Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Review and Questionnaire
2.2. Survey Respondents
2.3. Survey Procedures, Ethical Aspects, and Limitations of the Study
2.4. Statistical Analysis of the Sample
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Noncommunicable Diseases. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/noncommunicable-diseases (accessed on 10 January 2023).
- Deshaies, R.J. Multispecific drugs herald a new era of biopharmaceutical innovation. Nature 2020, 580, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Z. The history and advances in cancer immunotherapy: Understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell. Mol. Immunol. 2020, 17, 807–821. [Google Scholar] [CrossRef] [PubMed]
- Polverini, P.J.; Lingen, M.W. A History of Innovations in the Diagnosis and Treatment of Oral and Head and Neck Cancer. J. Dent. Res. 2019, 98, 489–497. [Google Scholar] [CrossRef]
- Bhinder, B.; Gilvary, C.; Madhukar, N.S.; Elemento, O. Artificial Intelligence in Cancer Research and Precision Medicine. Cancer Discov. 2021, 11, 900–915. [Google Scholar] [CrossRef]
- Ho, D. Artificial intelligence in cancer therapy. Science 2020, 367, 982–983. [Google Scholar] [CrossRef]
- Jones, O.T.; Calanzani, N.; Saji, S.; Duffy, S.W.; Emery, J.; Hamilton, W.; Singh, H.; de Wit, N.J.; Walter, F.M. Artificial Intelligence Techniques That May Be Applied to Primary Care Data to Facilitate Earlier Diagnosis of Cancer: Systematic Review. J. Med. Internet Res. 2021, 23, e23483. [Google Scholar] [CrossRef]
- Londhe, V.Y.; Bhasin, B. Artificial intelligence and its potential in oncology. Drug Discov. Today 2019, 24, 228–232. [Google Scholar] [CrossRef]
- Luchini, C.; Pea, A.; Scarpa, A. Artificial intelligence in oncology: Current applications and future perspectives. Br. J. Cancer 2022, 126, 4–9. [Google Scholar] [CrossRef]
- Davenport, T.; Kalakota, R. The potential for artificial intelligence in healthcare. Future Health J. 2019, 6, 94–98. [Google Scholar] [CrossRef]
