Assessing Psychological Morbidity in Cancer-Unaffected BRCA1/2 Pathogenic Variant Carriers: A Systematic Review
Abstract
1. Introduction
- How is the psychological morbidity in cancer-unaffected BRCA1/2 pathogenic variant carriers, both immediately after genetic test result disclosure and long-term?
- Which instruments are frequently employed to assess these psychological morbidities?
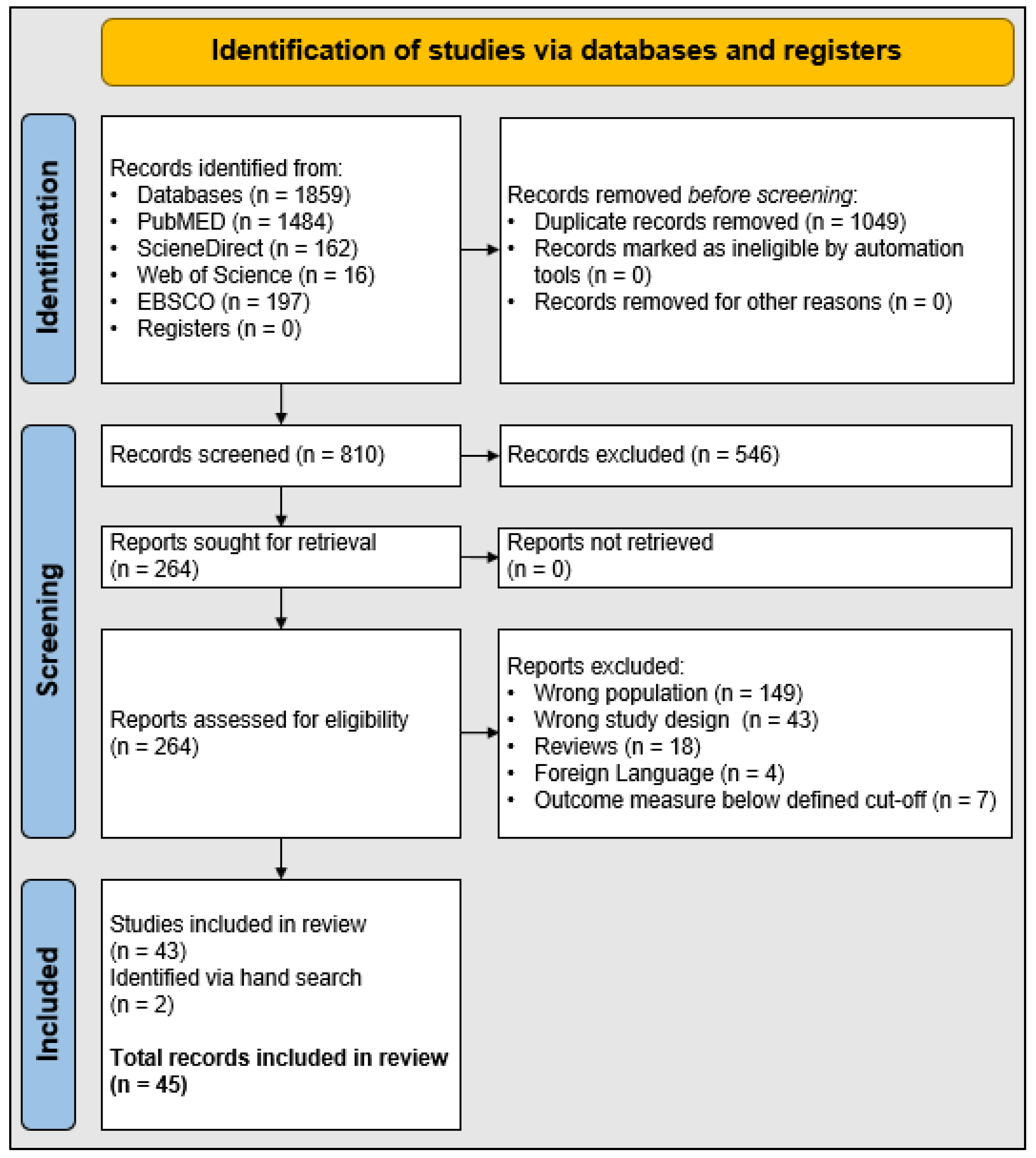
2. Materials and Methods
2.1. Eligibility Criteria
- Participants: the review focused on cancer-unaffected female adults (age ≥ 18 years) with a confirmed pathogenic variant in either BRCA1 or BRCA2.
- Intervention: no special intervention was specified.
- Comparison: studies that compared BRCA pathogenic variant carriers with women who received negative or inconclusive BRCA genetic test results, as well as studies that compared cancer-affected vs. cancer-unaffected pathogenic variant carriers were also included.
- Outcomes: the review included short-term and long-term psychological consequences that were measured with validated instruments.
- Study design: only quantitative studies, irrespective of study design (randomized or non-randomized trials, longitudinal cohort, cross-sectional, or case control), were included; qualitative studies were excluded from the present review.
