A Nomogram for Preoperative Prediction of the Risk of Lymph Node Metastasis in Patients with Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Methods
2.1. Participants
2.2. Statistical Analysis
3. Results
3.1. Baseline Information
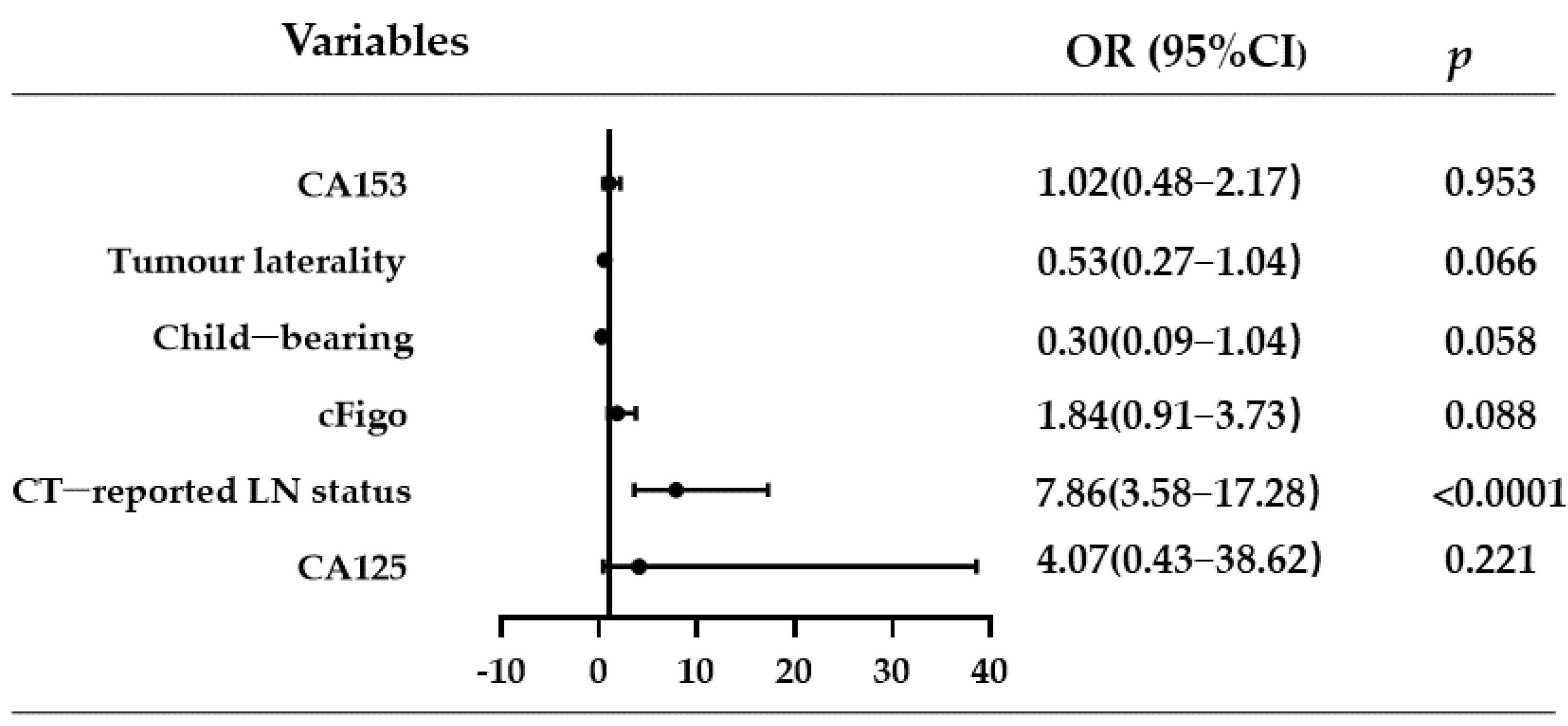
3.2. Univariate and Multivariate Analyses of LNM-Related Factors in Ovarian Cancer
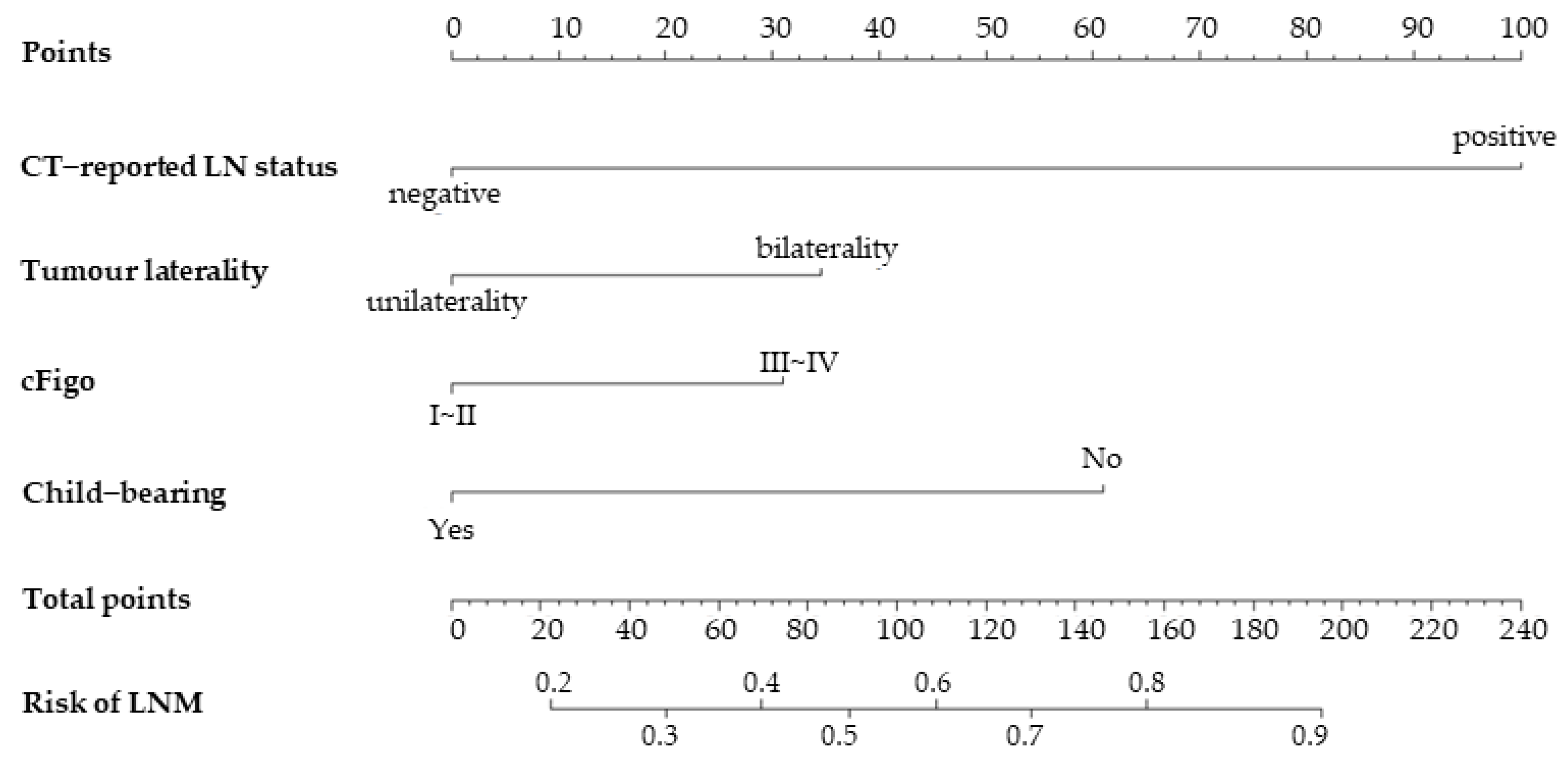
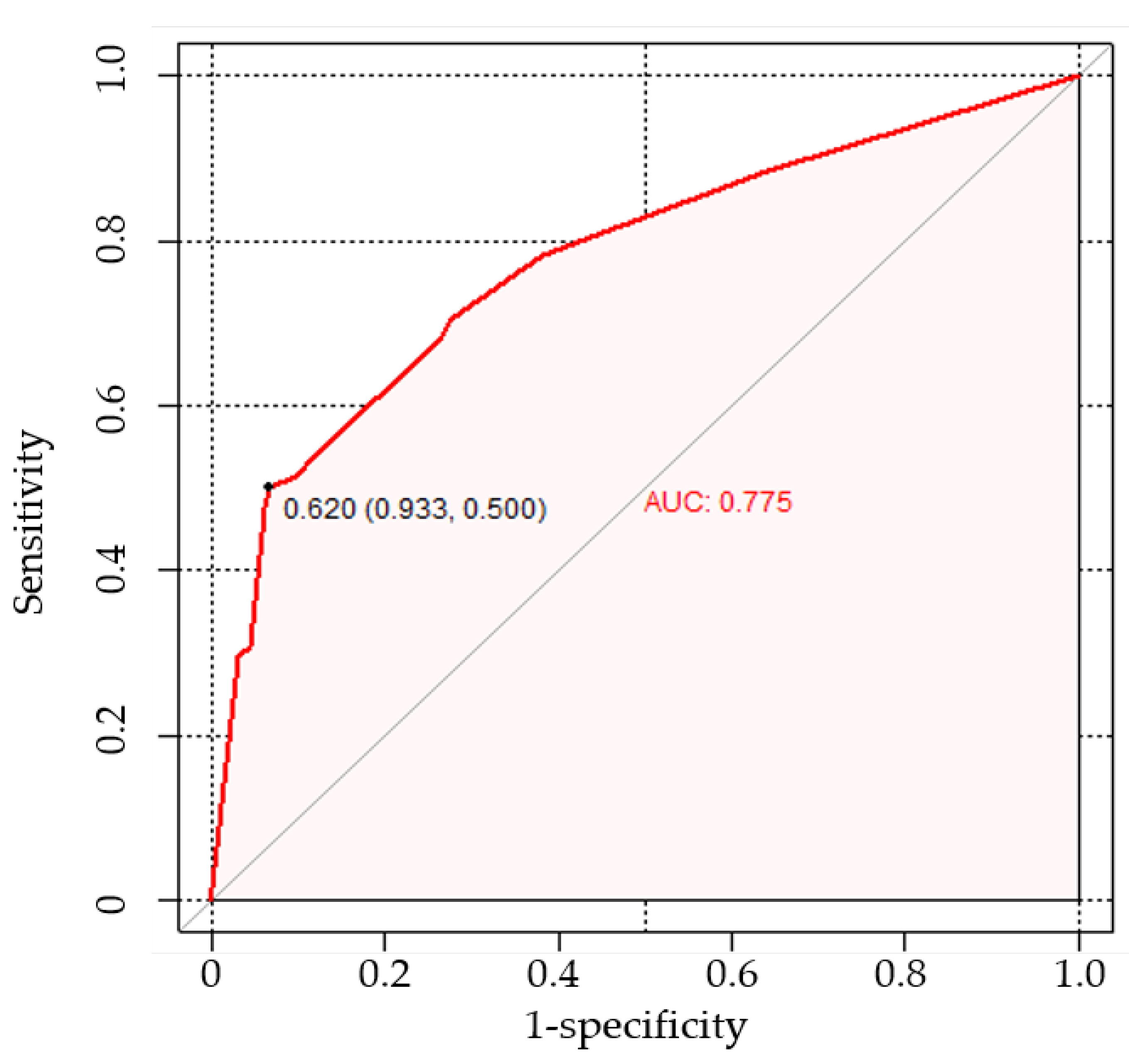
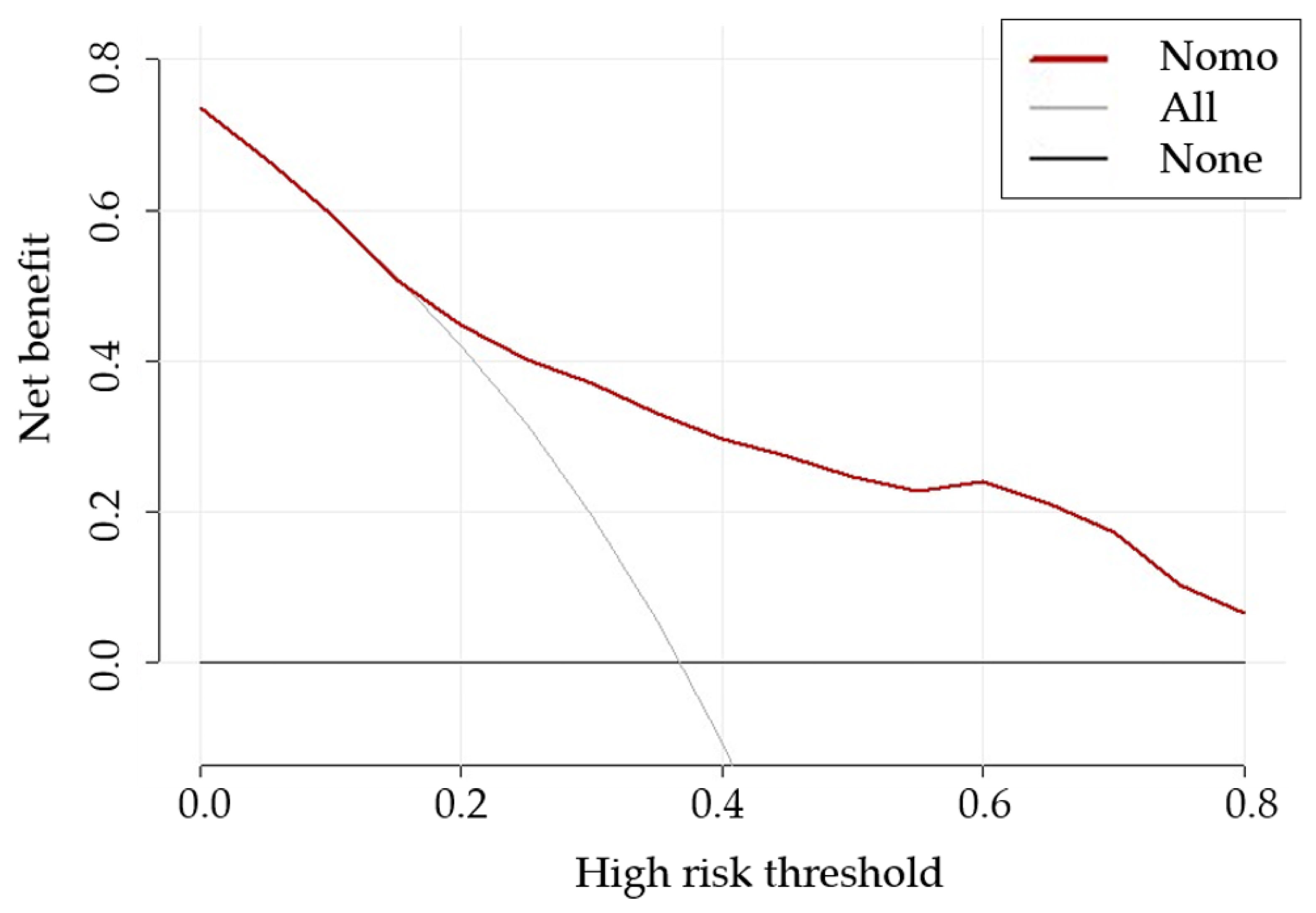
3.3. Nomogram Development and Evaluation
3.4. Incremental Predictive Value of CA125 to the Nomogram
3.5. Comparison with CT-Reported LN Status
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Prat, J. Ovarian carcinomas: Five distinct diseases with different origins, genetic alterations, and clinicopathological features. Virchows Archiv. 2012, 460, 237–249. [Google Scholar] [CrossRef]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9. [Google Scholar]
- Piver, M.S. Treatment of Ovarian Cancer at the Crossroads: −50 Years After Single-Agent Melphalan Chemotherapy. Oncology 2006, 20, 1157. [Google Scholar]
- Morice, P.; Joulie, F.; Camatte, S.; Atallah, D.; Rouzier, R.; Pautier, P.; Pomel, C.; Lhommé, C.; Duvillard, P.; Castaigne, D. Lymph node involvement in epithelial ovarian cancer: Analysis of 276 pelvic and paraaortic lymphadenectomies and surgical implications. J. Am. Coll. Surg. 2003, 197, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A randomized trial of lymphadenectomy in patients with advanced ovarian neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Kleppe, M.; Wang, T.; Van Gorp, T.; Slangen, B.; Kruse, A.J.; Kruitwagen, R. Lymph node metastasis in stages I and II ovarian cancer: A review. Gynecol. Oncol. 2011, 123, 610–614. [Google Scholar] [CrossRef]
- Prat, J.; FIGO Committee on Gynecologic Oncology. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int. J. Gynaecol. Obstet. 2014, 124, 1–5. [Google Scholar] [CrossRef]