- Lu, Y. Artificial intelligence: A survey on evolution, models, applications and future trends. J. Manag. Anal. 2019, 6, 1–29. [Google Scholar] [CrossRef]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q. Artificial intelligence in cancer diagnosis and prognosis: Opportunities and challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Darsey, J.A.; Ghosh, A.; Li, H.-Y.; Yang, M.Q.; Wang, S. Artificial Intelligence and Cancer Drug Development. Recent Pat. Anticancer. Drug Discov. 2022, 17, 2–8. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Lai, X.; Pan, Y.; Zheng, H.; Vera, J.; Liu, S.; Deng, S.; Zhang, L. Artificial intelligence in cancer target identification and drug discovery. Signal Transduct. Target. Ther. 2022, 7, 156. [Google Scholar] [CrossRef] [PubMed]
- Farina, E.; Nabhen, J.J.; Dacoregio, M.I.; Batalini, F.; Moraes, F.Y. An overview of artificial intelligence in oncology. Future Sci. OA 2022, 8, FSO787. [Google Scholar] [CrossRef]
- Mital, S.; Nguyen, H.V. Cost-effectiveness of using artificial intelligence versus polygenic risk score to guide breast cancer screening. BMC Cancer 2022, 22, 501. [Google Scholar] [CrossRef]
- Basurto-Hurtado, J.A.; Cruz-Albarran, I.A.; Toledano-Ayala, M.; Ibarra-Manzano, M.A.; Morales-Hernandez, L.A.; Perez-Ramirez, C.A. Diagnostic Strategies for Breast Cancer Detection: From Image Generation to Classification Strategies Using Artificial Intelligence Algorithms. Cancers 2022, 14, 3442. [Google Scholar] [CrossRef]
- Elemento, O.; Leslie, C.; Lundin, J.; Tourassi, G. Artificial intelligence in cancer research, diagnosis and therapy. Nat. Rev. Cancer 2021, 21, 747–752. [Google Scholar] [CrossRef]
- García-Pola, M.; Pons-Fuster, E.; Suárez-Fernández, C.; Seoane-Romero, J.; Romero-Méndez, A.; López-Jornet, P. Role of Artificial Intelligence in the Early Diagnosis of Oral Cancer. A Scoping Review. Cancers 2021, 13, 4600. [Google Scholar] [CrossRef]
- Bensoussan, Y.; Vanstrum, E.B.; Johns, M.M.; Rameau, A. Artificial Intelligence and Laryngeal Cancer: From Screening to Prognosis: A State of the Art Review. Otolaryngol.-Head Neck Surg. 2022, 168, 319–329. [Google Scholar] [CrossRef]
- Giulietti, M.; Cecati, M.; Sabanovic, B.; Scirè, A.; Cimadamore, A.; Santoni, M.; Montironi, R.; Piva, F. The Role of Artificial Intelligence in the Diagnosis and Prognosis of Renal Cell Tumors. Diagnostics 2021, 11, 206. [Google Scholar] [CrossRef]
