Longer Survival and Preserved Liver Function after Proton Beam Therapy for Patients with Unresectable Hepatocellular Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Etiology of Liver Diseases
2.3. Assessment for Hepatic Reserve Function
2.4. Proton Beam Therapy
2.5. Transcatheter Arterial Chemoembolization (TACE)
2.6. Percutaneous Radiofrequency Ablation
2.7. Evaluation of the Outcomes
2.8. Statistical Analyses
3. Results
3.1. Clinical Course of Representative Cases Treated by PBT or TACE + RFA
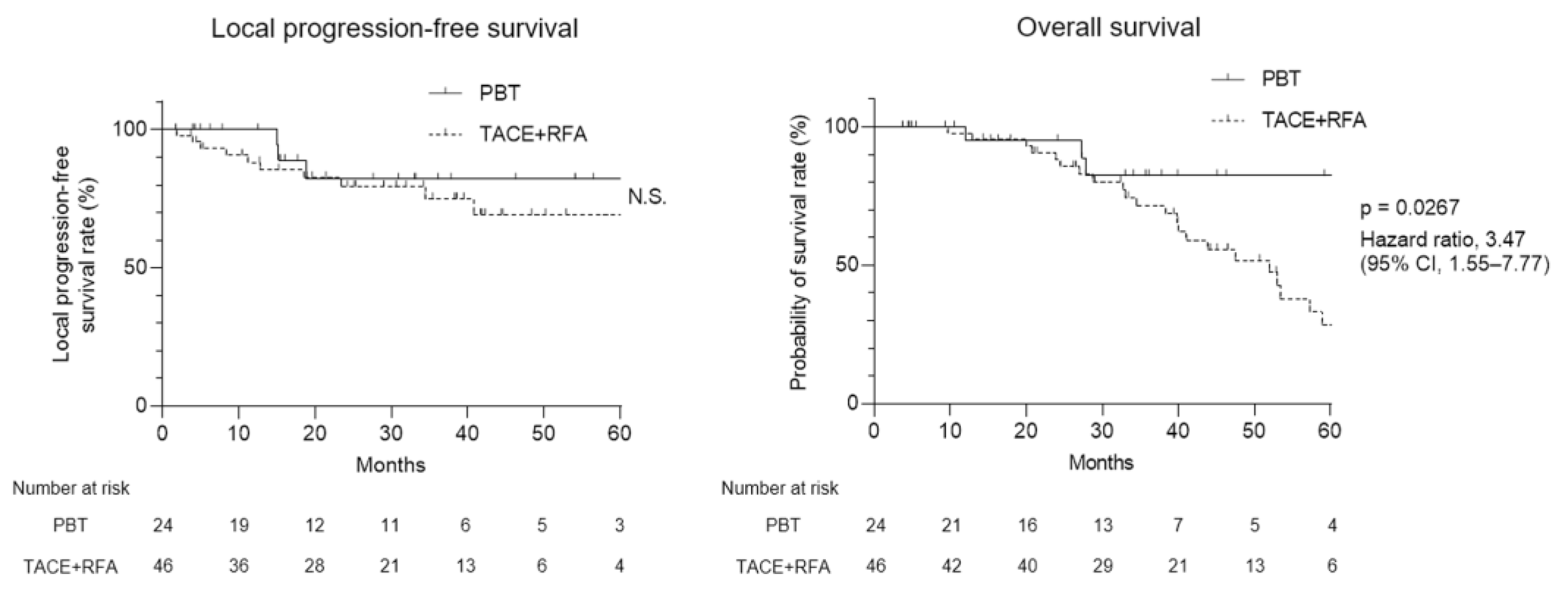
3.2. Therapeutic Efficacy of PBT and TACE + RFA
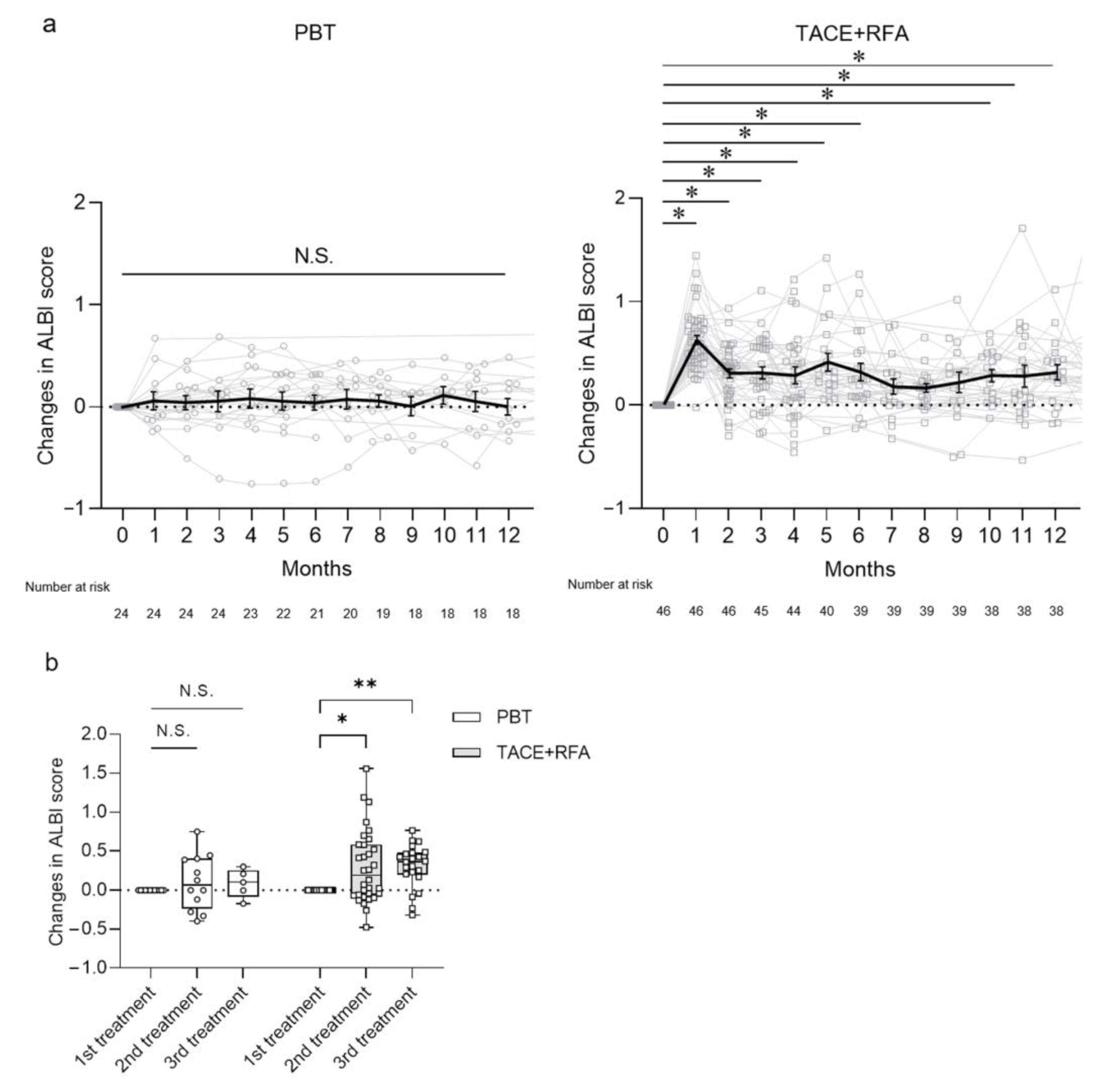
3.3. Long-Term Changes in Hepatic Reserve after Treatments
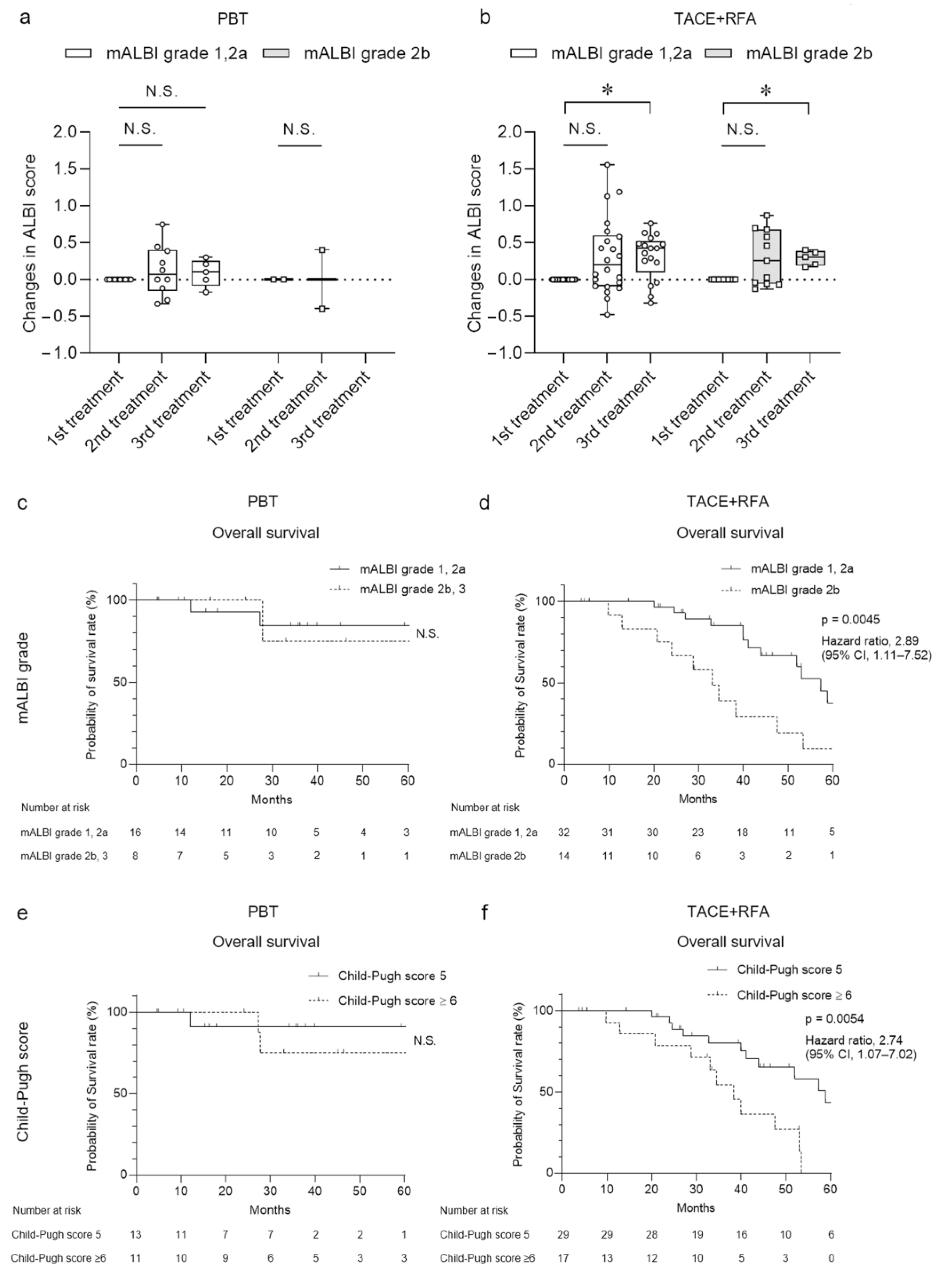
3.4. Clinical Course after Treatments Differentiated by Severity of Hepatic Reserve
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Sun, J.; Yang, X. Radiofrequency ablation-combined multimodel therapies for hepatocellular carcinoma: Current status. Cancer Lett. 2016, 370, 78–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kang, T.W.; Cha, D.I.; Song, K.D.; Lee, M.W.; Rhim, H.; Lim, H.K.; Sinn, D.H.; Kim, J.M.; Kim, K. Radiofrequency ablation vs. surgery for perivascular hepatocellular carcinoma: Propensity score analyses of long-term outcomes. J. Hepatol. 2018, 69, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Zou, Y.; Lyu, T.; Fan, Z.; Guan, H.; Song, L.; Tong, X.; Wang, J. Long-term outcomes of combined transarterial chemoembolization and radiofrequency ablation versus RFA monotherapy for single hepatocellular carcinoma ≤3 cm: Emphasis on local tumor progression. Int. J. Hyperth. 2022, 39, 1–7. [Google Scholar] [CrossRef]
