Injectable Synthetic Beta-Tricalcium Phosphate/Calcium Sulfate (GeneX) for the Management of Contained Defects Following Curettage of Benign Bone Tumours
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, C.J.; Brien, E.W. Early postoperative compilations of bone filling in curettage defects. J. Orthop. Surg. Res. 2019, 14, 261. [Google Scholar] [CrossRef]
- Goulet, J.A.; Senunas, L.E.; DeSilva, G.L.; Greenfield, M.L. Autogenous iliac crest bone graft. Complications and functional assessment. Clin. Orthop. Relat. Res. 1997, 339, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.M.; Leonidou, A.; Aslam-Pervez, N.; Hamza, A.; Panteliadis, P.; Heliotis, M.; Mantalaris, A.; Tsiridis, E. Biological therapy of bone defects: The immunology of bone allo-transplantation. Expert Opin. Biol. Ther. 2010, 10, 885–901. [Google Scholar] [CrossRef] [PubMed]
- Smeets, R.; Kolk, A.; Gerressen, M.; Driemel, O.; Maciejewski, O.; Hermanns-Sachweh, B.; Riediger, D.; Stein, J.M. A new biphasic osteoinductive calcium composite material with a negative Zeta potential for bone augmentation. Head Face Med. 2009, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Gava, N.F.; Engel, E.E. Treatment alternatives and clinical outcomes of bone filling after benign tumour curettage. A systematic review. Orthop. Traumatol. Surg. Res. 2022, 108, 102966. [Google Scholar] [CrossRef]
- Ogose, A.; Hotta, T.; Kawashima, H.; Kondo, N.; Gu, W.; Kamura, T.; Endo, N. Comparison of hydroxyapatite and beta tricalcium phosphate as bone substitutes after excision of bone tumors. J. Biomed. Mater. Res. B Appl. Biomater. 2005, 72, 94–101. [Google Scholar] [CrossRef]
- Parker, A.C.; Smith, J.K.; Courtney, H.S.; Haggard, W.O. Evaluation of two sources of calcium sulfate for a local drug delivery system: A pilot study. Clin. Orthop. Relat. Res. 2011, 469, 3008–3015. [Google Scholar] [CrossRef]
