Pain and Interventions in Stage IV Non-Small Cell Lung Cancer: A Province-Wide Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Study Cohort
2.4. Baseline Characteristics
2.5. Variables and Outcomes
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. Interventions for Pain
3.3. Factors Associated with Interventions for Pain
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Canadian Cancer Statistics Advisory Committee. Canadian Cancer Statistics 2018; Canadian Cancer Statistics Advisory Committee: Toronto, ON, Canada, 2018. [Google Scholar]
- Mercadante, S.; Vitrano, V. Pain in Patients with Lung Cancer: Pathophysiology and Treatment. Lung Cancer 2010, 68, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Potter, J.; Higginson, I.J. Pain Experienced by Lung Cancer Patients: A Review of Prevalence, Causes and Pathophysiology. Lung Cancer 2004, 43, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Paszat, L.; Qiu, F. End-of-Life Care in Lung Cancer Patients in Ontario: Aggressiveness of Care in the Population and a Description of Hospital Admissions. J. Pain Symptom Manag. 2008, 35, 267–274. [Google Scholar] [CrossRef]
- Sawhney, M.; Fletcher, G.G.; Rice, J.; Watt-Watson, J.; Rawn, T. Guidelines on Management of Pain in Cancer and/or Palliative Care; Cancer Care Ontario: Toronto, ON, Canada, 2017. [Google Scholar]
- Howell, D.; Molloy, S.; Wilkinson, K.; Green, E.; Orchard, K.; Wang, K.; Liberty, J. Patient-Reported Outcomes in Routine Cancer Clinical Practice: A Scoping Review of Use, Impact on Health Outcomes, and Implementation Factors. Ann. Oncol. 2015, 26, 1846–1858. [Google Scholar] [CrossRef]
- Kotronoulas, G.; Kearney, N.; Maguire, R.; Harrow, A.; Di Domenico, D.; Croy, S.; MacGillivray, S. What Is the Value of the Routine Use of Patient-Reported Outcome Measures toward Improvement of Patient Outcomes, Processes of Care, and Health Service Outcomes in Cancer Care? A Systematic Review of Controlled Trials. J. Clin. Oncol. 2014, 32, 1480–1501. [Google Scholar] [CrossRef]
- Basch, E.; Deal, A.M.; Kris, M.G.; Scher, H.I.; Hudis, C.A.; Sabbatini, P.; Rogak, L.; Bennett, A.V.; Dueck, A.C.; Atkinson, T.M.; et al. Symptom Monitoring with Patient-Reported Outcomes During Routine Cancer Treatment: A Randomized Controlled Trial. J. Clin. Oncol. 2016, 34, 557–565. [Google Scholar] [CrossRef]
- Barbera, L.; Sutradhar, R.; Seow, H.; Mittmann, N.; Howell, D.; Earle, C.C.; Li, Q.; Thiruchelvam, D. The Impact of Routine Edmonton Symptom Assessment System (ESAS) Use on Overall Survival in Cancer Patients: Results of a Population-Based Retrospective Matched Cohort Analysis. Cancer Med. 2020, 9, 7107–7115. [Google Scholar] [CrossRef]