2.2. Exclusion Criteria
2.3. Data Extraction, Data Synthesis, and Quality Assessment
3. Results
3.1. Study Characteristics
3.2. Distress Measures
3.2.1. Impact of Event Scale
3.2.2. Hospital Anxiety and Depression Scale—Anxiety Subscale
3.2.3. Cancer Worry Scale
3.2.4. Spielberger State-Trait Anxiety Inventory
3.2.5. Brief Symptom Inventory
3.2.6. General Health Questionnaire
3.2.7. Summary Distress Outcomes
3.3. Depression
3.3.1. Hospital Anxiety and Depression Scale—Depression Subscale
3.3.2. Center for Epidemiologic Studies Depression Scale
3.3.3. Beck Hopelessness Scale
3.3.4. Summary Depression Outcomes
3.4. Other Psychological Outcomes
3.4.1. Short Form Health Survey
3.4.2. Body Image Questionnaire
3.4.3. Summary Other Outcomes
3.5. Quality Assessment
4. Discussion
Limitations and Recommendations for Future Research
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Friedenson, B. The BRCA1/2 pathway prevents hematologic cancers in addition to breast and ovarian cancers. BMC Cancer 2007, 7, 152. [Google Scholar] [CrossRef]
- Tutt, A.; Ashworth, A. The relationship between the roles of BRCA genes in DNA repair and cancer predisposition. Trends Mol. Med. 2002, 8, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.-A.; Mooij, T.M.; Roos-Blom, M.-J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Evans, G.; Binchy, A.; Shenton, A.; Hopwood, P.; Craufurd, D. Comparison of proactive and usual approaches to offering predictive testing for BRCA1/2 mutations in unaffected relatives. Clin. Genet. 2009, 75, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Lerman, C.; Shields, A.E. Genetic testing for cancer susceptibility: The promise and the pitfalls. Nat. Rev. Cancer 2004, 4, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Bick, U.; Engel, C.; Krug, B.; Heindel, W.; Fallenberg, E.M.; Rhiem, K.; Maintz, D.; Golatta, M.; Speiser, D.; Rjosk-Dendorfer, D. High-risk breast cancer surveillance with MRI: 10-year experience from the German consortium for hereditary breast and ovarian cancer. Breast Cancer Res. Treat. 2019, 175, 217–228. [Google Scholar] [CrossRef]
- Hartmann, L.C.; Sellers, T.A.; Schaid, D.J.; Frank, T.S.; Soderberg, C.L.; Sitta, D.L.; Frost, M.H.; Grant, C.S.; Donohue, J.H.; Woods, J.E.; et al. Efficacy of Bilateral Prophylactic Mastectomy in BRCA1 and BRCA2 Gene Mutation Carriers. J. Natl. Cancer Inst. 2001, 93, 1633–1637. [Google Scholar] [CrossRef]
- Heemskerk-Gerritsen, B.A.; Menke-Pluymers, M.B.E.; Jager, A.; Tilanus-Linthorst, M.M.; Koppert, L.B.; Obdeijn, I.M.; van Deurzen, C.H.; Collee, J.M.; Seynaeve, C.; Hooning, M.J. Substantial breast cancer risk reduction and potential survival benefit after bilateral mastectomy when compared with surveillance in healthy BRCA1 and BRCA2 mutation carriers: A prospective analysis. Ann. Oncol. 2013, 24, 2029–2035. [Google Scholar] [CrossRef] [PubMed]
- Heemskerk-Gerritsen, B.A.M.; Jager, A.; Koppert, L.B.; Obdeijn, A.I.-M.; Collée, M.; Meijers-Heijboer, H.E.J.; Jenner, D.J.; Oldenburg, H.S.A.; van Engelen, K.; de Vries, J.; et al. Survival after bilateral risk-reducing mastectomy in healthy BRCA1 and BRCA2 mutation carriers. Breast Cancer Res. Treat. 2019, 177, 723–733. [Google Scholar] [CrossRef]
- den Heijer, M.; Seynaeve, C.; Timman, R.; Duivenvoorden, H.J.; Vanheusden, K.; Tilanus-Linthorst, M.; Menke-Pluijmers, M.B.E.; Tibben, A. Body image and psychological distress after prophylactic mastectomy and breast reconstruction in genetically predisposed women: A prospective long-term follow-up study. Eur. J. Cancer 2012, 48, 1263–1268. [Google Scholar] [CrossRef]
- Domchek, S.M. Risk-reducing mastectomy in BRCA1 and BRCA2 mutation carriers: A complex discussion. JAMA 2019, 321, 27. [Google Scholar] [CrossRef]
- Glassey, R.; Ives, A.; Saunders, C.; Musiello, T. Decision making, psychological wellbeing and psychosocial outcomes for high risk women who choose to undergo bilateral prophylactic mastectomy—A review of the literature. Breast 2016, 28, 130–135. [Google Scholar] [CrossRef]
- Altschuler, A.; Nekhlyudov, L.; Rolnick, S.J.; Greene, S.M.; Elmore, J.G.; West, C.N.; Herrinton, L.J.; Harris, E.L.; Fletcher, S.W.; Emmons, K.M.; et al. Positive, negative, and disparate—Women’s differing long-term psychosocial experiences of bilateral or contralateral prophylactic mastectomy. Breast J. 2008, 14, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.G.; Kesavan, N.; Lim, Y.; Gadde, S.; Hurley, E.; Massat, N.J.; Maxwell, A.J.; Ingham, S.; Eeles, R.; Leach, M.O.; et al. MRI breast screening in high-risk women: Cancer detection and survival analysis. Breast Cancer Res. Treat. 2014, 145, 663–672. [Google Scholar] [CrossRef] [PubMed]