- Hung, Y.W.; Ko, W.S.; Liu, W.H.; Chow, C.S.; Kwok, Y.Y.; Wong, C.W.; Tse, W.L.; Ho, P.C. Local review of treatment of hand enchondroma (artificial bone substitute versus autologous bone graft) in a tertiary referral centre: 13 years’ experience. Hong Kong Med. J. 2015, 21, 217–223. [Google Scholar] [CrossRef]
- Galois, L.; Mainard, D.; Delagoutte, J.P. Beta-tricalcium phosphate ceramic as a bone substitute in orthopaedic surgery. Int. Orthop. 2002, 26, 109–115. [Google Scholar] [CrossRef]
- Hirata, M.; Murata, H.; Takeshita, H.; Sakabe, T.; Tsuji, Y.; Kubo, T. Use of purified beta-tricalcium phosphate for filling defects after curettage of benign bone tumours. Int. Orthop. 2006, 30, 510–513. [Google Scholar] [CrossRef]
- Chung, H.; Kim, S.; Chung, S.H. Clinical Outcome of Beta-Tricalcium Phosphate Use for Bone Defects after Operative Treatment of Benign Tumors. Clin. Orthop. Surg. 2019, 11, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, R.W.; Lange, T.A. Granular tricalcium phosphate grafting of cavitary lesions in human bone. Clin. Orthop. Relat. Res. 1994, 306, 197–203. [Google Scholar]
- Kim, J.H.; Oh, J.H.; Han, I.; Kim, H.S.; Chung, S.W. Grafting using injectable calcium sulfate in bone tumor surgery: Comparison with demineralized bone matrix-based grafting. Clin. Orthop. Surg. 2011, 3, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.J.; Clayer, M. Aqueous calcium sulphate as bone graft for voids following open curettage of bone tumours. ANZ J. Surg. 2013, 83, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Xiao, L.; Fu, H.; Bi, D.; Ma, H.; Tong, P. Study on injectable and degradable cement of calcium sulphate and calcium phosphate for bone repair. J. Mater. Sci. Mater. Med. 2010, 21, 627–634. [Google Scholar] [CrossRef]