- Harter, P.; Gnauert, K.; Hils, R.; Lehmann, T.; Fisseler-Eckhoff, A.; Traut, A.; Du Bois, A. Pattern and clinical predictors of lymph node metastases in epithelial ovarian cancer. Int. J. Gynecol. Cancer 2007, 17, 1238–1244. [Google Scholar] [CrossRef] [PubMed]
- Khiewvan, B.; Torigian, D.A.; Emamzadehfard, S.; Paydary, K.; Salavati, A.; Houshmand, S.; Werner, T.J.; Alavi, A. An update on the role of PET/CT and PET/MRI in ovarian cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Gu, Z.-X.; Tao, X.-F.; Liu, S.-Y. Computer tomography, magnetic resonance imaging, and positron emission tomography or positron emission tomography/computer tomography for detection of metastatic lymph nodes in patients with ovarian cancer: A meta-analysis. Eur. J. Radiol. 2012, 81, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Mimoun, C.; Rouzier, R.; Benifla, J.L.; Fauconnier, A.; Huchon, C. Preoperative ct or pet/ct to assess pelvic and para-aortic lymph node status in epithelial ovarian cancer? A systematic review and meta-analysis. Diagnostics 2021, 11, 1748. [Google Scholar] [CrossRef] [PubMed]
- Dauwen, H.; Van Calster, B.; Deroose, C.; de Beeck, K.O.; Amant, F.; Neven, P.; Berteloot, P.; Leunen, K.; Timmerman, D.; Vergote, I. PET/CT in the staging of patients with a pelvic mass suspicious for ovarian cancer. Gynecol. Oncol. 2013, 131, 694–700. [Google Scholar] [CrossRef]
- Fischerova, D.; Burgetova, A. Imaging techniques for the evaluation of ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 697–720. [Google Scholar] [CrossRef] [PubMed]
- Tempany, C.M.; Zou, K.H.; Silverman, S.G.; Brown, D.L.; Kurtz, A.B.; McNeil, B.J. Staging of advanced ovarian cancer: Comparison of imaging modalities—Report from the Radiological Diagnostic Oncology Group. Radiology 2000, 215, 761–767. [Google Scholar] [CrossRef]
- Widschwendter, P.; Blersch, A.; Friedl, T.W.; Janni, W.; Kloth, C.; de Gregorio, A.; de Gregorio, N. CT scan in the prediction of lymph node involvement in ovarian cancer–a retrospective analysis of a tertiary gyneco-oncological unit. Geburtshilfe Frauenheilkd. 2020, 80, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Benoit, L.; Zerbib, J.; Koual, M.; Nguyen-Xuan, H.T.; Delanoy, N.; Le Frère-Belda, M.-A.; Bentivegna, E.; Bats, A.S.; Fournier, L.; Azaïs, H. What can we learn from the 10 mm lymph node size cut-off on the CT in advanced ovarian cancer at the time of interval debulking surgery? Gynecol. Oncol. 2021, 162, 667–673. [Google Scholar] [CrossRef]