- Hesse-Biber, S. The Genetic Testing Experience of BRCA-Positive Women: Deciding Between Surveillance and Surgery. Qual. Health Res. 2014, 24, 773–789. [Google Scholar] [CrossRef]
- Finch, A.; Evans, G.; Narod, S.A. BRCA carriers, prophylactic salpingo-oophorectomy and menopause: Clinical management considerations and recommendations. Women’s Health 2012, 8, 543–555. [Google Scholar] [PubMed]
- Marchetti, C.; de Felice, F.; Palaia, I.; Perniola, G.; Musella, A.; Musio, D.; Muzii, L.; Tombolini, V.; Panici, P.B. Risk-reducing salpingo-oophorectomy: A meta-analysis on impact on ovarian cancer risk and all cause mortality in BRCA 1 and BRCA 2 mutation carriers. BMC Women’s Health 2014, 14, 150. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.-L.; Wang, K.; Liu, Q.; Li, J.; Zhang, X.; Li, H.-Y. Risk Reduction and Survival Benefit of Risk-Reducing Salpingo-oophorectomy in Hereditary Breast Cancer: Meta-analysis and Systematic Review. Clin. Breast Cancer 2019, 19, e48–e65. [Google Scholar] [CrossRef]
- MacDonald, D.J.; Sarna, L.; Weitzel, J.N.; Ferrell, B. Women’s perceptions of the personal and family impact of genetic cancer risk assessment: Focus group findings. J. Genet. Counsel. 2010, 19, 148–160. [Google Scholar] [CrossRef]
- Lombardi, L.; Bramanti, S.M.; Babore, A.; Stuppia, L.; Trumello, C.; Antonucci, I.; Cavallo, A. Psychological aspects, risk and protective factors related to BRCA genetic testing: A review of the literature. Support Care Cancer 2019, 27, 3647–3656. [Google Scholar] [CrossRef]
- Dean, M. “It’s not if I get cancer, it’s when I get cancer”: BRCA-positive patients’(un) certain health experiences regarding hereditary breast and ovarian cancer risk. Soc. Sci. Med. 2016, 163, 21–27. [Google Scholar] [CrossRef]
- Possick, C.; Kestler-Peleg, M. BRCA and Motherhood: A Matter of Time and Timing. Qual. Health Res. 2019, 30, 825–835. [Google Scholar] [CrossRef]
- Glassey, R.; Hardcastle, S.J.; O’Connor, M.; Ives, A.; kConFab Investigators; Saunders, C. Perceived influence of psychological consultation on psychological well-being, body image, and intimacy following bilateral prophylactic mastectomy: A qualitative analysis. Psycho.-Oncol. 2018, 27, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Douglas, H.A.; Hamilton, R.J.; Grubs, R.E. The Effect of BRCA Gene Testing on Family Relationships: A Thematic Analysis of Qualitative Interviews. J. Genet. Counsel. 2009, 18, 418–435. [Google Scholar] [CrossRef] [PubMed]
- Meiser, B. Psychological impact of genetic testing for cancer susceptibility: An update of the literature. Psycho.-Oncol. 2005, 14, 1060–1074. [Google Scholar] [CrossRef]
- Hamilton, J.G.; Lobel, M.; Moyer, A. Emotional distress following genetic testing for hereditary breast and ovarian cancer: A meta-analytic review. Health Psychol. 2009, 28, 510–518. [Google Scholar] [CrossRef]
- Graves, K.D.; Wenzel, L.; Schwartz, M.D.; Luta, G.; Wileyto, P.; Narod, S.A.; Peshkin, B.N.; Marcus, A.; Cella, D.; Emsbo, S.P.; et al. Randomized Controlled Trial of a Psychosocial Telephone Counseling Intervention in BRCA1 and BRCA2 Mutation Carriers. Cancer Epidemiol. Biomark. Prev. 2010, 19, 648–654. [Google Scholar] [CrossRef]
- Boghosian, T.; McCuaig, J.M.; Carlsson, L.; Metcalfe, K.A. Psychosocial Interventions for Women with a BRCA1 or BRCA2 Mutation: A Scoping Review. Cancers 2021, 13, 1486. [Google Scholar] [CrossRef]
- Jeffers, L.; Reid, J.; Fitzsimons, D.; Morrison, P.J.; Dempster, M. Interventions to improve psychosocial well-being in female BRCA-mutation carriers following risk-reducing surgery. Cochrane Database Syst. Rev. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Ringwald, J.; Wochnowski, C.; Bosse, K.; Giel, K.E.; Schäffeler, N.; Zipfel, S.; Teufel, M. Psychological Distress, Anxiety, and Depression of Cancer-Affected BRCA1/2 Mutation Carriers: A Systematic Review. J. Genet. Counsel. 2016, 25, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Narod, S.A. Quality of life and health status after prophylactic salpingo-oophorectomy in women who carry a BRCA mutation: A review. Maturitas 2011, 70, 261–265. [Google Scholar] [CrossRef]
- Kautz-Freimuth, S.; Redaèlli, M.; Rhiem, K.; Vodermaier, A.; Krassuski, L.; Nicolai, K.; Schnepper, M.; Kuboth, V.; Dick, J.; Vennedey, V.; et al. Development of decision aids for female BRCA1 and BRCA2 mutation carriers in Germany to support preference-sensitive decision-making. BMC Med. Inform. Decis. Mak. 2021, 21, 180. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- da Costa Santos, C.M.; de Mattos Pimenta, C.A.; Nobre, M.R.C. The PICO strategy for the research question construction and evidence search. Rev. Lat. Am. Enferm. 2007, 15, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Amir-Behghadami, M.; Janati, A. Population, Intervention, Comparison, Outcomes and Study (PICOS) design as a framework to formulate eligibility criteria in systematic reviews. Emerg. Med. J. 2020, 37, 387. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef]