- Ren, Y.; Cao, Y.; Ma, H.; Kan, X.; Zhou, C.; Liu, J.; Shi, Q.; Feng, G.; Xiong, B.; Zheng, C. Improved clinical outcome using transarterial chemoembolization combined with radiofrequency ablation for patients in Barcelona clinic liver cancer stage A or B hepatocellular carcinoma regardless of tumor size: Results of a single-center retrospective case control study. BMC Cancer 2019, 19, 983. [Google Scholar] [CrossRef] [Green Version]
- Hatzidakis, A.; Muller, L.; Krokidis, M.; Kloeckner, R. Local and Regional Therapies for Hepatocellular Carcinoma and Future Combinations. Cancers 2022, 14, 2469. [Google Scholar] [CrossRef]
- Hiraoka, A.; Kumada, T.; Kudo, M.; Hirooka, M.; Koizumi, Y.; Hiasa, Y.; Tajiri, K.; Toyoda, H.; Tada, T.; Ochi, H.; et al. Hepatic Function during Repeated TACE Procedures and Prognosis after Introducing Sorafenib in Patients with Unresectable Hepatocellular Carcinoma: Multicenter Analysis. Dig. Dis. 2017, 35, 602–610. [Google Scholar] [CrossRef]
- Maesaka, K.; Sakamori, R.; Yamada, R.; Tahata, Y.; Imai, Y.; Oshita, M.; Ohkawa, K.; Kodama, T.; Hikita, H.; Tatsumi, T.; et al. Initial treatment response to transarterial chemoembolization as a predictive factor for Child-Pugh class deterioration prior to refractoriness in hepatocellular carcinoma. Hepatol. Res. 2020, 50, 1275–1283. [Google Scholar] [CrossRef]
- Bush, D.A.; Kayali, Z.; Grove, R.; Slater, J.D. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: A phase 2 prospective trial. Cancer 2011, 117, 3053–3059. [Google Scholar] [CrossRef]
- Hong, T.S.; Wo, J.Y.; Yeap, B.Y.; Ben-Josef, E.; McDonnell, E.I.; Blaszkowsky, L.S.; Kwak, E.L.; Allen, J.N.; Clark, J.W.; Goyal, L.; et al. Multi-Institutional Phase II Study of High-Dose Hypofractionated Proton Beam Therapy in Patients With Localized, Unresectable Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. J. Clin. Oncol. 2016, 34, 460–468. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Park, J.W.; Kim, Y.J.; Kim, B.H.; Woo, S.M.; Moon, S.H.; Kim, S.S.; Koh, Y.H.; Lee, W.J.; Park, S.J.; et al. Phase I dose-escalation study of proton beam therapy for inoperable hepatocellular carcinoma. Cancer Res. Treat. 2015, 47, 34–45. [Google Scholar] [CrossRef]
