Health Care Costs Attributable to Prostate Cancer in British Columbia, Canada: A Population-Based Cohort Study
Abstract
1. Introduction
2. Materials & Methods
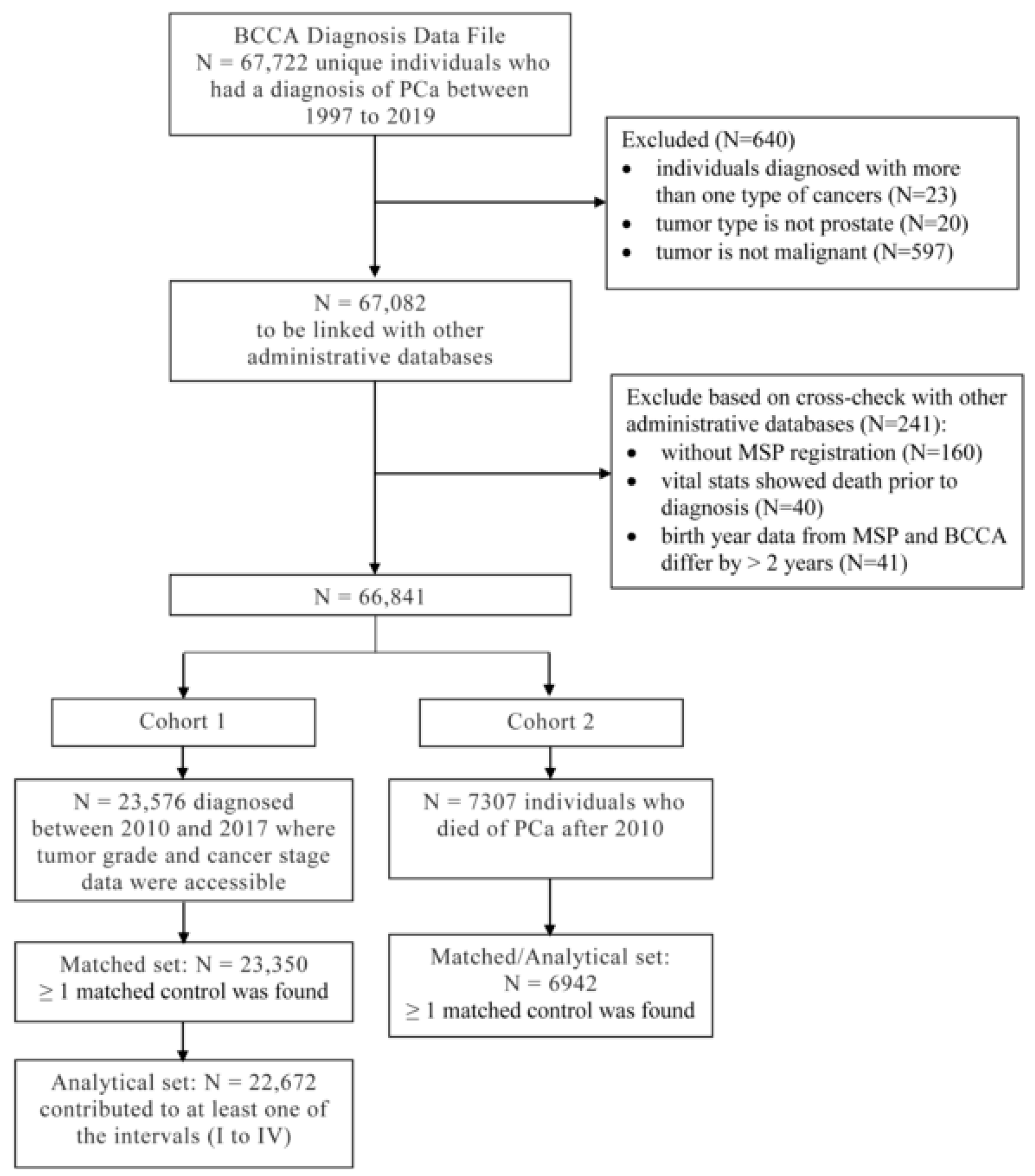
2.1. Data Source, Patient Cohorts, and Matched Controls
2.2. Outcome Measures
2.3. Grouping by Cancer Stage, Tumor Grade, and Treatment
2.4. Statistical Analysis
3. Results
3.1. Costs by Intervals
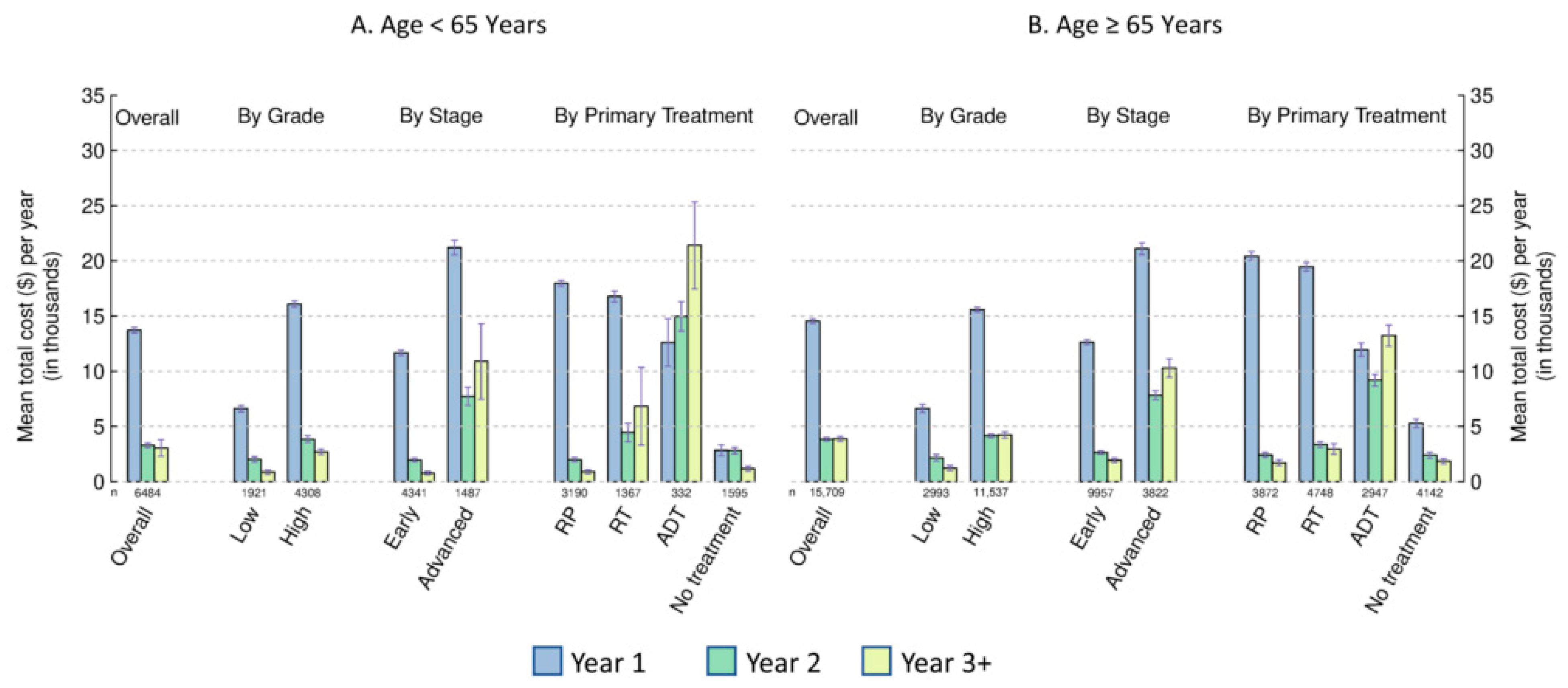
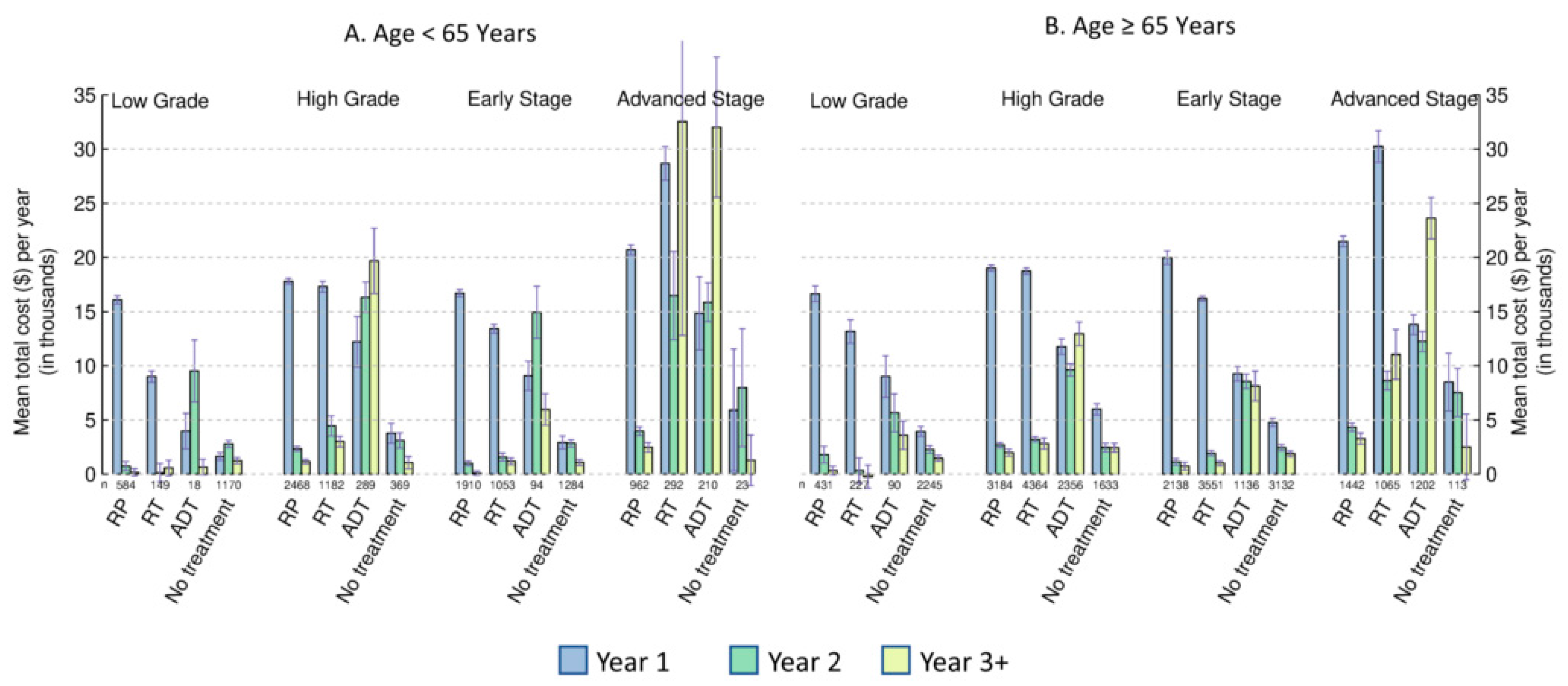
3.2. Costs by Age, Cancer Stage, and Tumor Grade
3.3. Costs by Primary Treatment
3.4. Subgroup and Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Canadian Cancer Society. Prostate Cancer Statistics. Available online: https://cancer.ca/en/cancer-information/cancer-types/prostate/statistics (accessed on 7 July 2022).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer Launches Lung Screening Program. Available online: http://www.bccancer.bc.ca/about/news-stories/stories/bc-cancer-launches-lung-screening-program (accessed on 7 July 2022).
- Canadian Cancer Society. How Do I Find a Colorectal Cancer Screening Program. Available online: https://cancer.ca/en/cancer-information/find-cancer-early/get-screened-for-colorectal-cancer/how-do-i-find-a-colorectal-cancer-screening-program (accessed on 7 July 2022).
- Lee, S. How Do I Find a Cervical Cancer Screening Program. Available online: https://cancer.ca/en/cancer-information/find-cancer-early/get-screened-for-cervical-cancer/how-do-i-find-a-cervical-cancer-screening-program (accessed on 8 November 2022).
- Canadian Cancer Society. How Do I Find a Breast Cancer Screening Program. Available online: https://cancer.ca/en/cancer-information/find-cancer-early/get-screened-for-breast-cancer/how-do-i-find-a-breast-cancer-screening-program (accessed on 7 July 2022).