- Borreani, C.; Manoukian, S.; Bianchi, E.; Brunelli, C.; Peissel, B.; Caruso, A.; Morasso, G.; Pierotti, M.A. The psychological impact of breast and ovarian cancer preventive options in BRCA1 and BRCA2 mutation carriers. Clin. Genet. 2014, 85, 7–15. [Google Scholar] [CrossRef]
- Brand, H.; Speiser, D.; Besch, L.; Roseman, J.; Kendel, F. Making Sense of a Health Threat: Illness Representations, Coping, and Psychological Distress among BRCA1/2 Mutation Carriers. Genes 2021, 12, 741. [Google Scholar] [CrossRef]
- Buchanan, A.H.; Voils, C.I.; Schildkraut, J.M.; Fine, C.; Horick, N.K.; Marcom, P.K.; Wiggins, K.; Skinner, C.S. Adherence to Recommended Risk Management among Unaffected Women with a BRCA Mutation. J. Genet. Counsel. 2017, 26, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, K.; Eisenberg, S.; Weltfreid, S.; Low, C.A.; Beran, T.M.; Stanton, A.L. Characterizing biased cancer-related cognitive processing: Relationships with BRCA1/2 genetic mutation status, personal cancer history, age, and prophylactic surgery. Health Psychol. 2014, 33, 1003–1011. [Google Scholar] [CrossRef]
- Claes, E.; Evers-Kiebooms, G.; Denayer, L.; Decruyenaere, M.; Boogaerts, A.; Philippe, K.; Legius, E. Predictive Genetic Testing for Hereditary Breast and Ovarian Cancer: Psychological Distress and Illness Representations 1 Year Following Disclosure. J. Genet. Counsel. 2005, 14, 349–363. [Google Scholar] [CrossRef]
- Croyle, R.; Smith, K.; Botkin, J.; Baty, B.J.; Nash, J. Psychological Responses to BRCA1 Mutation Testing: Preliminary Findings. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 1997, 16, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Dagan, E.; Gil, S. BRCA1/2 Mutation Carriers. J. Psychosoc. Oncol. 2004, 22, 93–106. [Google Scholar] [CrossRef]
- Dagan, E.; Shochat, T. Quality of life in asymptomatic BRCA1/2 mutation carriers. Prev. Med. 2009, 48, 193–196. [Google Scholar] [CrossRef]
- Dorval, M.; Drolet, M.; LeBlanc, M.; Maunsell, E.; Dugas, M.J.; Simard, J. Using the Impact of Event Scale to Evaluate Distress in the Context of Genetic Testing for Breast Cancer Susceptibility. Psychol. Rep. 2006, 98, 873–881. [Google Scholar] [CrossRef]
- Ertmański, S.; Metcalfe, K.A.; TrempaŁa, J.; GŁowacka, M.D.; Lubiński, J.; Narod, S.A.; Gronwald, J. Identification of Patients at High Risk of Psychological Distress After BRCA1 Genetic Testing. Genet. Test. Mol. Biomark. 2009, 13, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Metcalfe, K.A.; Chiang, J.; Elit, L.; McLaughlin, J.; Springate, C.; Esplen, M.J.; Demsky, R.; Murphy, J.; Rosen, B.; et al. The impact of prophylactic salpingo-oophorectomy on quality of life and psychological distress in women with a BRCA mutation. Psycho-Oncol. 2013, 22, 212–219. [Google Scholar] [CrossRef] [PubMed]
- Foster, C.; Watson, M.; Eeles, R.; Eccles, D.; Ashley, S.; Davidson, R.; Mackay, J.; Morrison, P.J.; Hopwood, P.; Evans, G.; et al. Predictive genetic testing for BRCA1/2 in a UK clinical cohort: Three-year follow-up. Br. J. Cancer 2007, 96, 718–724. [Google Scholar] [CrossRef] [PubMed]
- Geirdal, A.Ø.; Dahl, A.A. The relationship between coping strategies and anxiety in women from families with familial breast–ovarian cancer in the absence of demonstrated mutations. Psycho-Oncol. 2008, 17, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Geirdal, A.Ø.; Reichelt, J.G.; Dahl, A.A.; Heimdal, K.; Mæhle, L.; Stormorken, A.; Møller, P. Psychological distress in women at risk of hereditary breast/ovarian or HNPCC cancers in the absence of demonstrated mutations. Fam. Cancer 2005, 4, 121–126. [Google Scholar] [CrossRef]
- Gopie, J.P.; Mureau, M.A.M.; Seynaeve, C.; ter Kuile, M.M.; Menke-Pluymers, M.B.E.; Timman, R.; Tibben, A. Body image issues after bilateral prophylactic mastectomy with breast reconstruction in healthy women at risk for hereditary breast cancer. Fam. Cancer 2013, 12, 479–487. [Google Scholar] [CrossRef]