- Barbera, L.; Sutradhar, R.; Howell, D.; Sussman, J.; Seow, H.; Dudgeon, D.; Atzema, C.; Earle, C.; Husain, A.; Liu, Y.; et al. Does Routine Symptom Screening with ESAS Decrease ED Visits in Breast Cancer Patients Undergoing Adjuvant Chemotherapy? Support. Care Cancer 2015, 23, 3025–3032. [Google Scholar] [CrossRef]
- Hui, D.; Bruera, E. The Edmonton Symptom Assessment System 25 Years Later: Past, Present and Future Developments. J. Pain Symptom Manag. 2017, 53, 630–643. [Google Scholar] [CrossRef]
- Tjong, M.C.; Doherty, M.; Tan, H.; Chan, W.C.; Zhao, H.; Hallet, J.; Darling, G.; Kidane, B.; Wright, F.C.; Mahar, A.; et al. Province-Wide Analysis of Patient-Reported Outcomes for Stage IV Non-Small Cell Lung Cancer. Oncologist 2021, 26, e1800–e1811. [Google Scholar] [CrossRef] [PubMed]
- Levy, A.; O’Brien, B.; Sellors, C.; Grootendorst, F.; Willison, D. Coding Accuracy of Administrative Drug Claims in the Ontario Drug Benefit Database. Can. J. Clin. Pharmacol. 2003, 10, 67–71. [Google Scholar] [PubMed]
- Kralj, B. Measuring “Rurality” for Purposes of Health-Care Planning: An Empirical Measure for Ontario. Ont. Med. Rev. 2000, 67, 33–52. [Google Scholar]
- Azzalini, L.; Chabot-Blanchet, M.; Southern, D.A.; Nozza, A.; Wilton, S.B.; Graham, M.M.; Gravel, G.M.; Bluteau, J.P.; Rouleau, J.L.; Guertin, M.C.; et al. A Disease-Specific Comorbidity Index for Predicting Mortality in Patients Admitted to Hospital with a Cardiac Condition. Can. Med. Assoc. J. 2019, 191, E299–E307. [Google Scholar] [CrossRef] [PubMed]
- Jawitz, O.K.; Wang, Z.; Boffa, D.J.; Detterbeck, F.C.; Blasberg, J.D.; Kim, A.W. The Differential Impact of Preoperative Comorbidity on Perioperative Outcomes Following Thoracoscopic and Open Lobectomies. Eur. J. Cardiothorac. Surg. 2017, 51, 169–174. [Google Scholar] [CrossRef]
- Selby, D.; Cascella, A.; Gardiner, K.; Do, R.; Moravan, V.; Myers, J.; Chow, E. A Single Set of Numerical Cutpoints to Define Moderate and Severe Symptoms for the Edmonton Symptom Assessment System. J. Pain Symptom Manag. 2010, 39, 241–249. [Google Scholar] [CrossRef]
- van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients with Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef]
- Bubis, L.D.; Davis, L.; Mahar, A.; Barbera, L.; Li, Q.; Moody, L.; Karanicolas, P.; Sutradhar, R.; Coburn, N.G. Symptom Burden in the First Year After Cancer Diagnosis: An Analysis of Patient-Reported Outcomes. J. Clin. Oncol. 2018, 36, 1103–1111. [Google Scholar] [CrossRef]