- Chen, H.-z.; Wang X-r Zhao F-m Chen X-j Li X-s Ning, G.; Guo, Y.K. The Development and Validation of a CT-Based Radiomics Nomogram to Preoperatively Predict Lymph Node Metastasis in High-Grade Serous Ovarian Cancer. Front. Oncol. 2021, 11, 3362. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Zhang, L.; Guan, Z.; Yin, Y.; Ou, C.; Qian, H.; Tang, M.; Shen, A. Predictive value of indicator of CA125 combined with D-dimer (ICD) for lymph node metastasis in patients with ovarian cancer: A two center cohort study. J. Cancer 2022, 13, 2447. [Google Scholar] [CrossRef]
- Mimoun, C.; Paoletti, X.; Gaillard, T.; Crestani, A.; Benifla, J.-L.; Mezzadri, M.; Bendifallah, S.; Touboul, C.; Bricou, A.; Dabi, Y.; et al. Using a new diagnostic tool to predict lymph node metastasis in advanced epithelial ovarian cancer leads to simple lymphadenectomy decision rules: A multicentre study from the francogyn group. PLoS ONE 2021, 16, e0258783. [Google Scholar] [CrossRef]
- Bogani, G.; Tagliabue, E.; Ditto, A.; Signorelli, M.; Martinelli, F.; Casarin, J.; Chiappa, V.; Dondi, G.; Maggiore, U.L.R.; Scaffa, C.; et al. Assessing the risk of pelvic and para-aortic nodal involvement in apparent early-stage ovarian cancer: A predictors-and nomogram-based analyses. Gynecol. Oncol. 2017, 147, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Takeshima, N.; Hirai, Y.; Umayahara, K.; Fujiwara, K.; Takizawa, K.; Hasumi, K. Lymph node metastasis in ovarian cancer: Difference between serous and non-serous primary tumors. Gynecol. Oncol. 2005, 99, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Hoogendam, J.; Vlek, C.; Witteveen, P.; Verheijen, R.; Zweemer, R. Surgical lymph node assessment in mucinous ovarian carcinoma staging: A systematic review and meta-analysis. BJOG Int. J. Obstet. Gynaecol. 2017, 124, 370–378. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino Sr, R.B.; D’Agostino Jr, R.B.; Vasan, R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008, 27, 157–172. [Google Scholar] [CrossRef]