- Janssen, B.V.; Verhoef, S.; Wesdorp, N.J.; Huiskens, J.; de Boer, O.J.; Marquering, H.; Stoker, J.; Kazemier, G.; Besselink, M.G. Imaging-based Machine-learning Models to Predict Clinical Outcomes and Identify Biomarkers in Pancreatic Cancer: A Scoping Review. Ann. Surg. 2022, 275, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Liang, F.; Wang, S.; Zhang, K.; Liu, T.-J.; Li, J.-N. Development of artificial intelligence technology in diagnosis, treatment, and prognosis of colorectal cancer. World J. Gastrointest. Oncol. 2022, 14, 124–152. [Google Scholar] [CrossRef] [PubMed]
- Pei, Q.; Luo, Y.; Chen, Y.; Li, J.; Xie, D.; Ye, T. Artificial intelligence in clinical applications for lung cancer: Diagnosis, treatment and prognosis. Clin. Chem. Lab. Med. 2022, 60, 1974–1983. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, N.E.; Kerlavage, A.R. The potential of AI in cancer care and research. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188573. [Google Scholar] [CrossRef] [PubMed]
- Shreve, J.T.; Khanani, S.A.; Haddad, T.C. Artificial Intelligence in Oncology: Current Capabilities, Future Opportunities, and Ethical Considerations. Am. Soc. Clin. Oncol. Educ. Book 2022, 42, 1–10. [Google Scholar] [CrossRef]
- Hickman, S.E.; Baxter, G.C.; Gilbert, F.J. Adoption of artificial intelligence in breast imaging: Evaluation, ethical constraints and limitations. Br. J. Cancer 2021, 125, 15–22. [Google Scholar] [CrossRef]
- World Health Organization. Ethics and Governance of Artificial Intelligence for Health: WHO Guidance; Who Guidance: Geneva, Switzerland, 2021; Available online: https://apps.who.int/iris/bitstream/handle/10665/341996/9789240029200-eng.pdf (accessed on 10 January 2023).
- Chua, I.S.; Gaziel-Yablowitz, M.; Korach, Z.T.; Kehl, K.L.; Levitan, N.A.; Arriaga, Y.E.; Jackson, G.P.; Bates, D.W.; Hassett, M. Artificial intelligence in oncology: Path to implementation. Cancer Med. 2021, 10, 4138–4149. [Google Scholar] [CrossRef]
- Coccia, M. Deep learning technology for improving cancer care in society: New directions in cancer imaging driven by artificial intelligence. Technol. Soc. 2020, 60, 101198. [Google Scholar] [CrossRef]
- Shimizu, H.; Nakayama, K.I. Artificial intelligence in oncology. Cancer Sci. 2020, 111, 1452–1460. [Google Scholar] [CrossRef]
- Painuli, D.; Bhardwaj, S.; Köse, U. Recent advancement in cancer diagnosis using machine learning and deep learning techniques: A comprehensive review. Comput. Biol. Med. 2022, 146, 105580. [Google Scholar] [CrossRef]