- Graves, K.D.; Vegella, P.; Poggi, E.A.; Peshkin, B.N.; Tong, A.; Isaacs, C.; Finch, C.; Kelly, S.; Taylor, K.L.; Luta, G.; et al. Long-Term Psychosocial Outcomes of BRCA1/BRCA2 Testing: Differences across Affected Status and Risk-Reducing Surgery Choice. Cancer Epidemiol. Biomark. Prev. 2012, 21, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Isern, A.E.; Tengrup, I.; Loman, N.; Olsson, H.; Ringberg, A. Aesthetic outcome, patient satisfaction, and health-related quality of life in women at high risk undergoing prophylactic mastectomy and immediate breast reconstruction. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 1177–1187. [Google Scholar] [CrossRef]
- Isselhard, A.; Lautz, Z.; Töpper, M.; Rhiem, K.; Schmutzler, R.; Vitinius, F.; Fischer, H.; Berger-Höger, B.; Steckelberg, A.; Beifus, K.; et al. Coping Self-Efficacy and Its Relationship with Psychological Morbidity after Genetic Test Result Disclosure: Results from Cancer-Unaffected BRCA1/2 Mutation Carriers. Int. J. Environ. Res. Public Health 2023, 20, 1684. [Google Scholar] [CrossRef] [PubMed]
- Julian-Reynier, C.; Bouhnik, A.-D.; Mouret-Fourme, E.; Gauthier-Villars, M.; Berthet, P.; Lasset, C.; Fricker, J.-P.; Caron, O.; Gesta, P.; Luporsi, E.; et al. Time to prophylactic surgery in BRCA1/2 carriers depends on psychological and other characteristics. Genet. Med. 2010, 12, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Kinney, A.Y.; Bloor, L.E.; Mandal, D.; Simonsen, S.E.; Baty, B.J.; Holubkov, R.; Seggar, K.; Neuhausen, S.L.; Smith, K. The impact of receiving genetic test results on general and cancer-specific psychologic distress among members of an African-American kindred with a BRCA1 mutation. Cancer 2005, 104, 2508–2516. [Google Scholar] [CrossRef]
- Landau, C.; Lev-Ari, S.; Cohen-Mansfield, J.; Tillinger, E.; Geva, R.; Tarrasch, R.; Mitnik, I.; Friedman, E. Randomized controlled trial of Inquiry-Based Stress Reduction (IBSR) technique for BRCA1/2 mutation carriers. Psycho-Oncol. 2015, 24, 726–731. [Google Scholar] [CrossRef]
- Lapointe, J.; Dorval, M.; Nogués, C.; Fabre, R.; Julian-Reynier, C.; GENEPSO Cohort. Is the psychological impact of genetic testing moderated by support and sharing of test results to family and friends? Fam. Cancer 2013, 12, 601–610. [Google Scholar] [CrossRef]
- Lodder, L.; Frets, P.G.; Trijsburg, R.W.; Meijers-Heijboer, H.; Klijn, J.G.M.; Duivenvoorden, H.J.; Tibben, A.; Wagner, A.; van der Meer, C.A.; van den Ouweland, A.M.; et al. Psychological impact of receiving a BRCA1/BRCA2 test result. Am. J. Med. Genet. 2001, 98, 15–24. [Google Scholar] [CrossRef]
- Lodder, L.; Frets, P.G.; Trijsburg, R.W.; Meijers-Heijboer, H.; Klijn, J.G.M.; Seynaeve, C.; van Geel, A.N.; Tilanus, M.M.A.; Bartels, C.C.M.; Verhoog, L.C.; et al. One Year Follow-Up of Women Opting for Presymptomatic Testing for BRCA1 and BRCA2: Emotional Impact of the Test Outcome and Decisions on Risk Management (Surveillance or Prophylactic Surgery). Breast Cancer Res. Treat. 2002, 73, 97–112. [Google Scholar] [CrossRef]
- Low, C.A.; Bower, J.E.; Kwan, L.; Seldon, J. Benefit Finding in Response to BRCA1/2 Testing. Ann. Behav. Med. 2008, 35, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Madalinska, J.B.; van Beurden, M.; Bleiker, E.M.; Valdimarsdottir, H.; Lubsen-Brandsma, L.; Massuger, L.F.; Mourits, M.J.; Gaarenstroom, K.N.; van Dorst, E.B.; van der Putten, H.; et al. Predictors of Prophylactic Bilateral Salpingo-Oophorectomy Compared With Gynecologic Screening Use in BRCA1/2 Mutation Carriers. J. Clin. Oncol. 2007, 25, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Maheu, C.; Apostolidis, T.; Petri-Cal, A.; Mouret-Fourme, E.; Gauthier-Villars, M.; Lasset, C.; Berthet, P.; Fricker, J.-P.; Caron, O.; Luporsi, E.; et al. French women’s breast self-examination practices with time after undergoing BRCA1/2 genetic testing. Fam. Cancer 2012, 11, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Maheu, C.; Bouhnik, A.-D.; Nogués, C.; Mouret-Fourme, E.; Stoppa-Lyonnet, D.; Lasset, C.; Berthet, P.; Fricker, J.-P.; Caron, O.; Luporsi, E.; et al. Which factors predict proposal and uptake of psychological counselling after BRCA1/2 test result disclosure? Psycho-Oncol. 2014, 23, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Meiser, B.; Butow, P.N.; Friedlander, M.; Barratt, A.; Schnieden, V.; Watson, M.; Brown, J.; Tucker, K. Psychological impact of genetic testing in women from high-risk breast cancer families. Eur. J. Cancer 2002, 38, 2025–2031. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Mian, N.; Enmore, M.; Poll, A.; Llacuachaqui, M.; Nanda, S.; Sun, P.; Hughes, K.S.; Narod, S.A. Long-term follow-up of Jewish women with a BRCA1 and BRCA2 mutation who underwent population genetic screening. Breast Cancer Res. Treat. 2012, 133, 735–740. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Dennis, C.-L.; Poll, A.; Armel, S.; Demsky, R.; Carlsson, L.; Nanda, S.; Kiss, A.; Narod, S.A. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: A multisite, randomized, controlled trial. Genet. Med. 2017, 19, 330–336. [Google Scholar] [CrossRef]