- Larsson, S.; Hannink, G. Injectable bone-graft substitutes: Current products, their characteristics and indications, and new developments. Injury 2011, 42 (Suppl. S2), S30–S34. [Google Scholar] [CrossRef]
- Fillingham, Y.A.; Lenart, B.A.; Gitelis, S. Function after injection of benign bone lesions with a bioceramic. Clin. Orthop. Relat. Res. 2012, 470, 2014–2020. [Google Scholar] [CrossRef]
- Tan, V.; Evaniew, N.; Finlay, K.; Jurriaans, E.; Ghert, M.; Deheshi, B.; Parasu, N. Chronology of the Radiographic Appearances of the Calcium Sulfate-Calcium Phosphate Synthetic Bone Graft Composite Following Resection of Bone Tumors: A Follow-up Study of Postoperative Appearances. Can. Assoc. Radiol. J. 2016, 67, 21–27. [Google Scholar] [CrossRef]
- Friesenbichler, J.; Maurer-Ertl, W.; Sadoghi, P.; Pirker-Fruehauf, U.; Bodo, K.; Leithner, A. Adverse reactions of artificial bone graft substitutes: Lessons learned from using tricalcium phosphate geneX®. Clin. Orthop. Relat. Res. 2014, 472, 976–982. [Google Scholar] [CrossRef]
- Friesenbichler, J.; Maurer-Ertl, W.; Sadoghi, P.; Pirker-Fruehauf, U.; Bodo, K.; Leithner, A. Reply to the Letter to the Editor: Adverse reactions of artificial bone graft substitutes: Lessons learned from using tricalcium phosphate geneX®. Clin. Orthop. Relat. Res. 2014, 472, 767–768. [Google Scholar] [CrossRef]
- Lowery, K.; Chaturvedi, A.; Blomfield, M.; Sharma, H. Effectiveness of the management of bony articular collapse with bony defects in tibial plateau fractures with the use of GeneX: An absorbable calcium composite synthetic bone graft. J. Limb Lengthen. Recon. 2018, 4, 20–25. [Google Scholar] [CrossRef]