- Canadian Cancer Society. Prostate-Specific Antigen (PSA) Test. Available online: https://cancer.ca/en/treatments/tests-and-procedures/prostate-specific-antigen-psa-test (accessed on 7 July 2022).
- Liu, J.; Dong, B.; Qu, W.; Wang, J.; Xu, Y.; Yu, S.; Zhang, X. Using Clinical Parameters to Predict Prostate Cancer and Reduce the Unnecessary Biopsy among Patients with PSA in the Gray Zone. Sci. Rep. 2020, 10, 5157. [Google Scholar] [CrossRef] [PubMed]
- Pataky, R.; Gulati, R.; Etzioni, R.; Black, P.; Chi, K.N.; Coldman, A.J.; Pickles, T.; Tyldesley, S.; Peacock, S. Is Prostate Cancer Screening Cost-Effective? A Microsimulation Model of Prostate-Specific Antigen-Based Screening for British Columbia, Canada. Int. J. Cancer 2014, 135, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Bill-Axelson, A.; Holmberg, L.; Garmo, H.; Taari, K.; Busch, C.; Nordling, S.; Häggman, M.; Andersson, S.-O.; Andrén, O.; Steineck, G.; et al. Radical Prostatectomy or Watchful Waiting in Prostate Cancer—29-Year Follow-Up. N. Engl. J. Med. 2018, 379, 2319–2329. [Google Scholar] [CrossRef] [PubMed]
- Hamdy, F.C.; Donovan, J.L.; Lane, J.A.; Mason, M.; Metcalfe, C.; Holding, P.; Davis, M.; Peters, T.J.; Turner, E.L.; Martin, R.M.; et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N. Engl. J. Med. 2016, 375, 1415–1424. [Google Scholar] [CrossRef]
- Wilt, T.J.; Jones, K.M.; Barry, M.J.; Andriole, G.L.; Culkin, D.; Wheeler, T.; Aronson, W.J.; Brawer, M.K. Follow-up of Prostatectomy versus Observation for Early Prostate Cancer. N. Engl. J. Med. 2017, 377, 132–142. [Google Scholar] [CrossRef]
- Afferi, L.; Zamboni, S.; Baumeister, P.; Mordasini, L.; Mattei, A.; Moschini, M. Prostatectomy versus Watchful Waiting in Patients with Localized Prostate Cancer: The Survival Benefit Can Be Spotted in the Long Run. AME Med. J. 2019, 4, 22. [Google Scholar] [CrossRef]
- Sanghera, S.; Coast, J.; Martin, R.M.; Donovan, J.L.; Mohiuddin, S. Cost-Effectiveness of Prostate Cancer Screening: A Systematic Review of Decision-Analytical Models. BMC Cancer 2018, 18, 84. [Google Scholar] [CrossRef]
- Garg, V.; Gu, N.Y.; Borrego, M.E.; Raisch, D.W. A Literature Review of Cost-Effectiveness Analyses of Prostate-Specific Antigen Test in Prostate Cancer Screening. Expert Rev. Pharm. Outcomes Res. 2013, 13, 327–342. [Google Scholar] [CrossRef]
- Govers, T.M.; Hessels, D.; Vlaeminck-Guillem, V.; Schmitz-Dräger, B.J.; Stief, C.G.; Martinez-Ballesteros, C.; Ferro, M.; Borque-Fernando, A.; Rubio-Briones, J.; Sedelaar, J.P.M.; et al. Cost-Effectiveness of SelectMDx for Prostate Cancer in Four European Countries: A Comparative Modeling Study. Prostate Cancer Prostatic. Dis. 2019, 22, 101–109. [Google Scholar] [CrossRef]
- Beca, J.; Majeed, H.; Chan, K.K.W.; Hotte, S.J.; Loblaw, A.; Hoch, J.S. Cost-Effectiveness of Docetaxel in High-Volume Hormone-Sensitive Metastatic Prostate Cancer. Can. Urol. Assoc. J. 2019, 12, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Abreha, S.K. Model-Based Cost-Effectiveness Analysis of External Beam Radiation Therapy for the Treatment of Localized Prostate Cancer: A Systematic Review. Cost Eff. Resour. Alloc. 2019, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G.; Black, L.K. The Economic Burden of Prostate Cancer. BJU Int. 2011, 108, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Krahn, M.D.; Zagorski, B.; Laporte, A.; Alibhai, S.M.H.; Bremner, K.E.; Tomlinson, G.; Warde, P.; Naglie, G. Healthcare Costs Associated with Prostate Cancer: Estimates from a Population-Based Study. BJU Int. 2010, 105, 338–346. [Google Scholar] [CrossRef]