- Metcalfe, K.A.; Price, M.A.; Mansfield, C.; Hallett, D.C.; Lindeman, G.J.; Fairchild, A.; Posner, J.; Friedman, S.; Snyder, C.; Lynch, H.T.; et al. Predictors of long-term cancer-related distress among female BRCA1 and BRCA2 mutation carriers without a cancer diagnosis: An international analysis. British J. Cancer 2020, 123, 268–274. [Google Scholar] [CrossRef]
- O’Neill, S.M.; Rubinstein, W.S.; Sener, S.F.; Weissman, S.M.; Newlin, A.C.; West, D.K.; Ecanow, D.B.; Rademaker, A.W.; Edelman, R.R. Psychological impact of recall in high-risk breast MRI screening. Breast Cancer Res. Treat. 2009, 115, 365–371. [Google Scholar] [CrossRef]
- Reichelt, J.G.; Heimdal, K.; Møller, P.; Dahl, A.A. BRCA1 testing with definitive results: A prospective study of psychological distress in a large clinic-based sample. Fam. Cancer 2004, 3, 21–28. [Google Scholar] [CrossRef]
- Reichelt, J.G.; Møller, P.; Heimdal, K.; Dahl, A.A. Psychological and cancer-specific distress at 18 months post-testing in women with demonstrated BRCA1 mutations for hereditary breast/ovarian cancer. Fam. Cancer 2008, 7, 245–254. [Google Scholar] [CrossRef]
- Schwartz, M.D.; Peshkin, B.N.; Hughes, C.; Main, D.; Isaacs, C.; Lerman, C. Impact of BRCA1/BRCA2 Mutation Testing on Psychologic Distress in a Clinic-Based Sample. J. Clin. Oncol. 2002, 20, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Shochat, T.; Dagan, E. Sleep disturbances in asymptomatic BRCA1/2 mutation carriers: Women at high risk for breast–ovarian cancer. J. Sleep Res. 2010, 19, 333–340. [Google Scholar] [CrossRef]
- Smith, A.W.; Dougall, A.L.; Posluszny, D.M.; Somers, T.J.; Rubinstein, W.S.; Baum, A. Psychological distress and quality of life associated with genetic testing for breast cancer risk. Psycho-Oncol. 2008, 17, 767–773. [Google Scholar] [CrossRef]
- Spiegel, T.N.; Esplen, M.J.; Hill, K.A.; Wong, J.; Causer, P.A.; Warner, E. Psychological impact of recall on women with BRCA mutations undergoing MRI surveillance. Breast 2011, 20, 424–430. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, S.; Timmermans, D.; Meijers-Heijboer, H.; Tibben, A.; van Asperen, C.J.; Otten, W. Clinical Characteristics Affect the Impact of an Uninformative DNA Test Result: The Course of Worry and Distress Experienced by Women Who Apply for Genetic Testing for Breast Cancer. J. Clin. Oncol. 2006, 24, 3672–3677. [Google Scholar] [CrossRef] [PubMed]
- van Egdom, L.S.E.; de Kock, M.A.; Apon, I.; am Mureau, M.; Verhoef, C.; Hazelzet, J.A.; Koppert, L.B. Patient-Reported Outcome Measures may optimize shared decision-making for cancer risk management in BRCA mutation carriers. Breast Cancer 2020, 27, 426–434. [Google Scholar] [CrossRef]
- van Oostrom, I.; Meijers-Heijboer, H.; Lodder, L.; Duivenvoorden, H.J.; van Gool, A.R.; Seynaeve, C.; van der Meer, C.A.; Klijn, J.G.M.; van Geel, B.N.; Burger, C.W.; et al. Long-Term Psychological Impact of Carrying a BRCA1/2 Mutation and Prophylactic Surgery: A 5-Year Follow-Up Study. J. Clin. Oncol. 2003, 21, 3867–3874. [Google Scholar] [CrossRef]
- van Oostrom, I.; Meijers-Heijboer, H.; Duivenvoorden, H.J.; Bröcker-Vriends, A.H.J.T.; van Asperen, C.J.; Sijmons, R.H.; Seynaeve, C.; van Gool, A.R.; Klijn, J.G.M.; Tibben, A. The common sense model of self-regulation and psychological adjustment to predictive genetic testing: A prospective study. Psycho-Oncol. 2007, 16, 1121–1129. [Google Scholar] [CrossRef]
- van Roosmalen, M.S.; Stalmeier, P.; Verhoef, L.; Hoekstra-Weebers, J.; Oosterwijk, J.C.; Hoogerbrugge, N.; Moog, U.; van Daal, W. Impact of BRCA1/2 testing and disclosure of a positive test result on women affected and unaffected with breast or ovarian cancer. Am. J. Med. Genet. 2004, 124, 346–355. [Google Scholar] [CrossRef]
- Watson, M.; Foster, C.; Eeles, R.; Eccles, D.; Ashley, S.; Davidson, R.; Mackay, J.; Morrison, P.J.; Hopwood, P.; Evans, G.; et al. Psychosocial impact of breast/ovarian (BRCA 1/2) cancer-predictive genetic testing in a UK multi-centre clinical cohort. Br. J. Cancer 2004, 91, 1787–1794. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, M.; Wilner, N.; Alvarez, W. Impact of Event Scale: A measure of subjective stress. Psychosom. Med. 1979, 41, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.S. The impact of event scale: Revised. In Cross-Cultural Assessment of Psychological Trauma and PTSD; Springer: Berlin/Heidelberg, Germany, 2007; pp. 219–238. [Google Scholar]