- Shin, W.C.; Jang, J.H.; Jeong, J.Y.; Suh, K.T.; Moon, N.H. Effect of a synthetic osteoconductive bone graft substitute with zeta potential control (geneX®ds) in the treatment of intertrochanteric fracture: A single center experience of 115 consecutive proximal femoral nail antirotations. J. Orthop. Sci. 2019, 24, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Sung, T.W.; Lee, E.S.; Kim, O.G.; Heo, K.S.; Shon, W.Y. Usefulness of Synthetic Osteoconductive Bone Graft Substitute with Zeta Potential Control for Intramedullary Fixation with Proximal Femur Nail Antirotation in Osteoporotic Unstable Femoral Intertrochanteric Fracture. Hip Pelvis 2021, 33, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Zhu, X.S.; Chen, L.; Chen, C.M.; Mangham, D.C.; Coulton, L.A.; Aiken, S.S. Bone healing response to a synthetic calcium sulfate/β-tricalcium phosphate graft material in a sheep vertebral body defect model. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1911–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhan, B.L.; Ye, Z. Significance of percutaneous vertebroplasty with Genex in the treatment of thoracolumbar burst fractures. Zhongguo Gu Shang 2011, 24, 223–226. (In Chinese) [Google Scholar]
- Saadoun, S.; Macdonald, C.; Bell, B.A.; Papadopoulos, M.C. Dangers of bone graft substitutes: Lessons from using GeneX. J. Neurol. Neurosurg. Psychiatry 2011, 82, e3. [Google Scholar] [CrossRef] [PubMed]
- Welkerling, H.; Raith, J.; Kastner, N.; Marschall, C.; Windhager, R. Painful soft-tissue reaction to injectable Norian SRS calcium phosphate cement after curettage of enchondromas. J. Bone Jt. Surg. Br. 2003, 85, 238–239. [Google Scholar] [CrossRef] [PubMed]
- Cottalorda, J.; Bourelle, S. Current treatments of primary aneurysmal bone cysts. J. Pediatr. Orthop. B 2006, 15, 155–167. [Google Scholar] [CrossRef]
- Park, H.Y.; Yang, S.K.; Sheppard, W.L.; Hegde, V.; Zoller, S.D.; Nelson, S.D.; Federman, N.; Bernthal, N.M. Current management of aneurysmal bone cysts. Curr. Rev. Musculoskelet. Med. 2016, 9, 435–444. [Google Scholar] [CrossRef]