- Bubis, L.D.; Delibasic, V.; Davis, L.E.; Jeong, Y.; Chan, K.; Kosyachkova, E.; Mahar, A.; Karanicolas, P.; Coburn, N.G. Patient-Reported Symptoms in Metastatic Gastric Cancer Patients in the Last 6 Months of Life. Support. Care Cancer 2020, 29, 515–524. [Google Scholar] [CrossRef]
- Merchant, S.J.; Kong, W.; Brundage, M.; Booth, C.M. Symptom Evolution in Patients with Esophageal and Gastric Cancer Receiving Palliative Chemotherapy: A Population-Based Study. Ann. Surg. Oncol. 2020, 28, 79–87. [Google Scholar] [CrossRef]
- Bubis, L.D.; Davis, L.E.; Canaj, H.; Gupta, V.; Jeong, Y.; Barbera, L.; Li, Q.; Moody, L.; Karanicolas, P.J.; Sutradhar, R.; et al. Patient-Reported Symptom Severity Among 22,650 Cancer Outpatients in the Last Six Months of Life. J. Pain Symptom Manag. 2020, 59, 58–66.e4. [Google Scholar] [CrossRef]
- Hammad, A.; Davis, L.E.; Mahar, A.L.; Bubis, L.D.; Zhao, H.; Earle, C.C.; Barbera, L.; Hallet, J.; Coburn, N.G. Symptom Trajectories and Predictors of Severe Symptoms in Pancreatic Adenocarcinoma at the End-of-Life: A Population Based Analysis of 2538 Patients. HPB 2019, 21, 1744–1752. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.W.; Chen, Y.M.; Chao, J.Y.; Tsai, C.M.; Perng, R.P. Non-Small Cell Lung Cancer in Very Young and Very Old Patients. Chest 2000, 117, 354–357. [Google Scholar] [CrossRef] [PubMed]
- DeMaria, L.C.J.; Cohen, H.J. Characteristics of Lung Cancer in Elderly Patients. J. Gerontol. 1987, 42, 540–545. [Google Scholar] [CrossRef]
- Morris, J.N.; Mor, V.; Goldberg, R.J.; Sherwood, S.; Greer, D.S.; Hiris, J. The Effect of Treatment Setting and Patient Characteristics on Pain in Terminal Cancer Patients: A Report from the National Hospice Study. J. Chronic Dis. 1986, 39, 27–35. [Google Scholar] [CrossRef]
- Hirpara, D.H.; Gupta, V.; Davis, L.E.; Zhao, H.; Hallet, J.; Mahar, A.L.; Sutradhar, R.; Doherty, M.; Louie, A.V.; Kidane, B.; et al. Severe Symptoms Persist for Up to One Year after Diagnosis of Stage I-III Lung Cancer: An Analysis of Province-Wide Patient Reported Outcomes. Lung Cancer 2020, 142, 80–89. [Google Scholar] [CrossRef]
- Huhti, E.; Sutinen, S.; Relnila, A.; Poukkula, A.; Saloheimo, M. Lung Cancer in a Defined Geographical Area: History and Histological Types. Thorax 1980, 35, 660–667. [Google Scholar] [CrossRef]
- Quinn, K.L.; Wegier, P.; Stukel, T.A.; Huang, A.; Bell, C.M.; Tanuseputro, P. Comparison of Palliative Care Delivery in the Last Year of Life Between Adults with Terminal Noncancer Illness or Cancer. JAMA Netw. Open 2021, 4, e210677. [Google Scholar] [CrossRef]