- Chuong, M.; Kaiser, A.; Molitoris, J.; Mendez Romero, A.; Apisarnthanarax, S. Proton beam therapy for liver cancers. J. Gastrointest. Oncol. 2020, 11, 157–165. [Google Scholar] [CrossRef]
- Toramatsu, C.; Katoh, N.; Shimizu, S.; Nihongi, H.; Matsuura, T.; Takao, S.; Miyamoto, N.; Suzuki, R.; Sutherland, K.; Kinoshita, R.; et al. What is the appropriate size criterion for proton radiotherapy for hepatocellular carcinoma? A dosimetric comparison of spot-scanning proton therapy versus intensity-modulated radiation therapy. Radiat. Oncol. 2013, 8, 48. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.H.; Koh, Y.H.; Kim, B.H.; Kim, M.J.; Lee, J.H.; Park, B.; Park, J.W. Proton beam radiotherapy vs. radiofrequency ablation for recurrent hepatocellular carcinoma: A randomized phase III trial. J. Hepatol. 2021, 74, 603–612. [Google Scholar] [CrossRef]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Johnson, P.J.; Berhane, S.; Kagebayashi, C.; Satomura, S.; Teng, M.; Reeves, H.L.; O’Beirne, J.; Fox, R.; Skowronska, A.; Palmer, D.; et al. Assessment of liver function in patients with hepatocellular carcinoma: A new evidence-based approach-the ALBI grade. J. Clin. Oncol. 2015, 33, 550–558. [Google Scholar] [CrossRef]
- Hiraoka, A.; Michitaka, K.; Kumada, T.; Izumi, N.; Kadoya, M.; Kokudo, N.; Kubo, S.; Matsuyama, Y.; Nakashima, O.; Sakamoto, M.; et al. Validation and Potential of Albumin-Bilirubin Grade and Prognostication in a Nationwide Survey of 46,681 Hepatocellular Carcinoma Patients in Japan: The Need for a More Detailed Evaluation of Hepatic Function. Liver Cancer 2017, 6, 325–336. [Google Scholar] [CrossRef]