- Gibbs, C.P., Jr.; Hefele, M.C.; Peabody, T.D.; Montag, A.G.; Aithal, V.; Simon, M.A. Aneurysmal bone cyst of the extremities. Factors related to local recurrence after curettage with a high-speed burr. J. Bone Jt. Surg. Am. 1999, 81, 1671–1678. [Google Scholar] [CrossRef]
- Walden, M.J.; Murphey, M.D.; Vidal, J.A. Incidental enchondromas of the knee. AJR Am. J. Roentgenol. 2008, 190, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Lubahn, J.D.; Bachoura, A. Enchondroma of the Hand: Evaluation and Management. J. Am. Acad. Orthop. Surg. 2016, 24, 625–633. [Google Scholar] [CrossRef]
- Altay, M.; Bayrakci, K.; Yildiz, Y.; Erekul, S.; Saglik, Y. Secondary chondrosarcoma in cartilage bone tumors: Report of 32 patients. J. Orthop. Sci. 2007, 12, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Park, H.Y.; Joo, M.W.; Choi, Y.H.; Chung, Y.G.; Park, C.J. Simple curettage and allogeneic cancellous bone chip impaction grafting in solitary enchondroma of the short tubular bones of the hand. Sci. Rep. 2023, 13, 2081. [Google Scholar] [CrossRef]
- Horvai, A.; Unni, K.K. Premalignant conditions of bone. J. Orthop. Sci. 2006, 11, 412–423. [Google Scholar] [CrossRef]
- Kushchayeva, Y.S.; Kushchayev, S.V.; Glushko, T.Y.; Tella, S.H.; Teytelboym, O.M.; Collins, M.T.; Boyce, A.M. Fibrous dysplasia for radiologists: Beyond ground glass bone matrix. Insights Imaging 2018, 9, 1035–1056. [Google Scholar] [CrossRef]
- Feller, L.; Wood, N.H.; Khammissa, R.A.; Lemmer, J.; Raubenheimer, E.J. The nature of fibrous dysplasia. Head Face Med. 2009, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Riddle, N.D.; Bui, M.M. Fibrous dysplasia. Arch. Pathol. Lab. Med. 2013, 137, 134–138. [Google Scholar] [CrossRef]
- Diah, E.; Morris, D.E.; Lo, L.J.; Chen, Y.R. Cyst degeneration in craniofacial fibrous dysplasia: Clinical presentation and management. J. Neurosurg. 2007, 107, 504–508. [Google Scholar] [CrossRef]