- Majumder, A.; Sen, D. Artificial intelligence in cancer diagnostics and therapy: Current perspectives. Indian J. Cancer 2021, 58, 481–492. [Google Scholar] [CrossRef]
- Troyanskaya, O.; Trajanoski, Z.; Carpenter, A.; Thrun, S.; Razavian, N.; Oliver, N. Artificial intelligence and cancer. Nat. Cancer 2020, 1, 149–152. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Javed, Z.; Sadia, H.; Qureshi, I.A.; Irshad, A.; Ahmed, R.; Malik, K.; Raza, S.; Abbas, A.; Pezzani, R.; et al. Clinical applications of artificial intelligence and machine learning in cancer diagnosis: Looking into the future. Cancer Cell Int. 2021, 21, 270. [Google Scholar] [CrossRef] [PubMed]
- Kann, B.H.; Hosny, A.; Aerts, H.J.W.L. Artificial intelligence for clinical oncology. Cancer Cell 2021, 39, 916–927. [Google Scholar] [CrossRef]
- Zhang, S.M.; Wang, Y.J.; Zhang, S.T. Accuracy of artificial intelligence-assisted detection of esophageal cancer and neoplasms on endoscopic images: A systematic review and meta-analysis. J. Dig. Dis. 2021, 22, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Posner, R.; Wang, T.; Yang, C.; Nabavi, S. Applying deep learning in digital breast tomosynthesis for automatic breast cancer detection: A review. Med. Image Anal. 2021, 71, 102049. [Google Scholar] [CrossRef] [PubMed]
- Gastounioti, A.; Desai, S.; Ahluwalia, V.S.; Conant, E.F.; Kontos, D. Artificial intelligence in mammographic phenotyping of breast cancer risk: A narrative review. Breast Cancer Res. 2022, 24, 14. [Google Scholar] [CrossRef]
- Hildebrand, L.A.; Pierce, C.J.; Dennis, M.; Paracha, M.; Maoz, A. Artificial Intelligence for Histology-Based Detection of Microsatellite Instability and Prediction of Response to Immunotherapy in Colorectal Cancer. Cancers 2021, 13, 391. [Google Scholar] [CrossRef] [PubMed]
- Mitsala, A.; Tsalikidis, C.; Pitiakoudis, M.; Simopoulos, C.; Tsaroucha, A.K. Artificial Intelligence in Colorectal Cancer Screening, Diagnosis and Treatment. A New Era. Curr. Oncol. 2021, 28, 1581–1607. [Google Scholar] [CrossRef]
- Rezayi, S.; R Niakan Kalhori, S.; Saeedi, S. Effectiveness of Artificial Intelligence for Personalized Medicine in Neoplasms: A Systematic Review. Biomed Res. Int. 2022, 2022, 7842566. [Google Scholar] [CrossRef] [PubMed]