- Mizuhata, M.; Takamatsu, S.; Shibata, S.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; Sasaki, M.; et al. Respiratory-gated Proton Beam Therapy for Hepatocellular Carcinoma Adjacent to the Gastrointestinal Tract without Fiducial Markers. Cancers 2018, 10, 58. [Google Scholar] [CrossRef] [Green Version]
- Shibata, S.; Takamatsu, S.; Yamamoto, K.; Mizuhata, M.; Bou, S.; Sato, Y.; Kawamura, M.; Asahi, S.; Tameshige, Y.; Maeda, Y.; et al. Proton Beam Therapy without Fiducial Markers Using Four-Dimensional CT Planning for Large Hepatocellular Carcinomas. Cancers 2018, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Komatsu, S.; Fukumoto, T.; Demizu, Y.; Miyawaki, D.; Terashima, K.; Sasaki, R.; Hori, Y.; Hishikawa, Y.; Ku, Y.; Murakami, M. Clinical results and risk factors of proton and carbon ion therapy for hepatocellular carcinoma. Cancer 2011, 117, 4890–4904. [Google Scholar] [CrossRef]
- Llovet, J.M.; Brú, C.; Bruix, J. Prognosis of hepatocellular carcinoma: The BCLC staging classification. Semin. Liver Dis. 1999, 19, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Reig, M.; Bruix, J. Hepatocellular carcinoma. Lancet 2018, 391, 1301–1314. [Google Scholar] [CrossRef] [PubMed]
- Kudo, M.; Chung, H.; Osaki, Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan Integrated Staging Score (JIS score). J. Gastroenterol. 2003, 38, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Llovet, J.M.; Villanueva, A.; Marrero, J.A.; Schwartz, M.; Meyer, T.; Galle, P.R.; Lencioni, R.; Greten, T.F.; Kudo, M.; Mandrekar, S.J.; et al. Trial Design and Endpoints in Hepatocellular Carcinoma: AASLD Consensus Conference. Hepatology 2021, 73 (Suppl. 1), 158–191. [Google Scholar] [CrossRef] [PubMed]
- Forner, A.; Llovet, J.M.; Bruix, J. Hepatocellular carcinoma. Lancet 2012, 379, 1245–1255. [Google Scholar] [CrossRef]