- Krahn, M.D.; Bremner, K.E.; Zagorski, B.; Alibhai, S.M.H.; Chen, W.; Tomlinson, G.; Mitsakakis, N.; Naglie, G. Health Care Costs for State Transition Models in Prostate Cancer. Med. Decis. Mak. 2014, 34, 366–378. [Google Scholar] [CrossRef]
- Magnani, C.J.; Bievre, N.; Baker, L.C.; Brooks, J.D.; Blayney, D.W.; Hernandez-Boussard, T. Real-World Evidence to Estimate Prostate Cancer Costs for First-Line Treatment or Active Surveillance. Eur. Urol. Open Sci. 2021, 23, 20–29. [Google Scholar] [CrossRef]
- Tang, C.; Lei, X.; Smith, G.L.; Pan, H.Y.; Hess, K.; Chen, A.; Hoffman, K.E.; Chapin, B.F.; Kuban, D.A.; Anscher, M.; et al. Costs and Complications After a Diagnosis of Prostate Cancer Treated With Time-Efficient Modalities: An Analysis of National Medicare Data. Pract. Radiat. Oncol. 2020, 10, 282–292. [Google Scholar] [CrossRef]
- Trogdon, J.G.; Falchook, A.D.; Basak, R.; Carpenter, W.R.; Chen, R.C. Total Medicare Costs Associated With Diagnosis and Treatment of Prostate Cancer in Elderly Men. JAMA Oncol. 2019, 5, 60–66. [Google Scholar] [CrossRef]
- Garbens, A.; Wallis, C.J.D.; Matta, R.; Kodama, R.; Herschorn, S.; Narod, S.; Nam, R.K. The Cost of Treatment and Its Related Complications for Men Who Receive Surgery or Radiation Therapy for Prostate Cancer. Can. Urol. Assoc. J. 2018, 13, E236–E248. [Google Scholar] [CrossRef]
- BC Cancer. BC Cancer Registry Data (2021). Data Extract. BC Cancer (2021). Available online: http://www.bccancer.bc.ca/health-professionals/professional-resources/bc-cancer-registry/request-registry-data (accessed on 28 September 2021).
- Population Data BC. BC Cancer Registry Data (2021). V2. Data Extract. BC Cancer (2021). Available online: https://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Population Data BC. British Columbia Ministry of Health [Creator] (2021): Medical Services Plan (MSP) Payment Information File. V2. Data Extract. MOH (2021). Available online: http://www.popdata.bc.ca/data (accessed on 28 September 2021).
- BC Ministry of Health. BC Ministry of Health [Creator] (2021): PharmaNet. V2. Data Extract. Data Stewardship Committee (2021). Available online: https://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Population Data BC. Canadian Institute for Health Information [Creator] (2021): Discharge Abstract Database (Hospital Separations). V2. Data Extract. MOH (2021). Available online: https://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Population Data BC. Canadian Institute for Health Information [Creator] (2021): National Ambulatory Care Reporting System. V2. Data Extract. MOH (2021). Available online: https://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Population Data BC. British Columbia Ministry of Health [Creator] (2021): Vital Events Deaths. Data Extract. MOH (2021). Available online: https://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Population Data BC. British Columbia Ministry of Health [Creator] (2021): Consolidation File (MSP Registration & Premium Billing). V2. Data Extract. MOH (2021). Available online: http://www.popdata.bc.ca/data (accessed on 28 September 2021).
- Yabroff, K.R.; Warren, J.L.; Brown, M.L. Costs of Cancer Care in the USA: A Descriptive Review. Nat. Rev. Clin. Oncol. 2007, 4, 643–656. [Google Scholar] [CrossRef]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef] [PubMed]
- CIHI. Cost of a Standard Hospital Stay. Available online: https://yourhealthsystem.cihi.ca/hsp/inbrief%3Bjsessionid=TI0N-pfyq6TvKmMp4qn+pXIw.yhs?lang=en#!/indicators/015/cost-of-a-standard-hospital-stay/;mapC1;mapLevel2;/ (accessed on 6 February 2018).