- Sullivan, D.R.; Chan, B.; Lapidus, J.A.; Ganzini, L.; Hansen, L.; Carney, P.A.; Fromme, E.K.; Marino, M.; Golden, S.E.; Vranas, K.C.; et al. Association of Early Palliative Care Use with Survival and Place of Death Among Patients With Advanced Lung Cancer Receiving Care in the Veterans Health Administration. JAMA Oncol. 2019, 5, 1702–1709. [Google Scholar] [CrossRef]
- Temel, J.S.; Greer, J.A.; Muzikansky, A.; Gallagher, E.R.; Admane, S.; Jackson, V.A.; Dahlin, C.M.; Blinderman, C.D.; Jacobsen, J.; Pirl, W.F.; et al. Early Palliative Care for Patients with Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2010, 363, 733–742. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, D.; Tanuseputro, P.; Perez, R.; Pond, G.R.; Seow, H.-Y. Early Initiation of Palliative Care Is Associated with Reduced Late-Life Acute-Hospital Use: A Population-Based Retrospective Cohort Study. Palliat. Med. 2019, 33, 150–159. [Google Scholar] [CrossRef]
- Seow, H.; Barbera, L.C.; McGrail, K.; Burge, F.; Guthrie, D.M.; Lawson, B.; Chan, K.K.W.; Peacock, S.J.; Sutradhar, R. Effect of Early Palliative Care on End-of-Life Health Care Costs: A Population-Based, Propensity Score–Matched Cohort Study. JCO Oncol. Pract. 2022, 18, e183–e192. [Google Scholar] [CrossRef] [PubMed]
- Barbera, L.; Sutradhar, R.; Earle, C.C.; Howell, D.; Mittman, N.; Li, Q.; Thiruchelvam, D.; Seow, H. The Impact of Routine Edmonton Symptom Assessment System Use on Receiving Palliative Care Services: Results of a Population-Based Retrospective-Matched Cohort Analysis. BMJ Support. Palliat. Care 2020. [Google Scholar] [CrossRef] [PubMed]
- Goldie, C.L.; Nguyen, P.; Robinson, A.G.; Goldie, C.E.; Kircher, C.E.; Hanna, T.P. Quality of End-of-Life Care for People with Advanced Non-Small Cell Lung Cancer in Ontario: A Population-Based Study. Curr. Oncol. 2021, 28, 3297–3315. [Google Scholar] [CrossRef] [PubMed]
- Tanuseputro, P.; Budhwani, S.; Bai, Y.Q.; Wodchis, W.P. Palliative Care Delivery across Health Sectors: A Population-Level Observational Study. Palliat. Med. 2017, 31, 247–257. [Google Scholar] [CrossRef]
- Conlon, M.S.C.; Caswell, J.M.; Santi, S.A.; Ballantyne, B.; Meigs, M.L.; Knight, A.; Earle, C.C.; Hartman, M. Access to Palliative Care for Cancer Patients Living in a Northern and Rural Environment in Ontario, Canada: The Effects of Geographic Region and Rurality on End-of-Life Care in a Population-Based Decedent Cancer Cohort. Clin. Med. Insights. Oncol. 2019, 13, 1179554919829500. [Google Scholar] [CrossRef]