- Yan, K.; Chen, M.H.; Yang, W.; Wang, Y.B.; Gao, W.; Hao, C.Y.; Xing, B.C.; Huang, X.F. Radiofrequency ablation of hepatocellular carcinoma: Long-term outcome and prognostic factors. Eur. J. Radiol. 2008, 67, 336–347. [Google Scholar] [CrossRef]
- Iezzi, R.; Pompili, M.; Posa, A.; Coppola, G.; Gasbarrini, A.; Bonomo, L. Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J. Gastroenterol. 2016, 22, 1935–1942. [Google Scholar] [CrossRef]
- Yang, Y.; Yu, H.; Qi, L.; Liu, C.; Feng, Y.; Qi, J.; Li, J.; Zhu, Q. Combined radiofrequency ablation or microwave ablation with transarterial chemoembolization can increase efficiency in intermediate-stage hepatocellular carcinoma without more complication: A systematic review and meta-analysis. Int. J. Hyperth. 2022, 39, 455–465. [Google Scholar] [CrossRef]
- Miksad, R.A.; Ogasawara, S.; Xia, F.; Fellous, M.; Piscaglia, F. Liver function changes after transarterial chemoembolization in US hepatocellular carcinoma patients: The LiverT study. BMC Cancer 2019, 19, 795. [Google Scholar] [CrossRef] [Green Version]
- Garwood, E.R.; Fidelman, N.; Hoch, S.E.; Kerlan, R.K., Jr.; Yao, F.Y. Morbidity and mortality following transarterial liver chemoembolization in patients with hepatocellular carcinoma and synthetic hepatic dysfunction. Liver Transpl. 2013, 19, 164–173. [Google Scholar] [CrossRef]
- Petersen, J.B.; Lassen, Y.; Hansen, A.T.; Muren, L.P.; Grau, C.; Hoyer, M. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol. 2011, 50, 823–828. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, S.; Oshiro, Y.; Nakayama, H.; Fukuda, K.; Mizumoto, M.; Abei, M.; Shoda, J.; Matsuzaki, Y.; Thono, E.; Tokita, M.; et al. Proton beam therapy for large hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2010, 76, 460–466. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | PBT (n = 24) | TACE + RFA (n = 46) | p Value |
|---|---|---|---|
| Age, years | |||
| Median (range) | 74 (54–88) | 74 (57–85) | 0.478 * |
| <65 | 2 (8.3) | 3 (6.5) | >0.999 † |