- Stanton, R.P. Surgery for fibrous dysplasia. J. Bone Miner. Res. 2006, 21 (Suppl. S2), P105–P109. [Google Scholar] [CrossRef]
- Keijser, L.C.; Van Tienen, T.G.; Schreuder, H.W.; Lemmens, J.A.; Pruszczynski, M.; Veth, R.P. Fibrous dysplasia of bone: Management and outcome of 20 cases. J. Surg. Oncol. 2001, 76, 157–166. [Google Scholar] [CrossRef] [PubMed]
- DiCaprio, M.R.; Enneking, W.F. Fibrous dysplasia. Pathophysiology, evaluation, and treatment. J. Bone Jt. Surg. Am. 2005, 87, 1848–1864. [Google Scholar] [CrossRef] [PubMed]
- Hamad, A.T.; Kumar, A.; Anand Kumar, C. Intraosseous epidermoid cyst of the finger phalanx: A case report. J. Orthop. Surg. 2006, 14, 340–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.Y.; Eisler, J.; Springfield, D.; Klein, M.J. Intraosseous epidermoid inclusion cyst in a great toe. A case report and review of the literature. Arch. Pathol. Lab. Med. 2003, 127, e298–e300. [Google Scholar] [CrossRef]
- Patel, K.; Bhuiya, T.; Chen, S.; Kenan, S.; Kahn, L. Epidermal inclusion cyst of phalanx: A case report and review of the literature. Skeletal. Radiol. 2006, 35, 861–863. [Google Scholar] [CrossRef]
- Memon, F.; Panjwani, T.R.; Patankar, H. Intraosseous Epidermoid Inclusion Cyst of Distal Phalanx: A Rare Entity. J. Clin. Diagn. Res. 2016, 10, RJ01–RJ02. [Google Scholar] [CrossRef]


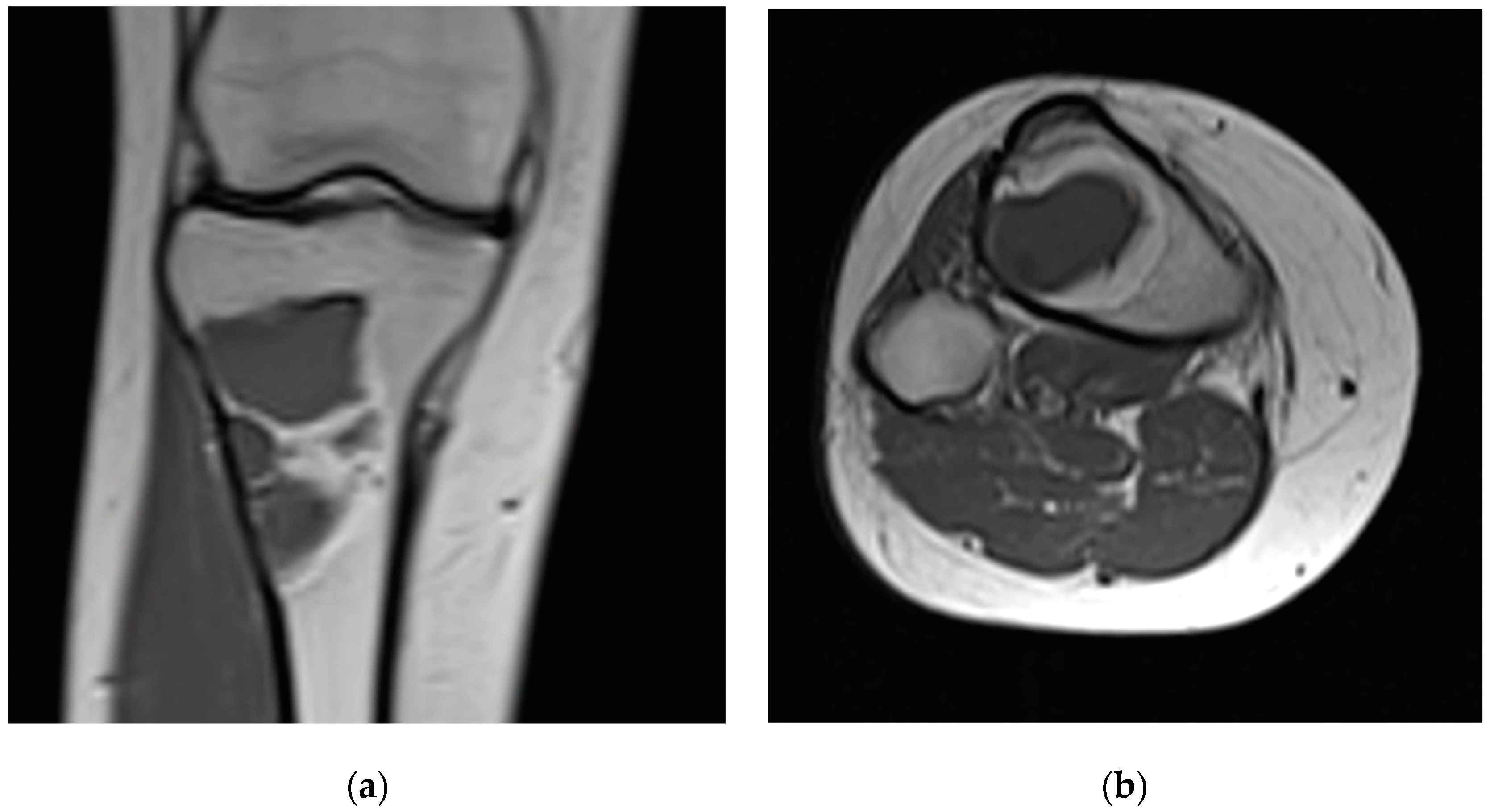

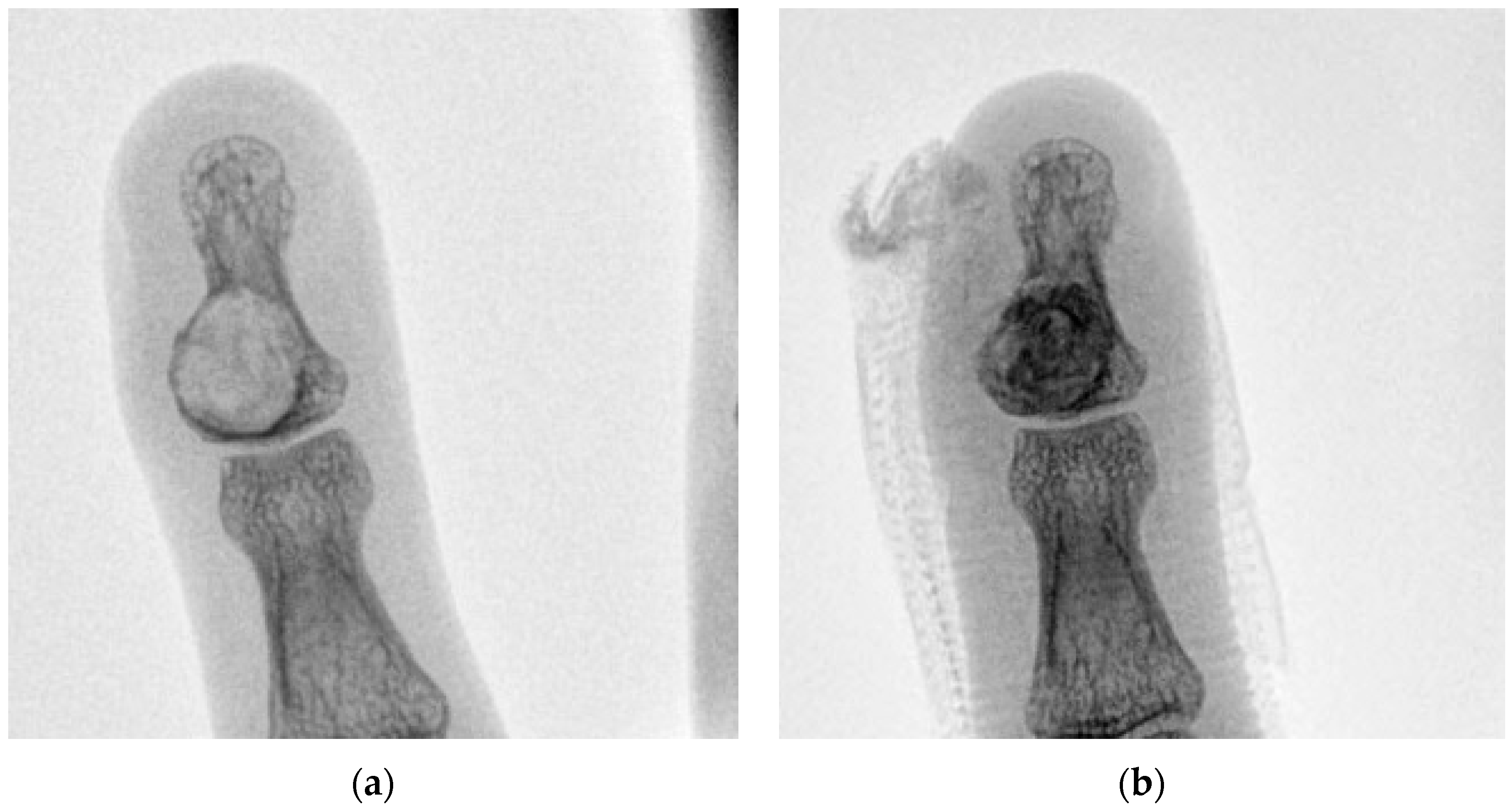
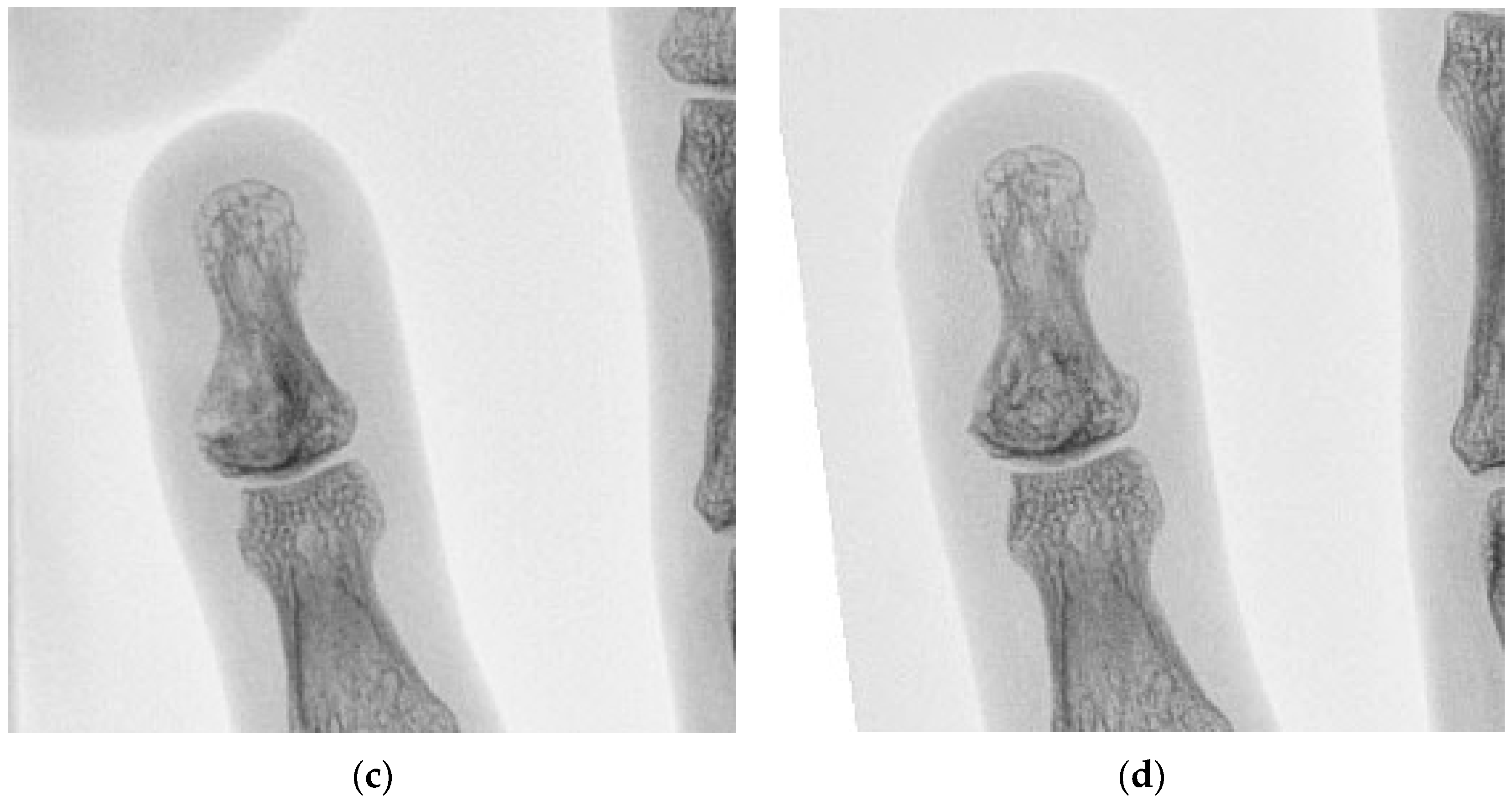
| Case | Age/Sex | ASA | Lesion Site | Diameter (mm) | Tissue Diagnosis | Follow-Up (Months) |
|---|---|---|---|---|---|---|
| 1 | 23 M | 2 | Right foot 4th metatarsal | 20 | Aneurysmal bone cyst | 30 |
| 2 | 34 M | 1 | Left middle finger P1 | 32 | Enchondroma | 24 |