- Nir, G.; Karimi, D.; Goldenberg, S.L.; Fazli, L.; Skinnider, B.F.; Tavassoli, P.; Turbin, D.; Villamil, C.F.; Wang, G.; Thompson, D.J.S.; et al. Comparison of Artificial Intelligence Techniques to Evaluate Performance of a Classifier for Automatic Grading of Prostate Cancer from Digitized Histopathologic Images. JAMA Netw. Open 2019, 2, e190442. [Google Scholar] [CrossRef] [PubMed]
- Ström, P.; Kartasalo, K.; Olsson, H.; Solorzano, L.; Delahunt, B.; Berney, D.M.; Bostwick, D.G.; Evans, A.J.; Grignon, D.J.; Humphrey, P.A.; et al. Artificial intelligence for diagnosis and grading of prostate cancer in biopsies: A population-based, diagnostic study. Lancet Oncol. 2020, 21, 222–232. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Wei, S.; Anaokar, J.; Uzzo, R.; Kutikov, A. Kidney cancer management 3.0: Can artificial intelligence make us better? Curr. Opin. Urol. 2021, 31, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, J.; Higgins, D.; Mazo Vargas, C.; Watson, W.; Mooney, C.; Rahman, A.; Aspell, N.; Connolly, A.; Aura Gonzalez, C.; Gallagher, W. Future of biomarker evaluation in the realm of artificial intelligence algorithms: Application in improved therapeutic stratification of patients with breast and prostate cancer. J. Clin. Pathol. 2021, 74, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kenner, B.J.; Abrams, N.D.; Chari, S.T.; Field, B.F.; Goldberg, A.E.; Hoos, W.A.; Klimstra, D.S.; Rothschild, L.J.; Srivastava, S.; Young, M.R.; et al. Early Detection of Pancreatic Cancer: Applying Artificial Intelligence to Electronic Health Records. Pancreas 2021, 50, 916–922. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; Antoniou, A.C.; Fruk, L.; Rosenfeld, N. The future of early cancer detection. Nat. Med. 2022, 28, 666–677. [Google Scholar] [CrossRef]
- Xiao, Z.; Ji, D.; Li, F.; Li, Z.; Bao, Z. Application of Artificial Intelligence in Early Gastric Cancer Diagnosis. Digestion 2022, 103, 69–75. [Google Scholar] [CrossRef]
- Mota, F.; Braga, L.; Rocha, L.; Cabral, B. 3D and 4D bioprinted human model patenting and the future of drug development. Nat. Biotechnol. 2020, 38, 689–694. [Google Scholar] [CrossRef]
- Pereira Cabral, B.; Bonventre, J.V.; Wieringa, F.; Mota, F.B. Probing expert opinions on the future of kidney replacement therapies. Artif. Organs 2021, 45, 79–87. [Google Scholar] [CrossRef]