| ≥65 | 22 (91.7) | 43 (93.5) | |
| Gender | |||
| Male | 16 (66.7) | 33 (71.7) | 0.785 † |
| Female | 8 (33.3) | 13 (28.3) | |
| ECOG performance status | |||
| 0 | 21 (87.5) | 37 (80.4) | 0.526 † |
| 1 | 3 (12.5) | 9 (19.6) | |
| Etiology | |||
| HBV | 5 (20.8) | 2 (4.3) | 0.077 ‡ |
| HCV | 7 (29.2) | 20 (43.5) | |
| NBNC | 12 (50.0) | 24 (52.2) | |
| AFP, ng/mL # | 4.3 (2.1–13,099.0) | 8.2 (1.1–1922.9) | 0.242 * |
| <10 | 16 (66.7) | 25 (54.3) | 0.444 † |
| ≥10 | 8 (33.3) | 21 (45.7) | |
| DCP, mAU/mL # | 29 (8–22,694) | 38 (7–50,929) | 0.837 * |
| <100 | 16 (66.7) | 33 (71.7) | 0.785 † |
| ≥100 | 8 (33.3) | 13 (28.3) | |
| Child–Pugh score | |||
| 5 | 13 (54.2) | 29 (63.0) | 0.608 † |
| ≥6 | 11 (45.8) | 17 (37.0) | |
| mALBI grade | |||
| 1, 2a | 16 (66.7) | 32 (69.6) | 0.794 † |
| 2b, 3 | 8 (33.3) | 14 (30.4) | |
| Tumor size, cm # | 2.7 (1.2–9.3) | 2.7 (1.4–7.0) | 0.557 * |
| <3 | 12 (50.0) | 29 (63.0) | 0.318 † |
| ≥3 | 12 (50.0) | 17 (37.0) | |
| Number of treated lesion(s) | |||
| 1 | 22 (91.7) | 34 (73.9) | 0.201 ‡ |
| 2 | 2 (8.3) | 11 (23.9) | |
| 3 | 0 (0.0) | 1 (2.2) | |
| Vascular invasion | |||
| Vp 0,1 | 23 (95.8) | 46 (100.0) | 0.343 † |
| Vp 2 | 1 (4.2) | 0 (0.0) | |
| BCLC stage | |||
| 0 | 9 (37.5) | 4 (8.7) | 0.147 † |
| A | 7 (29.2) | 34 (73.9) | |
| B | 5 (20.8) | 7 (15.2) | |
| C | 3 (12.5) | 1 (2.2) | |
| Post treatment to target lesion(s) | |||
| No | 21 (87.5) | 37 (80.4) | 0.526 † |
| Yes | 3 (12.5) | 9 (19.6) | |
| TACE | 1 (4.2) | 1 (2.2) | |
| TACE, RFA | 0 (0.0) | 3 (6.5) | |
| TACE, RFA, TKI, HAIC | 0 (0.0) | 1 (2.2) | |
| TACE, TKI or Atezo + Bev | 2 (8.3) | 1 (2.2) | |
| TACE, HAIC | 0 (0.0) | 1 (2.2) | |
| TKI | 0 (0.0) | 1 (2.2) | |
| TKI, HAIC | 0 (0.0) | 1 (2.2) | |
| Post treatment to non-target lesion(s) | |||
| No | 17 (70.8) | 19 (41.3) | 0.025 † |
| Yes | 7 (29.2) | 27 (58.7) | |