- Pencina, M.J.; D’Agostino Sr, R.B.; Steyerberg, E.W. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat. Med. 2011, 30, 11–21. [Google Scholar] [CrossRef]
- Zhang, Z.; Rousson, V.; Lee, W.-C.; Ferdynus, C.; Chen, M.; Qian, X.; Guo, Y. Decision curve analysis: A technical note. Ann. Transl. Med. 2018, 6, 308. [Google Scholar] [CrossRef]
- Trimbos, J.B.; Vergote, I.; Bolis, G.; Vermorken, J.B.; Mangioni, C.; Madronal, C.; Franchi, M.; Tateo, S.; Zanetta, G.; Scarfone, G.; et al. Impact of adjuvant chemotherapy and surgical staging in early-stage ovarian carcinoma: European Organisation for Research and Treatment of Cancer–Adjuvant ChemoTherapy In Ovarian Neoplasm trial. J. Natl. Cancer Inst. 2003, 95, 113–125. [Google Scholar] [CrossRef]
- Trimbos, B.; Timmers, P.; Pecorelli, S.; Coens, C.; Ven, K.; Van der Burg, M.; Casado, A. Surgical staging and treatment of early ovarian cancer: Long-term analysis from a randomized trial. J. Natl. Cancer Inst. 2010, 102, 982–987. [Google Scholar] [CrossRef]
- Du Bois, A.; Reuss, A.; Harter, P.; Pujade-Lauraine, E.; Ray-Coquard, I.; Pfisterer, J. Potential role of lymphadenectomy in advanced ovarian cancer: A combined exploratory analysis of three prospectively randomized phase III multicenter trials. J. Clin. Oncol. 2010, 28, 1733–1739. [Google Scholar] [CrossRef] [PubMed]
- Paik, E.S.; Shim, M.; Choi, H.J.; Lee, Y.-Y.; Kim, T.-J.; Lee, J.-W.; Kim, B.-G.; Bae, D.-S.; Choi, C.H. Impact of lymphadenectomy on survival after recurrence in patients with advanced ovarian cancer without suspected lymph node metastasis. Gynecol. Oncol. 2016, 143, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Aletti, G.D.; Dowdy, S.; Podratz, K.C.; Cliby, W.A. Role of lymphadenectomy in the management of grossly apparent advanced stage epithelial ovarian cancer. Am. J. Obstet. Gynecol. 2006, 195, 1862–1868. [Google Scholar] [CrossRef]