- Braga, L.A.M.; Filho, C.G.C.; Mota, F.B. Future of genetic therapies for rare genetic diseases: What to expect for the next 15 years? Ther. Adv. Rare Dis. 2022. [Google Scholar] [CrossRef]
- Rocha, L.F.M.; Braga, L.A.M.; Mota, F.B. Gene Editing for Treatment and Prevention of Human Diseases: A Global Survey of Gene Editing-Related Researchers. Hum. Gene Ther. 2020, 31, 852–862. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Moradpoor, H.; Salari, F.; Mosharraf, R.; Raissi, S.; Shirani, M. Patient satisfaction with occlusal scheme of conventional complete dentures. J. Oral. Rehabil. 2020, 47, 494–500. [Google Scholar] [CrossRef]
- Pereira Cabral, B.; Da Graça Derengowski Fonseca, M.; Batista Mota, F. What is the future of cancer care? A technology foresight assessment of experts’ expectations. Econ. Innov. New Technol. 2019, 28, 635–652. [Google Scholar] [CrossRef]
- Mota, F.; Braga, L.A.M.; Cabral, B.P.P.; Conte Filho, C.G. What is the future of lab-on-a-chip diagnostic devices? Assessing changes in experts’ expectations over time. FS 2021, 23, 640–654. [Google Scholar] [CrossRef]
- Pu, B.; Li, K.; Li, S.; Zhu, N. Automatic Fetal Ultrasound Standard Plane Recognition Based on Deep Learning and IIoT. IEEE Trans. Ind. Inf. 2021, 17, 7771–7780. [Google Scholar] [CrossRef]
- Sone, K.; Toyohara, Y.; Taguchi, A.; Miyamoto, Y.; Tanikawa, M.; Uchino-Mori, M.; Iriyama, T.; Tsuruga, T.; Osuga, Y. Application of artificial intelligence in gynecologic malignancies: A review. J. Obstet. Gynaecol. Res. 2021, 47, 2577–2585. [Google Scholar] [CrossRef]
- Hunter, B.; Hindocha, S.; Lee, R.W. The Role of Artificial Intelligence in Early Cancer Diagnosis. Cancers 2022, 14, 1524. [Google Scholar] [CrossRef]
- Lang, Q.; Zhong, C.; Liang, Z.; Zhang, Y.; Wu, B.; Xu, F.; Cong, L.; Wu, S.; Tian, Y. Six application scenarios of artificial intelligence in the precise diagnosis and treatment of liver cancer. Artif. Intell. Rev. 2021, 54, 5307–5346. [Google Scholar] [CrossRef]
- Abdelaziz Ismael, S.A.; Mohammed, A.; Hefny, H. An enhanced deep learning approach for brain cancer MRI images classification using residual networks. Artif. Intell. Med. 2020, 102, 101779. [Google Scholar] [CrossRef] [PubMed]