| TACE | 3 (12.5) | 3 (6.5) | |
| TACE, TKI | 0 (0.0) | 0 (0.0) | |
| TACE, TKI, HAIC | 1 (4.2) | 1 (2.2) | |
| TACE, RFA (PEIT) | 0 (0.0) | 7 (15.2) | |
| RFA (PEIT) | 1 (4.2) | 15 (32.6) | |
| PBT | 1 (4.2) | 0 (0.0) | |
| Atezo + Bev | 1 (4.2) | 1 (2.2) | |
| Follow-up time, months) | |||
| Median (range) | 34.9 (4.6–92.5) | 38.8 (3.7–110.8) | 0.329 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nosaka, T.; Matsuda, H.; Sugata, R.; Akazawa, Y.; Takahashi, K.; Naito, T.; Ohtani, M.; Kinoshita, K.; Tsujikawa, T.; Sato, Y.; et al. Longer Survival and Preserved Liver Function after Proton Beam Therapy for Patients with Unresectable Hepatocellular Carcinoma. Curr. Oncol. 2023, 30, 3915-3926. https://doi.org/10.3390/curroncol30040296
Nosaka T, Matsuda H, Sugata R, Akazawa Y, Takahashi K, Naito T, Ohtani M, Kinoshita K, Tsujikawa T, Sato Y, et al. Longer Survival and Preserved Liver Function after Proton Beam Therapy for Patients with Unresectable Hepatocellular Carcinoma. Current Oncology. 2023; 30(4):3915-3926. https://doi.org/10.3390/curroncol30040296
Chicago/Turabian StyleNosaka, Takuto, Hidetaka Matsuda, Ryotaro Sugata, Yu Akazawa, Kazuto Takahashi, Tatsushi Naito, Masahiro Ohtani, Kazuyuki Kinoshita, Tetsuya Tsujikawa, Yoshitaka Sato, and et al. 2023. "Longer Survival and Preserved Liver Function after Proton Beam Therapy for Patients with Unresectable Hepatocellular Carcinoma" Current Oncology 30, no. 4: 3915-3926. https://doi.org/10.3390/curroncol30040296
APA StyleNosaka, T., Matsuda, H., Sugata, R., Akazawa, Y., Takahashi, K., Naito, T., Ohtani, M., Kinoshita, K., Tsujikawa, T., Sato, Y., Maeda, Y., Tamamura, H., & Nakamoto, Y. (2023). Longer Survival and Preserved Liver Function after Proton Beam Therapy for Patients with Unresectable Hepatocellular Carcinoma. Current Oncology, 30(4), 3915-3926. https://doi.org/10.3390/curroncol30040296