- Ercelep, O.; Ozcelik, M.; Gumus, M. Association of lymphadenectomy and survival in epithelial ovarian cancer. Curr. Probl. Cancer 2019, 43, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Maggioni, A.; Panici, P.B.; Dell’Anna, T.; Landoni, F.; Lissoni, A.; Pellegrino, A.; Rossi, R.S.; Chiari, S.; Campagnutta, E.; Greggi, S.; et al. Randomised study of systematic lymphadenectomy in patients with epithelial ovarian cancer macroscopically confined to the pelvis. Br. J. Cancer 2006, 95, 699–704. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.; Lang, J. Survival analysis of lymph node resection in ovarian cancer: A population-based study. Front. Oncol. 2020, 10, 355. [Google Scholar] [CrossRef]
- Deng, T.; Huang, Q.; Wan, T.; Luo, X.; Feng, Y.; Huang, H.; Liu, J. The impact of lymph node dissection on survival in patients with clinical early-stage ovarian cancer. J. Gynecol. Oncol. 2021, 32, e40. [Google Scholar] [CrossRef]
- Armstrong, D.K.; Alvarez, R.D.; Bakkum-Gamez, J.N.; Barroilhet, L.; Behbakht, K.; Berchuck, A.; Berek, J.S.; Chen, L.M.; Cristea, M.; DeRosa, M.; et al. NCCN guidelines insights: Ovarian cancer, version 1.2019: Featured updates to the NCCN guidelines. J. Natl. Compr. Cancer Netw. 2019, 17, 896–909. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; Bois, A.; Ledermann, J.; McCluggage, W.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef]
- Sudolmuş, S.; Köroğlu, N.; Yıldırım, G.; Ülker, V.; Gülkılık, A.; Dansuk, R. Can CA-125 predict lymph node metastasis in epithelial ovarian cancers in Turkish population? Dis. Markers 2014, 2014, 492537. [Google Scholar] [CrossRef]
- Bachmann, C.; Kraemer, B.; Brucker, S.Y.; Staebler, A.; Fend, F.; Wallwiener, D.; Grischke, E.M.; Rothmund, R. Relevance of pelvic and para-aortic node metastases in early-stage ovarian cancer. Anticancer Res. 2014, 34, 6735–6738. [Google Scholar] [PubMed]