- Balkenende, L.; Teuwen, J.; Mann, R.M. Application of Deep Learning in Breast Cancer Imaging. Semin. Nucl. Med. 2022, 52, 584–596. [Google Scholar] [CrossRef] [PubMed]
- Karaman, A.; Pacal, I.; Basturk, A.; Akay, B.; Nalbantoglu, U.; Coskun, S.; Sahin, O.; Karaboga, D. Robust real-time polyp detection system design based on YOLO algorithms by optimizing activation functions and hyper-parameters with artificial bee colony (ABC). Expert Syst. Appl. 2023, 221, 119741. [Google Scholar] [CrossRef]
- Smith, H. Clinical AI: Opacity, accountability, responsibility and liability. AI Soc. 2021, 36, 535–545. [Google Scholar] [CrossRef]
- Reddy, S. Explainability and artificial intelligence in medicine. Lancet Digit. Health 2022, 4, e214–e215. [Google Scholar] [CrossRef]
- Felmingham, C.M.; Adler, N.R.; Ge, Z.; Morton, R.L.; Janda, M.; Mar, V.J. The Importance of Incorporating Human Factors in the Design and Implementation of Artificial Intelligence for Skin Cancer Diagnosis in the Real World. Am. J. Clin. Dermatol. 2021, 22, 233–242. [Google Scholar] [CrossRef]
- Vobugari, N.; Raja, V.; Sethi, U.; Gandhi, K.; Raja, K.; Surani, S.R. Advancements in Oncology with Artificial Intelligence—A Review Article. Cancers 2022, 14, 1349. [Google Scholar] [CrossRef]
- Johnson, K.B.; Wei, W.-Q.; Weeraratne, D.; Frisse, M.E.; Misulis, K.; Rhee, K.; Zhao, J.; Snowdon, J.L. Precision Medicine, AI, and the Future of Personalized Health Care. Clin. Transl. Sci. 2021, 14, 86–93. [Google Scholar] [CrossRef]
- Alami, H.; Lehoux, P.; Auclair, Y.; de Guise, M.; Gagnon, M.-P.; Shaw, J.; Roy, D.; Fleet, R.; Ag Ahmed, M.A.; Fortin, J.-P. Artificial Intelligence and Health Technology Assessment: Anticipating a New Level of Complexity. J. Med. Internet Res. 2020, 22, e17707. [Google Scholar] [CrossRef]
- Ozair, F.F.; Jamshed, N.; Sharma, A.; Aggarwal, P. Ethical issues in electronic health records: A general overview. Perspect. Clin. Res. 2015, 6, 73–76. [Google Scholar] [CrossRef]
- Almaghrabi, N.S.; Bugis, B.A. Patient Confidentiality of Electronic Health Records: A Recent Review of the Saudi Literature. Dr. Sulaiman Al Habib Med. J. 2022, 4, 126–135. [Google Scholar] [CrossRef]

| Topic | Alternatives | References |
|---|---|---|
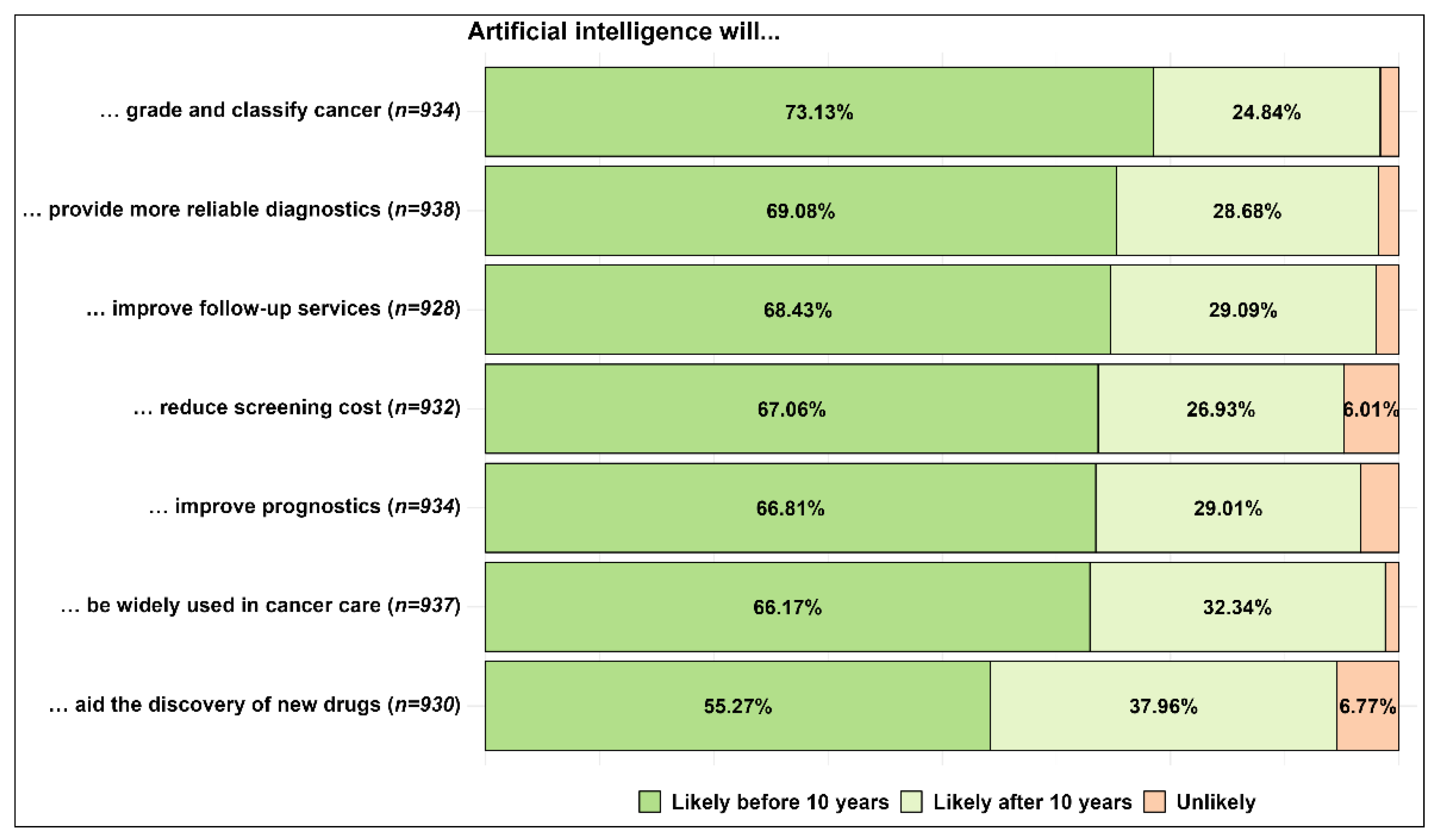
| Broad use of artificial intelligence in the future | Likely before or after ten years; unlikely | [7,10,13,19,34,35] |