- Chen, A.B.; Cronin, A.; Weeks, J.C.; Chrischilles, E.A.; Malin, J.; Hayman, J.A.; Schrag, D. Palliative Radiation Therapy Practice in Patients with Metastatic Non-Small-Cell Lung Cancer: A Cancer Care Outcomes Research and Surveillance Consortium (CanCORS) Study. J. Clin. Oncol. 2013, 31, 558–564. [Google Scholar] [CrossRef]
- Hayman, J.A.; Abrahamse, P.H.; Lakhani, I.; Earle, C.C.; Katz, S.J. Use of Palliative Radiotherapy among Patients with Metastatic Non-Small-Cell Lung Cancer. Int. J. Radiat. Oncol. Biol. Phys. 2007, 69, 1001–1007. [Google Scholar] [CrossRef]
- Dohm, A.; Diaz, R.; Nanda, R.H. The Role of Radiation Therapy in the Older Patient. Curr. Oncol. Rep. 2021, 23, 11. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zhou, S.; Groome, P.; Tyldesley, S.; Zhang-Solomans, J.; Mackillop, W.J. Factors Affecting the Use of Palliative Radiotherapy in Ontario. J. Clin. Oncol. 2001, 19, 137–144. [Google Scholar] [CrossRef]
- Palma, D.A.; Olson, R.; Harrow, S.; Gaede, S.; Louie, A.V.; Haasbeek, C.; Mulroy, L.; Lock, M.; Rodrigues, G.B.; Yaremko, B.P.; et al. Stereotactic Ablative Radiotherapy versus Standard of Care Palliative Treatment in Patients with Oligometastatic Cancers (SABR-COMET): A Randomised, Phase 2, Open-Label Trial. Lancet 2019, 393, 2051–2058. [Google Scholar] [CrossRef] [PubMed]
- Kazazian, K.; Bogach, J.; Johnston, W.; Ng, D.; Swallow, C.J. Challenges in Virtual Collection of Patient-Reported Data: A Prospective Cohort Study Conducted in COVID-19 Era. Support. Care Cancer 2022, 30, 7535–7544. [Google Scholar] [CrossRef] [PubMed]
- Wagner, L.I.; Schink, J.; Bass, M.; Patel, S.; Diaz, M.V.; Rothrock, N.; Pearman, T.; Gershon, R.; Penedo, F.J.; Rosen, S.; et al. Bringing PROMIS to Practice: Brief and Precise Symptom Screening in Ambulatory Cancer Care. Cancer 2015, 121, 927–934. [Google Scholar] [CrossRef]
- Dhiliwal, S.; Salins, N.; Deodhar, J.; Rao, R.; Muckaden, M.A. Pilot Testing of Triage Coding System in Home-Based Palliative Care Using Edmonton Symptom Assessment Scale. Indian J. Palliat. Care 2016, 22, 19–24. [Google Scholar] [CrossRef] [PubMed]

| Characteristics | None or Mild Pain ESAS Score (n = 4151) | Moderate-to-Severe Pain ESAS Score (n = 9008) | Standardized Difference |
|---|---|---|---|
| Sex | |||
| Female | 2038 (49.1%) | 4388 (48.7%) | 0.01 |
| Male | 2113 (50.9%) | 4620 (51.3%) | 0.01 |
| Age | 70 (63–78) | 67 (59–74) | 0.30 |
| Residence | |||
| Major urban | 2634 (63.5%) | 5986 (66.5%) | 0.06 |
| Non-major urban | 1156 (27.8%) | 2362 (26.2%) | 0.04 |