- Powless, C.A.; Aletti, G.D.; Bakkum-Gamez, J.N.; Cliby, W.A. Risk factors for lymph node metastasis in apparent early-stage epithelial ovarian cancer: Implications for surgical staging. Gynecol. Oncol. 2011, 122, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Park, N.H.; Chung, H.H.; Kim, J.W.; Song, Y.S.; Kang, S.B. Significance of preoperative serum CA-125 levels in the prediction of lymph node metastasis in epithelial ovarian cancer. Acta Obstet. Gynecol. Scand. 2008, 87, 1136–1142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Sun, J.-Y.; Wu, S.-G.; Wang, X.; He, Z.-Y.; Chen, Q.-H.; Li, F.-Y. Risk factors for lymph node metastasis in ovarian cancer: Implications for systematic lymphadenectomy. Int. J. Surg. 2016, 29, 123–127. [Google Scholar] [CrossRef]
- Pereira, A.; Magrina, J.F.; Rey, V.; Cortes, M.; Magtibay, P.M. Pelvic and aortic lymph node metastasis in epithelial ovarian cancer. Gynecol. Oncol. 2007, 105, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Ditto, A.; Martinelli, F.; Reato, C.; Kusamura, S.; Solima, E.; Fontanelli, R.; Haeusler, E.; Raspagliesi, F. Systematic para-aortic and pelvic lymphadenectomy in early stage epithelial ovarian cancer: A prospective study. Ann. Surg. Oncol. 2012, 19, 3849–3855. [Google Scholar] [CrossRef]
- Haller, H.; Mamula, O.; Krasevic, M.; Rupcic, S.; Fischer, A.B.; Eminovic, S.; Manestar, M.; Perovic, D. Frequency and distribution of lymph node metastases in epithelial ovarian cancer: Significance of serous histology. Int. J. Gynecol. Cancer 2011, 21. [Google Scholar] [CrossRef]
- Poole, E.M.; Merritt, M.A.; Jordan, S.J.; Yang, H.P.; Hankinson, S.E.; Park, Y.; Rosner, B.; Webb, P.M.; Cramer, D.W.; Wentzensen, N.; et al. Hormonal and reproductive risk factors for epithelial ovarian cancer by tumor aggressiveness. Cancer Epidemiol. Biomark. Prev. 2013, 22, 429–437. [Google Scholar] [CrossRef]
- Stewart, L.M.; Spilsbury, K.; Jordan, S.; Stewart, C.; Holman, C.A.J.; Powell, A.; Reekie, J.; Cohen, P. Risk of high-grade serous ovarian cancer associated with pelvic inflammatory disease, parity and breast cancer. Cancer Epidemiol. 2018, 55, 110–116. [Google Scholar] [CrossRef]