| 3 | 17 F | 1 | Right proximal tibia | 58 | Fibrous dysplasia | 22 |
| 4 | 27 F | 2 | Left foot 2nd metatarsal | 24 | Enchondroma | 23 |
| 5 | 36 M | 1 | Left little finger P3 | 10 | Enchondroma | 25 |
| 6 | 25 F | 2 | Right os calcis | 33 | Aneurysmal bone cyst | 28 |
| 7 | 71 M | 2 | Right middle finger P3 | 20 | Epidermoid cyst | 27 |
| 8 | 23 F | 1 | Left hand 2nd metacarpal | 27 | Aneurysmal bone cyst | 23 |
| 9 | 27 F | 1 | Left tibial diaphysis | 41 | Fibrous dysplasia | 23 |
| 10 | 75 F | 2 | Right medial femoral condyle | 20 | Enchondroma | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razii, N.; Docherty, L.M.; Halai, M.; Mahendra, A.; Gupta, S. Injectable Synthetic Beta-Tricalcium Phosphate/Calcium Sulfate (GeneX) for the Management of Contained Defects Following Curettage of Benign Bone Tumours. Curr. Oncol. 2023, 30, 3697-3707. https://doi.org/10.3390/curroncol30040281
Razii N, Docherty LM, Halai M, Mahendra A, Gupta S. Injectable Synthetic Beta-Tricalcium Phosphate/Calcium Sulfate (GeneX) for the Management of Contained Defects Following Curettage of Benign Bone Tumours. Current Oncology. 2023; 30(4):3697-3707. https://doi.org/10.3390/curroncol30040281
Chicago/Turabian StyleRazii, Nima, Laura M. Docherty, Mansur Halai, Ashish Mahendra, and Sanjay Gupta. 2023. "Injectable Synthetic Beta-Tricalcium Phosphate/Calcium Sulfate (GeneX) for the Management of Contained Defects Following Curettage of Benign Bone Tumours" Current Oncology 30, no. 4: 3697-3707. https://doi.org/10.3390/curroncol30040281
APA StyleRazii, N., Docherty, L. M., Halai, M., Mahendra, A., & Gupta, S. (2023). Injectable Synthetic Beta-Tricalcium Phosphate/Calcium Sulfate (GeneX) for the Management of Contained Defects Following Curettage of Benign Bone Tumours. Current Oncology, 30(4), 3697-3707. https://doi.org/10.3390/curroncol30040281