- Snaith, P.; Zigmond, A.S. Hospital anxiety and depression scale (HADS). In Handbook of Psychiatric Measures; American Psychiatric Association: Washington, DC, USA, 2000; pp. 547–548. [Google Scholar]
- Lerman, C.; Trock, B.; Rimer, B.K.; Jepson, C.; Brody, D.; Boyce, A. Psychological side effects of breast cancer screening. Health Psychol. Off. J. Div. Health Psychol. Am. Psychol. Assoc. 1991, 10, 259–267. [Google Scholar] [CrossRef]
- Lerman, C.; Daly, M.; Masny, A.; Balshem, A. Attitudes about genetic testing for breast-ovarian cancer susceptibility. J. Clin. Oncol. 1994, 12, 843–850. [Google Scholar] [CrossRef]
- Spielberger, C.D.; Gonzalez-Reigosa, F.; Martinez-Urrutia, A.; Natalicio, L.F.; Natalicio, D.S. The State-Trait Anxiety Inventory. Interam. J. Psychol. 1971, 5, 3–4. [Google Scholar]
- Derogatis, L.R.; Melisaratos, N. The Brief Symptom Inventory: An introductory report. Psychol. Med. 1983, 13, 595–605. [Google Scholar] [CrossRef]
- Goldberg, D.P.; Hillier, V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef]
- Beck, A.T.; Weissman, A.; Lester, D.; Trexler, L. The measurement of pessimism: The Hopelessness Scale. J. Consult. Clin. Psychol. 1974, 42, 861. [Google Scholar] [CrossRef]
- Sheehan, T.J.; Fifield, J.; Reisine, S.; Tennen, H. The Measurement Structure of the Center for Epidemiologic Studies Depression Scale. J. Personal. Assess. 1995, 64, 507–521. [Google Scholar] [CrossRef]
- Ware, J.E.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Cull, A.M. The assessment of sexual function in cancer patients. Eur. J. Cancer 1992, 28, 1680–1686. [Google Scholar] [CrossRef]
- Donnelly, L.S.; Watson, M.; Moynihan, C.; Bancroft, E.; Evans, D.; Eeles, R.; Lavery, S.; Ormondroyd, E. Reproductive decision-making in young female carriers of a BRCA mutation. Hum. Reprod. 2013, 28, 1006–1012. [Google Scholar] [CrossRef]
- McGaughey, A. Body Image After Bilateral Prophylactic Mastectomy: An Integrative Literature Review. J. Midwifery Women’s Health 2006, 51, e45–e49. [Google Scholar] [CrossRef]
- Torrisi, C. Body Image in BRCA-Positive Young Women Following Bilateral Risk-Reducing Mastectomy: A Review of the Literature. Front. Psychol. 2021, 12, 5592. [Google Scholar] [CrossRef] [PubMed]
- Yusufov, M.; Bober, S.L. Sexual Health in the Era of Cancer Genetic Testing: A Systematic Review. Sex. Med. Rev. 2020, 8, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Antoniou, A.C.; Casadei, S.; Heikkinen, T.; Barrowdale, D.; Pylkäs, K.; Roberts, J.; Lee, A.; Subramanian, D.; de Leeneer, K.; Fostira, F.; et al. Breast-Cancer Risk in Families with Mutations in PALB2. N. Engl. J. Med. 2014, 371, 497–506. [Google Scholar] [CrossRef] [PubMed]
| First Author, Year | Country | Participant Characteristics 1 | Study Design, Follow-up Length | BHS | BIQ | BSI | CES-D | CWS | HADS | IES | GHQ | SF-12/36 | STAI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Borreani et al., 2014 [37] | Italy | n = 27 Age range: 26–75 | Longitudinal, 15 months | √ | √ | √ | |||||||
| Brand et al., 2021 [38] | Germany | n = 48 Age mean: 40 | Cross-sectional | √ | |||||||||
| Buchanan et al., 2017 [39] | U.S. | n = 97 Age range: 25–40+ | Cross-sectional | √ | √ | √ | |||||||
| Carpenter et al., 2014 [40] | U.S. | n = 26 Age mean: 42.9 | Experimental | √ | |||||||||
| Claes et al., 2005 [41] | Belgium | n = 34 Age range: 19–61 | Longitudinal, 1 year | √ | √ | ||||||||
| Croyle et al., 1997 [42] | U.S. | n = 13 Age range: 19–83 | Longitudinal, 2 years | √ | √ | ||||||||
| Dagan and Gil, 2004 [43] | Israel | n = 36 Age mean: 54.1 | Retrospective | √ | |||||||||
| Dagan and Shochat, 2009 [44] | Israel | n = 17 Age mean: 51.4 | Case control | √ | √ | √ | |||||||
| Dorval et al., 2006 [45] | Canada | n = 19 Age mean: 48 | Longitudinal, 36 months | √ | |||||||||
| Ertmanski et al., 2009 [46] | Poland | n = 56 Age range: 18–56+ | Longitudinal, 1 year | √ | √ | ||||||||
| Finch et al., 2013 [47] | Canada | n = 59 Age range: 35–69 | Longitudinal, 1 year | √ | √ | √ | |||||||
| Foster et al., 2007 [48] | U.K. | n = 53 Age range: 23–72 | Longitudinal, 3 years | √ | √ | ||||||||
| Geirdal and Dahl, 2008 [49] | Norway | n = 68 Age mean: 42 | Cross-sectional | √ | |||||||||
| Geirdal et al., 2005 [50] | Norway | n = 68 Age mean: 42 | Cross-sectional | √ | √ | √ | √ | ||||||
| Gopie et al., 2013 [51] | Netherlands | n = 44 Age mean: 37.1 | Longitudinal, 21.7 months | √ | √ | √ | |||||||
| Graves et al., 2012 [52] | U.S. | n = 47 Age mean: 54.1 | Longitudinal, 5 years | √ | √ | √ | |||||||
| Isern et al., 2008 [53] | Sweden | n = 27 Age range: 25–51 | Longitudinal, 42 months | √ | √ | ||||||||