- Poole, E.M.; Konstantinopoulos, P.A.; Terry, K.L. Prognostic implications of reproductive and lifestyle factors in ovarian cancer. Gynecol. Oncol. 2016, 142, 574–587. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, A.; Gultekin, M.; Dursun, P.; Dogan, N.U.; Aksan, G.; Guven, S.; Velipasaoglu, M.; Yuce, K. Metastatic lymph node number in epithelial ovarian carcinoma: Does it have any clinical significance? Gynecol. Oncol. 2008, 108, 428–432. [Google Scholar] [CrossRef] [PubMed]

| All (n = 212) | LNM Negative (n = 134) | LNM Positive (n = 78) | |
|---|---|---|---|
| Age | |||
| Mean ± SD | 50.8 (9.4) | 50.9 (9.3) | 50.7 (9.7) |
| Range | 27–75 | 27–75 | 28–75 |
| Child-bearing | |||
| No | 13 (6.1) | 6 (4.5) | 7 (9.0) |
| Yes | 199 (93.9) | 128 (95.5) | 71 (91.0) |
| Histological type | |||
| High-grade serous cancer | 124 (58.5) | 69 (51.5) | 55 (70.5) |
| Clear cell carcinoma | 34 (16.0) | 25 (18.7) | 9 (11.5) |
| Endometrial cancer | 25 (11.8) | 23 (17.2) | 2 (2.6) |
| Low-grade serous cancer | 9 (4.2) | 5 (3.7) | 4 (5.1) |
| Mixed carcinoma | 5 (2.4) | 3 (2.2) | 2 (2.6) |
| Other | 15 (7.1) | 9 (6.7) | 6 (7.7) |
| Pelvic LNM | |||
| Negative | 157 (74.1) | 134 (100) | 23 (29.5) |
| Positive | 55 (25.9) | 0 (0.0) | 55 (70.5) |
| Para-aortic LNM | |||
| Negative | 145 (68.4) | 134 (100) | 11 (14.1) |
| Positive | 67 (31.6) | 0 (0.0) | 67 (85.9) |
| Peritoneal washings | |||
| Negative | 94 (44.3) | 66 (49.3) | 28 (35.9) |
| Positive | 101 (47.6) | 60 (44.8) | 41 (52.6) |
| Unknown | 17 (8. 2) | 8 (5.9) | 9 (11.5) |
| CT-reported LN status | |||
| Negative | 162 (76.4) | 122 (91.0) | 40 (51.3) |
| Positive | 50 (23.6) | 12 (9.0) | 38 (48.7) |
| cFigo | |||
| I–II | 89 (42.0) | 70 (52.2) | 19 (24.4) |
| III–IV | 123 (58.0) | 64 (47.8) | 59 (75.6) |
| Laterality | |||
| Unilaterality | 122 (57.5) | 87 (64.9) | 35 (44.9) |
| Bilaterality | 90 (42.5) | 47 (35.1) | 43 (55.1) |
| Ascites | |||
| Negative | 87 (41.0) | 55 (41.0) | 32 (41.0) |
| Positive | 125 (59.0) | 79 (59.0) | 46 (59.0) |
| CA125 | |||
| <35 U/mL | 13 (6.1) | 12 (9.0) | 1 (1.3) |