| Possible artificial intelligence applications in cancer care in the future | More reliable diagnostics | [8,13,18,19,23,25,36,38] |
| Reduce screening cost | [20,21,34,39,40,41,42,43] | |
| Improve follow-up services | [9,10,37] | |
| Discovery of new drugs | [9,10,14,15] | |
| Grade and classify cancer | [22,30,44,45,46] | |
| Improve prognostics | [19,22,24,36,47] | |
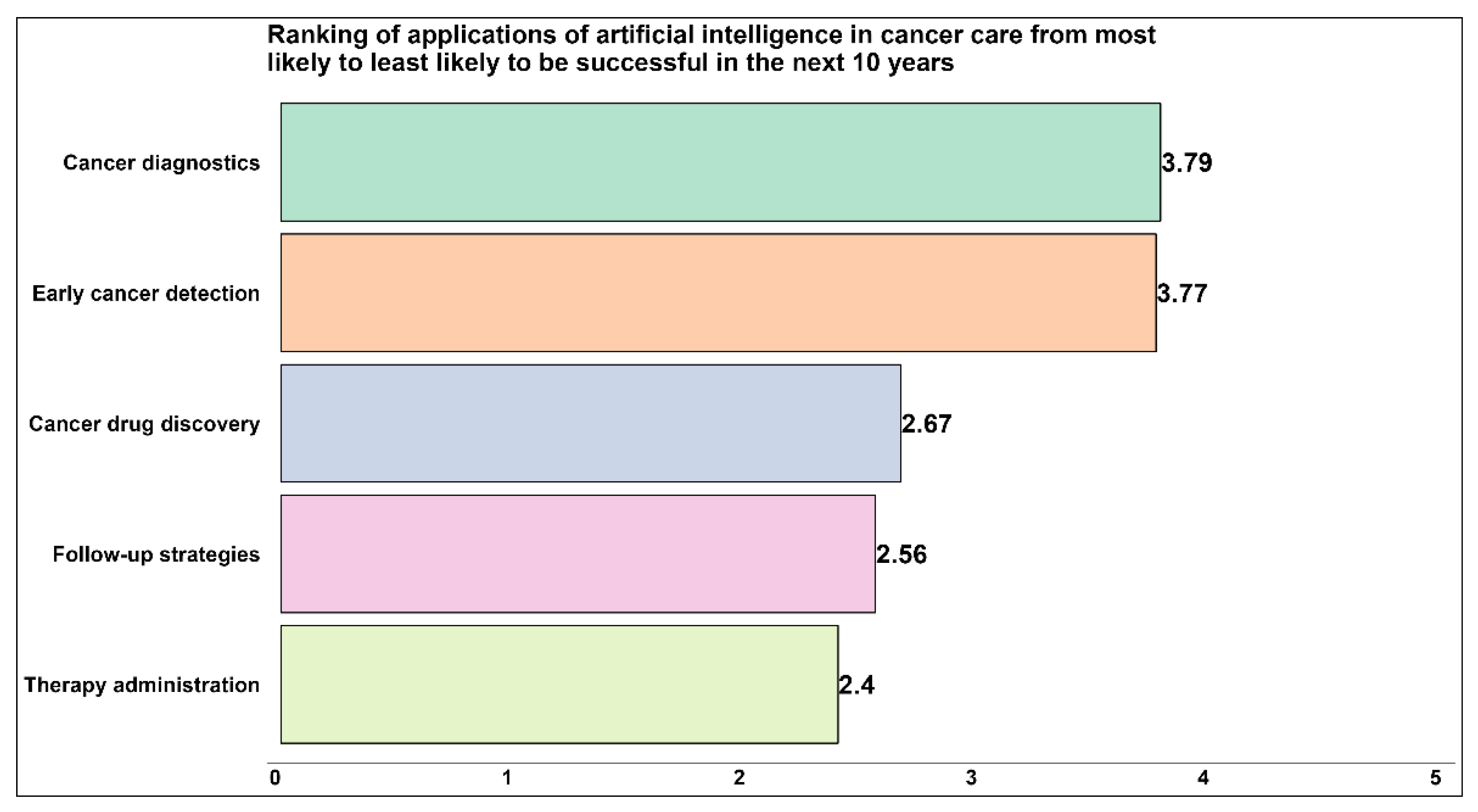
| Possible applications of artificial intelligence in cancer care | Drug discovery | [14,15] |
| Early detection | [20,48,49,50] | |
| Diagnostics | [8,13,18,19,22,24,25,33,36,42] | |
| Therapy administration | [7,15,19,34] | |
| Follow-up strategies | [6,7,10,19] | |
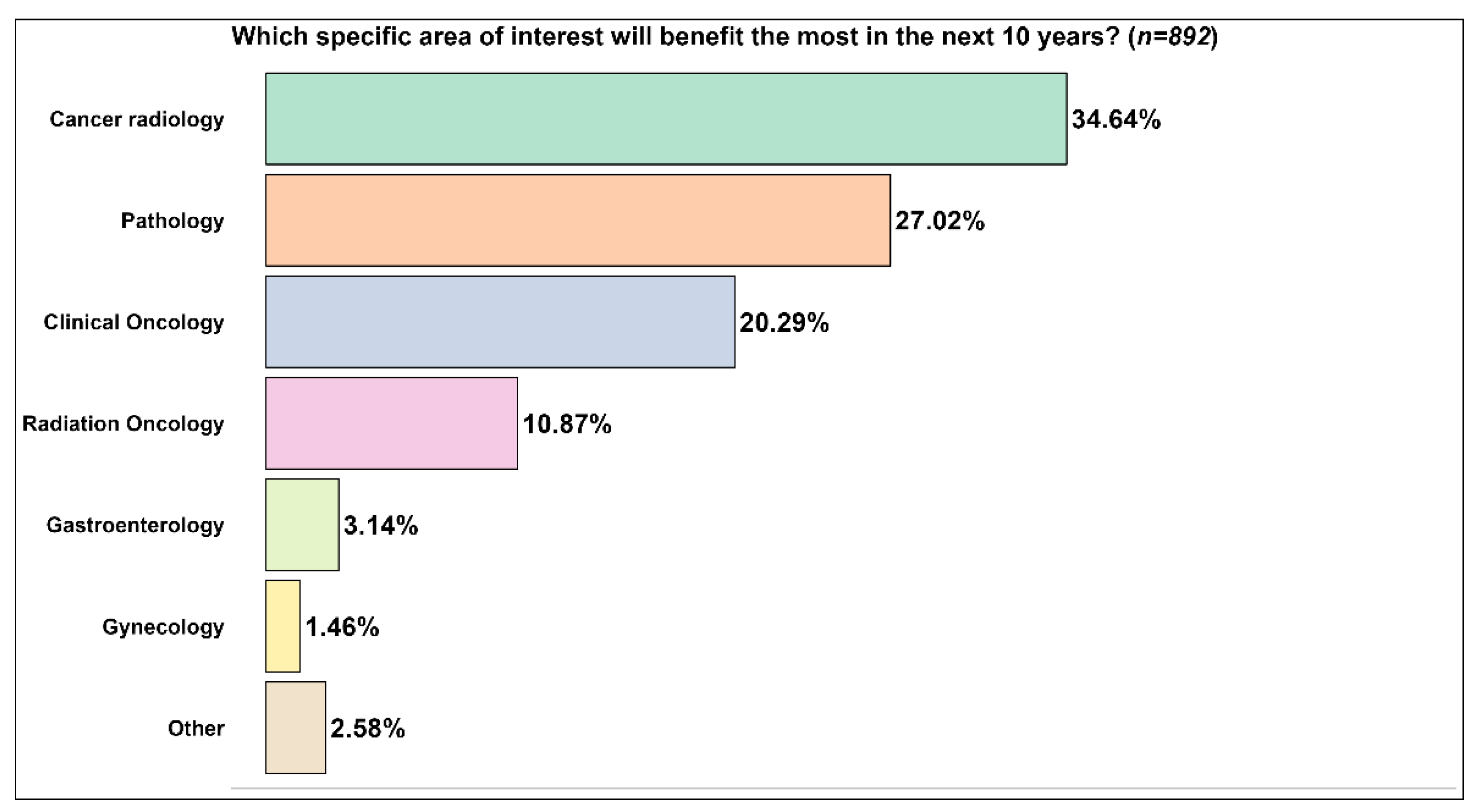
| Specific areas of interest for future developments of artificial intelligence applications in cancer care | Pathology, clinical oncology, radiation oncology, gastroenterology, gynecology | [10] |
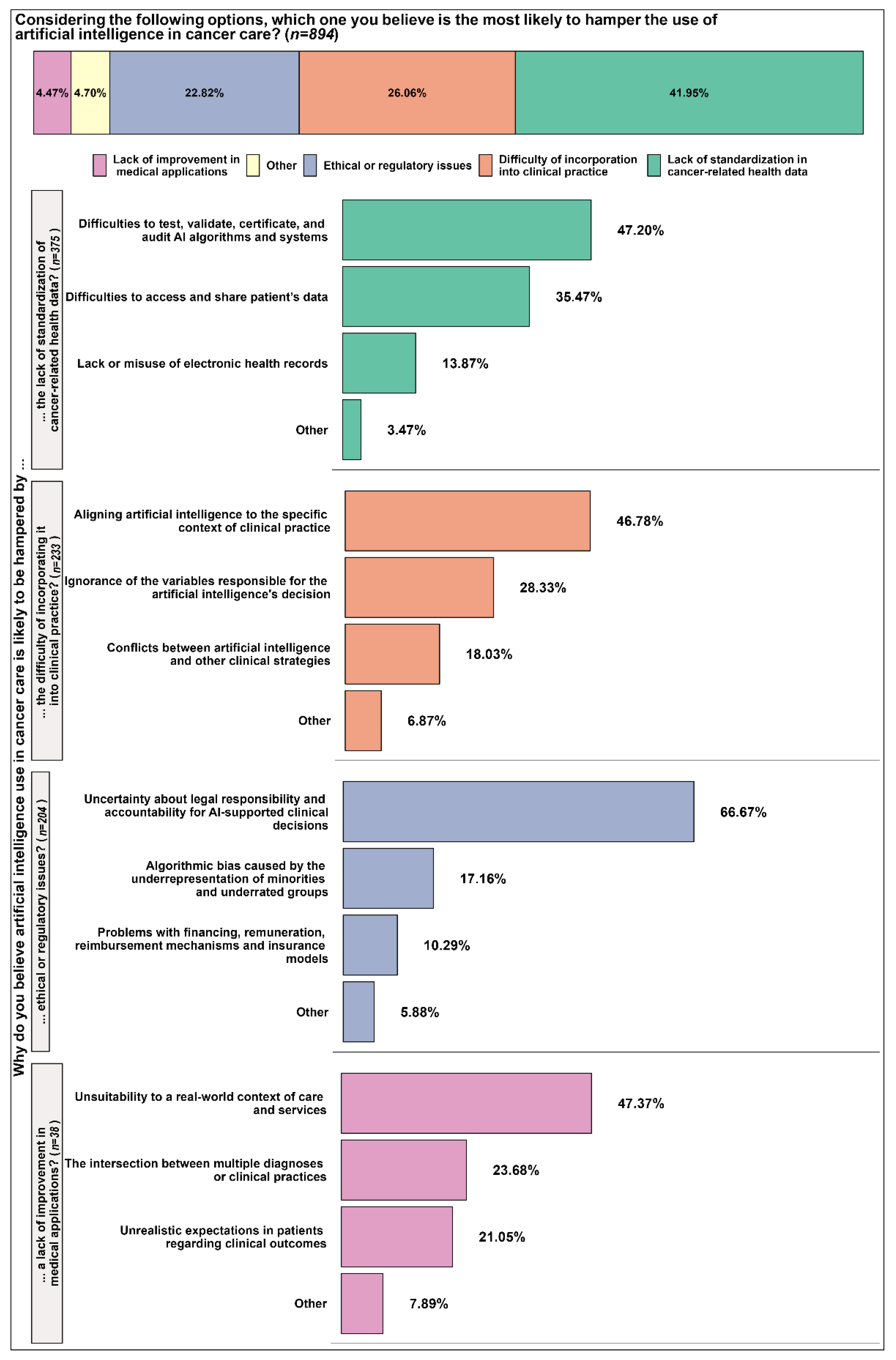
| Factors hampering artificial intelligence adoption in cancer care | The difficulty of incorporation into clinical practice | [10,16,19,26,27,30,32] |
| Ethical or regulatory issues | [10,16,26,27,28,29] | |
| Lack of improvement in medical applications | [10,16,26,27,30,31] | |
| Lack of standardization in cancer-related health data | [10,16,19,26,27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabral, B.P.; Braga, L.A.M.; Syed-Abdul, S.; Mota, F.B. Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers. Curr. Oncol. 2023, 30, 3432-3446. https://doi.org/10.3390/curroncol30030260
Cabral BP, Braga LAM, Syed-Abdul S, Mota FB. Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers. Current Oncology. 2023; 30(3):3432-3446. https://doi.org/10.3390/curroncol30030260
Chicago/Turabian StyleCabral, Bernardo Pereira, Luiza Amara Maciel Braga, Shabbir Syed-Abdul, and Fabio Batista Mota. 2023. "Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers" Current Oncology 30, no. 3: 3432-3446. https://doi.org/10.3390/curroncol30030260
APA StyleCabral, B. P., Braga, L. A. M., Syed-Abdul, S., & Mota, F. B. (2023). Future of Artificial Intelligence Applications in Cancer Care: A Global Cross-Sectional Survey of Researchers. Current Oncology, 30(3), 3432-3446. https://doi.org/10.3390/curroncol30030260






