicines Agency. 2017. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-good-pharmacovigilance-practices-module-v-risk-management-systems-rev-2_en.pdf (accessed on 3 February 2023).
- Guideline on Good Pharmacovigilance Practices (GVP): Module VI—Collection, Management and Submission of Reports of Suspected Adverse Reactions to Medicinal Products (Rev 2). European Medicines Agency. 2017. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/guideline-good-pharmacovigilance-practices-gvp-module-vi-collection-management-submission-reports_en.pdf (accessed on 25 March 2023).
- Pan American Health Organization. Good Pharmacovigilance Practices for the Americas. PANDRH Technical Document Nº 5 Washington D.C. 2011. Available online: https://www.paho.org/hq/dmdocuments/2011/Series-Red-PARF---5-Eng.pdf (accessed on 25 March 2023).
- Common Terminology Criteria for Adverse Events (CTCAE). Available online: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm (accessed on 3 February 2023).
- RECIST 1.1 Guidelines. Available online: https://recist.eortc.org/recist-1-1-2/ (accessed on 3 February 2023).
- Peters, S.; Camidge, D.R.; Shaw, A.T.; Gadgeel, S.; Ahn, J.S.; Kim, D.W.; Ou, S.I.; Pérol, M.; Dziadziuszko, R.; Rosell, R.; et al. Alectinib versus Crizotinib in Untreated ALK-Positive Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 829–838. [Google Scholar] [CrossRef]
- Mayer, R.J.; Van Cutsem, E.; Falcone, A.; Yoshino, T.; Garcia-Carbonero, R.; Mizunuma, N.; Yamazaki, K.; Shimada, Y.; Tabernero, J.; Komatsu, Y.; et al. Randomized trial of TAS-102 for refractory metastatic colorectal cancer. N. Engl. J. Med. 2015, 372, 1909–1919. [Google Scholar] [CrossRef]
- Bahri, P.; Pariente, A. Systematising Pharmacovigilance Engagement of Patients, Healthcare Professionals and Regulators: A Practical Decision Guide Derived from the International Risk Governance Framework for Engagement Events and Discourse. Drug Saf. 2021, 44, 1193–1208. [Google Scholar] [CrossRef]
- O’Callaghan, J.; Griffin, B.T.; Morris, J.M.; Bermingham, M. Knowledge of adverse drug reaction reporting and the Pharmacovigilance of biological medicines: A survey of healthcare professionals in Ireland. BioDrugs 2018, 32, 267–280. [Google Scholar] [CrossRef]
- Summary of Product Characteristics LONSURF®. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/lonsurf-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics NINLARO®. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/ninlaro-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics REVLIMID®. 2007. Available online: https://www.ema.europa.eu/en/documents/product-information/revlimid-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics CABOMETYX®. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/cabometyx-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics CAPRELSA®. 2012. Available online: https://www.ema.europa.eu/en/documents/product-information/caprelsa-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics IBRANCE®. 2016. Available online: https://www.ema.europa.eu/en/documents/product-information/ibrance-epar-product-information_en.pdf (accessed on 4 February 2023).
- Summary of Product Characteristics KISQALI®. 2017. Available online: https://www.ema.europa.eu/en/documents/product-information/kisqali-epar-product-information_en.pdf (accessed on 4 February 2023).
- Braal, C.L.; Jongbloed, E.M.; Wilting, S.M.; Mathijssen, R.H.J.; Koolen, S.L.W.; Jager, A. Inhibiting CDK4/6 in Breast Cancer with Palbociclib, Ribociclib, and Abemaciclib: Similarities and Differences. Drugs 2021, 81, 317–331. [Google Scholar] [CrossRef]
- Masuda, N.; Ohe, Y.; Gemma, A.; Kusumoto, M.; Yamada, I.; Ishii, T.; Yamamoto, N. Safety and effectiveness of alectinib in a real-world surveillance study in patients with ALK-positive non-small-cell lung cancer in Japan. Cancer Sci. 2019, 110, 1401–1407. [Google Scholar] [CrossRef]
- Summary of Product Characteristics ALESCENSA®. 2017. Available online: https://www.ema.europa.eu/en/documents/product-information/alecensa-epar-product-information_en.pdf (accessed on 4 February 2023).
- Camidge, D.R.; Dziadziuszko, R.; Peters, S.; Mok, T.; Noe, J.; Nowicka, M.; Gadgeel, S.M.; Cheema, P.; Pavlakis, N.; de Marinis, F.; et al. Updated efficacy and safety data and impact of the EML4-ALK fusion variant on the efficacy of alectinib in untreated ALK-Positive advanced non-small cell lung cancer in the global phase III ALEX study. J. Thorac. Oncol. 2019, 14, 1233–1243. [Google Scholar] [CrossRef]
- Makimoto, G.; Kawakado, K.; Nakanishi, M.; Tamura, T.; Kuyama, S. Successful Treatment with Lorlatinib after the Development of Alectinib-Induced Liver Damage in ALK-Positive Non-Small-Cell Lung Cancer: A Case Report. Case Rep. Oncol. 2021, 14, 197–201. [Google Scholar] [CrossRef]
- Anderson, S.; Kiernan, M.; Ho, P.J. Lenalidomide-related progressive multifocal leukoencephalopathy: A Case Report and review of drug-related cases in multiple myeloma. Clin. Lymphoma Myeloma Leuk. 2019, 19, e169–e171. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Halabi, S.; Sanford, B.L.; Hahn, O.; Michaelson, M.D.; Walsh, M.K.; Feldman, D.R.; Olencki, T.; Picus, J.; Small, E.J.; et al. Cabozantinib Versus Sunitinib as Initial Targeted Therapy for Patients With Metastatic Renal Cell Carcinoma of Poor or Intermediate Risk: The Alliance A031203 CABOSUN Trial. J. Clin. Oncol. 2017, 35, 591–597. [Google Scholar] [CrossRef]
- Brigo, F.; Pagani, E.; Tezzon, F.; Masi, E.; Nardone, R. Lenalidomide-associated progressive multifocal leukoencephalopathy. Leuk. Lymphoma 2017, 58, 2514–2515. [Google Scholar] [CrossRef]
- Ruiz-Heredia, Y.; Sanchez-Vega, B.; Barrio, S.; Linares, M.; Rapado, I.; Braggio, E.; Stewart, K.; Folgueira, M.D.; Ramos, A.; Collado, L.; et al. Concurrent progressive multifocal leukoencephalopathy and central nervous system infiltration by multiple myeloma: A case report. J. Oncol. Pharm. Pract. 2019, 25, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Felip, E.; Solomon, B.J.; Thurm, H.; Peltz, G.; Chioda, M.D.; Shaw, A.T. Clinical Management of Adverse Events Associated with Lorlatinib. Oncologist 2019, 24, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhu, V.W.; Lin, Y.T.; Kim, D.W.; Loong, H.H.; Nagasaka, M.; To, H.; Ang, Y.L.; Ock, C.Y.; Tchekmedyian, N.; Ou, S.I.; et al. An International Real-World Analysis of the Efficacy and Safety of Lorlatinib Through Early or Expanded Access Programs in Patients With Tyrosine Kinase Inhibitor-Refractory ALK-Positive or ROS1-Positive NSCLC. J. Thorac. Oncol. 2020, 15, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Summary of Product Characteristics LORVIQUA®. 2019. Available online: https://www.ema.europa.eu/en/documents/product-information/lorviqua-epar-product-information_en.pdf (accessed on 4 February 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).