| Isselhard et al., 2023 [54] | Germany | n = 130 Age range: 24–60 | Cross-sectional | √ | √ | ||||||||
| Julian-Reynier et al., 2010 [55] | France | n = 244 Age range: <30–50+ | Longitudinal, 60 months | √ | √ | √ | |||||||
| Kinney et al., 2005 [56] | U.S. | n = 19 Age range: <40–50+ | Longitudinal, 1 year | √ | √ | √ | √ | ||||||
| Landau et al., 2015 [57] | Israel | n = 56 Age mean: 49.6 | Intervention, 12 weeks | √ | √ | ||||||||
| Lapointe et al., 2013 [58] | France | n = 221 Age range: 20–60 | Longitudinal, 2 years | √ | √ | ||||||||
| Lodder et al., 2001 [59] | Netherlands | n = 25 Age range: 19–68 | Longitudinal, 1–3 weeks | √ | √ | ||||||||
| Lodder et al., 2002 [60] | Netherlands | n = 26 Age mean: 38.8 | Longitudinal, 12 months | √ | √ | √ | |||||||
| Low et al., 2008 [61] | U.S. | n = 7 Age mean: 44.7 | Longitudinal, 6 months | √ | |||||||||
| Madalinska et al., 2007 [62] | Netherlands | n = 160 Age range: 35–50+ | Longitudinal, 12 months | √ | √ | √ | |||||||
| Maheu et al., 2012 [63] | France | n = 217 Age range: <35–50+ | Longitudinal, 2 years | √ | √ | ||||||||
| Maheu et al., 2014 [64] | France | n = 232 Age mean: 40.7 | Longitudinal, 12 months | √ | √ | ||||||||
| Meiser et al., 2002 [65] | Australia | n = 30 Age mean: 40 | Longitudinal, 12 months | √ | √ | ||||||||
| Metcalfe et al., 2012 [66] | Canada | n = 22 Age range: 25–70 | Longitudinal, 2 years | √ | |||||||||
| Metcalfe et al., 2017 [67] | Canada | n = 150 Age range: 25–60 | RCT, 12 months | √ | |||||||||
| Metcalfe et al., 2020 [68] | Canada | n = 576 Age range: 25–55 | Cross-sectional | √ | |||||||||
| O’Neill et al., 2009 [69] | U.S. | n = 14 Age range: 27–68 | Longitudinal, 1 year | √ | |||||||||
| Reichelt et al., 2004 [70] | Norway | n = 80 Age mean: 43.9 | Longitudinal, 6 weeks | √ | √ | √ | √ | ||||||
| Reichelt et al., 2008 [71] | Norway | n = 58 Age mean: 45.4 | Longitudinal, 18 months | √ | √ | √ | |||||||
| Schwartz et al., 2002 [72] | U.S. | n = 35 Age mean: 45 | Longitudinal | √ | |||||||||
| Shochat and Dagan, 2010 [73] | Israel | n = 17 Age mean: 51.4 | Cross-sectional | √ | √ | ||||||||
| Smith et al., 2008 [74] | U.S. | n = 20 Age range: 22–70 | Longitudinal, 6 months | √ | √ | √ | √ | ||||||
| Spiegel et al., 2011 [75] | U.S. | n = 51 Age range: 25–60 | Longitudinal, 6 months | √ | √ | ||||||||
| Van Dijk et al., 2006 [76] | Netherlands | n = 22 Age range: <30–50+ | Longitudinal, 6 months | √ | √ | ||||||||
| Van Egdom et al., 2020 [77] | Netherlands | n = 96 Age mean: 41.4 | Cross-sectional | √ | |||||||||
| Van Oostrom et al., 2003 [78] | Netherlands | n = 23 Age mean: 41.9 | Longitudinal, 4–6 years | √ | √ | √ | √ | ||||||
| Van Oostrom et al., 2007 [79] | Netherlands | n = 49 Age mean: 42.3 | Longitudinal, 12 months | √ | √ | ||||||||
| Van Roosmalen et al., 2004 [80] | Netherlands | n = 68 Age mean: 37.6 | Longitudinal, 2 weeks | √ | √ | √ | |||||||
| Watson et al., 2004 [81] | U.K. | n = 91 Age range: 23–72 | Longitudinal, 12 months | √ | √ | √ |
| General Outcome | Specific Measure |
|---|---|
| Distress | Impact of Event Scale (IES) [82,83] Hospital Anxiety and Depression Scale (HADS) [84] |
| Cancer Worry Scale (CWS) [85,86] | |
| Spielberger State-Trait Anxiety Inventory (STAI) [87] Brief Symptom Inventory (BSI) [88] | |
| General Health Questionnaire (GHQ) [89] | |
| Depression | Hospital Anxiety and Depression Scale (HADS) [84] |
| Beck’s Hopelessness Scale (BHS) [90] | |
| Center for Epidemiologic Studies Depression Scale (CES-D) [91] | |
| Other | Short Form Health Survey (SF-36/SF-12) [92,93] |
| Body Image Questionnaire (BIQ) [60] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isselhard, A.; Lautz, Z.; Rhiem, K.; Stock, S. Assessing Psychological Morbidity in Cancer-Unaffected BRCA1/2 Pathogenic Variant Carriers: A Systematic Review. Curr. Oncol. 2023, 30, 3590-3608. https://doi.org/10.3390/curroncol30040274
Isselhard A, Lautz Z, Rhiem K, Stock S. Assessing Psychological Morbidity in Cancer-Unaffected BRCA1/2 Pathogenic Variant Carriers: A Systematic Review. Current Oncology. 2023; 30(4):3590-3608. https://doi.org/10.3390/curroncol30040274
Chicago/Turabian StyleIsselhard, Anna, Zoë Lautz, Kerstin Rhiem, and Stephanie Stock. 2023. "Assessing Psychological Morbidity in Cancer-Unaffected BRCA1/2 Pathogenic Variant Carriers: A Systematic Review" Current Oncology 30, no. 4: 3590-3608. https://doi.org/10.3390/curroncol30040274
APA StyleIsselhard, A., Lautz, Z., Rhiem, K., & Stock, S. (2023). Assessing Psychological Morbidity in Cancer-Unaffected BRCA1/2 Pathogenic Variant Carriers: A Systematic Review. Current Oncology, 30(4), 3590-3608. https://doi.org/10.3390/curroncol30040274