| Rural | 345 (8.3%) | 629 (7.0%) | 0.05 |
| Neighborhood Income Quintile | |||
| Q1 | 849 (20.5%) | 1968 (21.8%) | 0.03 |
| Q2 | 942 (22.7%) | 2019 (22.4%) | 0.01 |
| Q3 | 829 (20.0%) | 1744 (19.4%) | 0.02 |
| Q4 | 757 (18.2%) | 1694 (18.8%) | 0.01 |
| Q5 (highest income) | 759 (18.3%) | 1558 (17.3%) | 0.03 |
| Elixhauser Comorbidity Index | |||
| 4 or more | 3871 (93.3%) | 8450 (93.8%) | 0.02 |
| Less than 4 | 280 (6.7%) | 558 (6.2%) | 0.02 |
| Year of Diagnosis | |||
| 2007 to 2012 | 1652 (39.8%) | 3502 (38.9%) | 0.02 |
| 2013 to 2018 | 2499 (60.2%) | 5506 (61.1%) | 0.02 |
| Number of ESAS questionnaires | 2 (1–6) | 6 (2–14) | 0.60 |
| Systemic Therapy | 2165 (52.2%) | 5686 (63.1%) | 0.22 |
| Death | 3703 (89.2%) | 8204 (91.1%) | 0.06 |
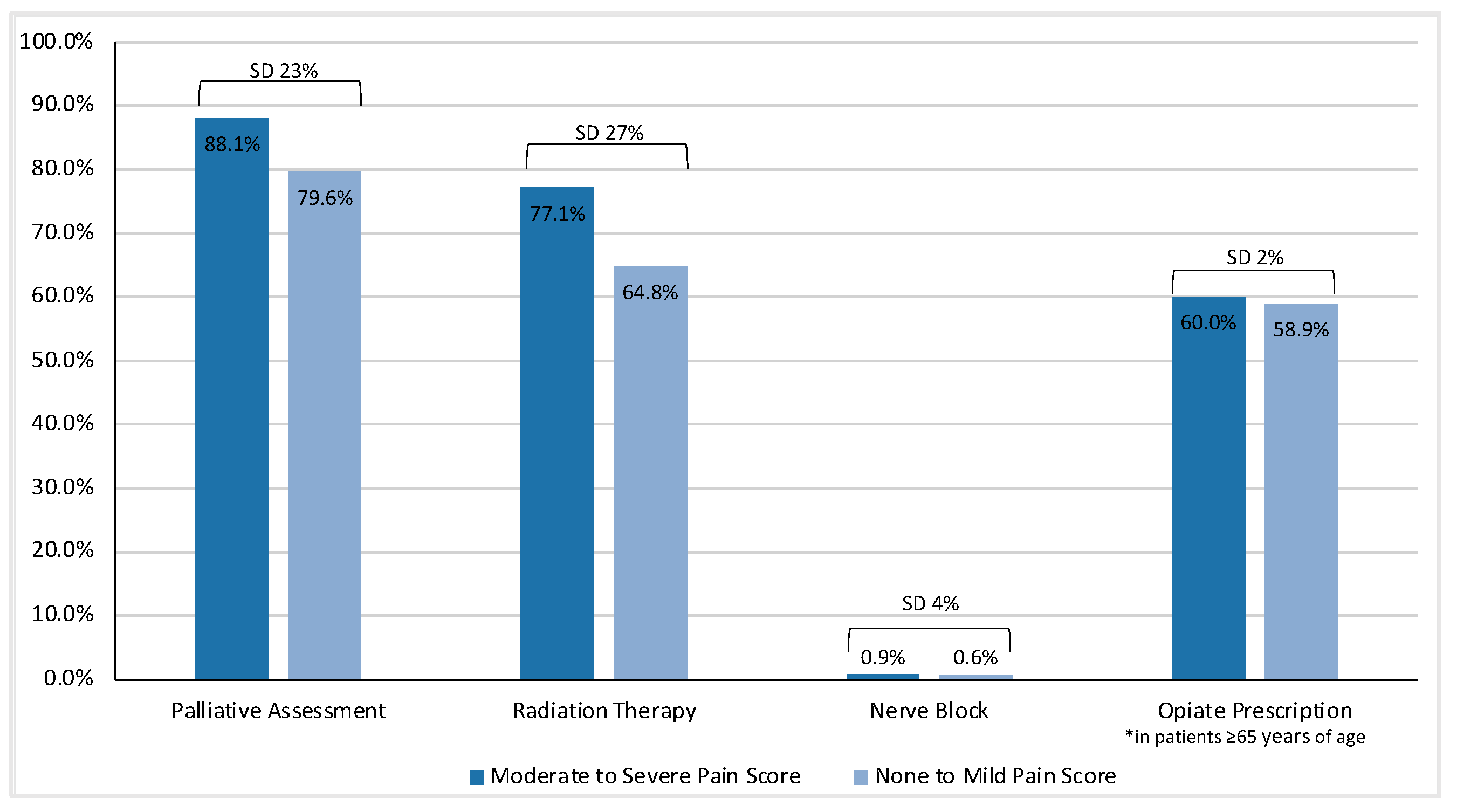
| Interventions | Total Cohort | None or Mild Pain ESAS Score | Moderate-to-Severe Pain ESAS Score | Standardized Difference |
|---|---|---|---|---|
| Intervention for Pain | n = 13,159 | n = 4151 | n = 9008 | |
| Yes | 12,410 (94.3%) | 3785 (91.2%) | 8625 (95.7%) | 0.19 |
| No | 749 (5.7%) | 366 (8.8%) | 383 (4.3%) | |
| Palliative Care | 0.23 0.05 0.06 0.07 | |||
| Assessment | ||||
| Yes | 11,239 (85.4%) | 3303 (79.6%) | 7936 (88.1%) | |
| Inpatient | 275 (2.4%) | 101 (3.1%) | 174 (2.2%) | |
| Outpatient | 6616 (58.9%) | 2008 (60.8%) | 4608 (58.1%) | |
| Both | 4348 (38.7%) | 1194 (36.1%) | 3154 (39.7%) | |
| No | 1920 (14.6%) | 848 (20.4%) | 1072 (11.9%) | |
| Radiation Therapy | ||||
| Yes | 9635 (73.2%) | 2691 (64.8%) | 6944 (77.1%) | 0.27 |
| No | 3524 (26.8%) | 1460 (35.2%) | 2064 (22.9%) | |
| Nerve Block | ||||
| Yes | 106 (0.8%) | 24 (0.6%) | 82 (0.9%) | 0.04 |
| No | 13,053 (99.2%) | 4127 (99.4%) | 8926 (99.1%) | |
| For patients ≥65 | n = 2863 | n = 5198 | 0.02 | |
| Opiate Prescription | ||||
| Yes | 4806 (59.6%) | 1685 (58.9%) | 3121 (60.0%) | |
| No | 3255 (40.4%) | 1178 (41.1%) | 2077 (40.0%) |
| Characteristics | Palliative Assessment | Radiation Therapy | Nerve Block | Use of Opiates (65+) |
|---|---|---|---|---|
| Sex | ||||
| Male | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Female | 1.006 (0.991–1.022) | 0.991 (0.969–1.013) | 1.782 (1.139–2.787) | 1.014 (0.969–1.060) |
| Age (years) | ||||
| 18–59 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 60–69 | 0.997 (0.977–1.017) | 0.976 (0.951–1.001) | 1.009 (0.586–1.735) | 1.0 (reference) |
| 70–79 | 1.000 (0.98–1.021) | 0.902 (0.875–0.929) | 0.586 (0.309–1.111) | 0.980 (0.932–1.032) |
| 80 and older | 1.001 (0.974–1.028) | 0.861 (0.822–0.901) | 1.005 (0.488–2.068) | 0.989 (0.926–1.056) |