| ≥35 U/mL | 199 (93.9) | 122 (91.0) | 77 (98.7) |
| CA153 | |||
| <25 U/mL | 73 (34.4) | 51 (38.1) | 22 (28.2) |
| ≥25 U/mL | 139 (65.6) | 83 (61.9) | 56 (71.8) |
| CA199 | |||
| <35 U/mL | 135 (63.7) | 86 (64.2) | 49 (62.8) |
| ≥35 U/mL | 77 (36.3) | 48 (35.8) | 29 (37.2) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Age | 1.00 | (0.97–1.03) | 0.905 | |||
| Child-bearing | 0.48 | (0.15–1.47) | 0.197 | 0.30 | (0.09–1.04) | 0.058 |
| Ascites | 1.00 | (0.57–1.77) | 0.998 | |||
| Tumour laterality | 2.89 | (1.62–5.16) | <0.0001 | 0.53 | (0.27–1.04) | 0.066 |
| cFigo | 3.4 | (1.83–6.30) | <0.0001 | 1.84 | (0.91–3.73) | 0.088 |
| CT-reported LN status | 9.66 | (4.61–20.26) | <0.0001 | 7.86 | (3.58–17.28) | <0.0001 |
| CA125 | 7.57 | (0.97–59.42) | 0.054 | 4.07 | (0.43–38.62) | 0.221 |
| CA153 | 1.56 | (0.86–2.86) | 0.147 | 1.02 | (0.48–2.17) | 0.953 |
| CA199 | 1.06 | (0.59–1.89) | 0.843 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, H.; Yang, F.; Zheng, X.; Pan, B.; Ju, M.; Xu, S.; Zheng, M. A Nomogram for Preoperative Prediction of the Risk of Lymph Node Metastasis in Patients with Epithelial Ovarian Cancer. Curr. Oncol. 2023, 30, 3289-3300. https://doi.org/10.3390/curroncol30030250
Xiang H, Yang F, Zheng X, Pan B, Ju M, Xu S, Zheng M. A Nomogram for Preoperative Prediction of the Risk of Lymph Node Metastasis in Patients with Epithelial Ovarian Cancer. Current Oncology. 2023; 30(3):3289-3300. https://doi.org/10.3390/curroncol30030250
Chicago/Turabian StyleXiang, Huiling, Fan Yang, Xiaojing Zheng, Baoyue Pan, Mingxiu Ju, Shijie Xu, and Min Zheng. 2023. "A Nomogram for Preoperative Prediction of the Risk of Lymph Node Metastasis in Patients with Epithelial Ovarian Cancer" Current Oncology 30, no. 3: 3289-3300. https://doi.org/10.3390/curroncol30030250
APA StyleXiang, H., Yang, F., Zheng, X., Pan, B., Ju, M., Xu, S., & Zheng, M. (2023). A Nomogram for Preoperative Prediction of the Risk of Lymph Node Metastasis in Patients with Epithelial Ovarian Cancer. Current Oncology, 30(3), 3289-3300. https://doi.org/10.3390/curroncol30030250