| Residence | ||||
| Major urban | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Non-major urban | 0.953 (0.935–0.97) | 1.006 (0.981–1.032) | 1.052 (0.657–1.686) | 0.972 (0.922–1.024) |
| Rural | 0.864 (0.828–0.902) | 1.015 (0.972–1.061) | 0.177 (0.025–1.273) | 0.995 (0.91–1.088) |
| Neighborhood Income Quintile | ||||
| Q1 | 0.978 (0.955–1.001) | 0.972 (0.937–1.007) | 0.578 (0.292–1.144) | 0.961 (0.895–1.031) |
| Q2 | 0.973 (0.950–0.997) | 0.98 (0.946–1.015) | 0.638 (0.334–1.221) | 0.959 (0.895–1.029) |
| Q3 | 0.992 (0.968–1.016) | 0.982 (0.947–1.018) | 0.885 (0.475–1.649) | 0.957 (0.891–1.028) |
| Q4 | 0.987 (0.963–1.011) | 1.014 (0.980–1.050) | 0.552 (0.271–1.125) | 0.969 (0.901–1.042) |
| Q5 (highest) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| Elixhauser Cormorbidity Index | ||||
| Less than 4 | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) | 1.0 (reference) |
| 4 or more | 0.96 (0.926–0.996) | 0.903 (0.853–0.957) | 1.354 (0.595–3.083) | 0.921 (0.844–1.006) |
| Year of Diagnosis | ||||
| 2007 to 2018 | 1.003 (1.000–1.005) | 0.970 (0.966–0.974) | 0.977 (0.909–1.049) | 0.998 (0.991–1.006) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, V.S.; Tjong, M.C.; Chan, W.C.; Yan, M.; Delibasic, V.; Darling, G.; Davis, L.E.; Doherty, M.; Hallet, J.; Kidane, B.; et al. Pain and Interventions in Stage IV Non-Small Cell Lung Cancer: A Province-Wide Analysis. Curr. Oncol. 2023, 30, 3461-3472. https://doi.org/10.3390/curroncol30030262
Tan VS, Tjong MC, Chan WC, Yan M, Delibasic V, Darling G, Davis LE, Doherty M, Hallet J, Kidane B, et al. Pain and Interventions in Stage IV Non-Small Cell Lung Cancer: A Province-Wide Analysis. Current Oncology. 2023; 30(3):3461-3472. https://doi.org/10.3390/curroncol30030262
Chicago/Turabian StyleTan, Vivian S., Michael C. Tjong, Wing C. Chan, Michael Yan, Victoria Delibasic, Gail Darling, Laura E. Davis, Mark Doherty, Julie Hallet, Biniam Kidane, and et al. 2023. "Pain and Interventions in Stage IV Non-Small Cell Lung Cancer: A Province-Wide Analysis" Current Oncology 30, no. 3: 3461-3472. https://doi.org/10.3390/curroncol30030262
APA StyleTan, V. S., Tjong, M. C., Chan, W. C., Yan, M., Delibasic, V., Darling, G., Davis, L. E., Doherty, M., Hallet, J., Kidane, B., Mahar, A., Mittmann, N., Parmar, A., Tan, H., Wright, F. C., Coburn, N. G., & Louie, A. V. (2023). Pain and Interventions in Stage IV Non-Small Cell Lung Cancer: A Province-Wide Analysis. Current Oncology, 30(3), 3461-3472. https://doi.org/10.3390/curroncol30030262