- Peterson, S.; Wickham, M.; Lavergne, R.; Beaumier, J.; Ahuja, M.; Mooney, D.; McGrail, K. Methods to Comprehensively Identify Emergency Department Visits Using Administrative Data in British Columbia; UBC Centre for Health Services and Policy Research: Vancouver, BC, Canada, 2021. [Google Scholar]
- Health Sector Information, Analysis and Reporting (HSIAR) Division, British Columbia Ministry of Health. The Health System Matrix Version “10.1:” Understanding the Health Care Needs of the Population of British Columbia; British Columbia Ministry of Health: Victoria, BC, Canada, 2020.
- Yong, J.H.E.; McGowan, T.; Redmond-Misner, R.; Beca, J.; Warde, P.; Gutierrez, E.; Hoch, J.S. Estimating the Costs of Intensity-Modulated and 3-Dimensional Conformal Radiotherapy in Ontario. Curr. Oncol. 2016, 23, e228–e238. [Google Scholar] [CrossRef]
- Canadian Institute for Health Information. National Prescription Drug Utilization Information System (NPDUIS) Metadata. Available online: https://www.cihi.ca/en/national-prescription-drug-utilization-information-system-metadata (accessed on 30 January 2018).
- CADTH. Pan-Canadian Oncology Drug Review Final Economic Guidance Report—Enzalutamide (Xtandi) for Metastatic Castration-Resistant Prostate Cancer. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr-xtandi-mcrpc-fn-egr.pdf (accessed on 19 October 2022).
- CADTH. Pan-Canadian Oncology Drug Review Initial Economic Guidance Report—Pembrolizumab (Keytruda) for Metastatic Urothelial Carcinoma. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_pembrolizumab_keytruda_muc_in_egr.pdf (accessed on 19 October 2022).
- Ministry of Health. Negotiated Agreements with the Doctors of BC—Province of British Columbia. Available online: https://www2.gov.bc.ca/gov/content/health/practitioner-professional-resources/msp/negotiated-agreements-with-the-doctors-of-bc (accessed on 8 November 2022).
- Carrière, G.; Sanmartin, C.; Murison, P.; Trudeau, R.; Trainor, C.; Pelletier, C.; Farrar, N.; Snow, A.; Bryan, S.; Newman, K. Canadian Cancer Treatment Linkage Project; Catalogue No. 11-633-X—No. 016; Statistics Canada: Ottawa, ON, Canada, 2018.
- Statistics Canada. Consumer Price Index, Annual Average, Not Seasonally Adjusted. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=1810000501 (accessed on 7 July 2022).
- De Oliveira, C.; Pataky, R.; Bremner, K.E.; Rangrej, J.; Chan, K.K.W.; Cheung, W.Y.; Hoch, J.S.; Peacock, S.; Krahn, M.D. Estimating the Cost of Cancer Care in British Columbia and Ontario: A Canadian Inter-Provincial Comparison. Healthc. Policy 2017, 12, 95–108. [Google Scholar] [CrossRef] [PubMed]
- Alternative Payments. Available online: https://www.doctorsofbc.ca/advocacy-policy/negotiations/agreements-contracts/alternative-payments (accessed on 8 July 2022).
- El-Adam, S.; Ahuja, M.; Lavergne, R.; Peterson, S.; McGrail, K. Alternative Payment Plan Remuneration Trends in British Columbia; UBC Centre for Health Services and Policy Research: Vancouver, BC, Canada, 2020. [Google Scholar]
- Population Data BC. Home and Community Care Data Set. Available online: https://www.popdata.bc.ca/data/health/hcc (accessed on 8 July 2022).
- Timilshina, N.; Ouellet, V.; Alibhai, S.M.H.; Mes-Masson, A.-M.; Delvoye, N.; Drachenberg, D.; Finelli, A.; Jammal, M.-P.; Karakiewicz, P.; Lapointe, H.; et al. Analysis of Active Surveillance Uptake for Low-Risk Localized Prostate Cancer in Canada: A Canadian Multi-Institutional Study. World J. Urol. 2017, 35, 595–603. [Google Scholar] [CrossRef] [PubMed]
- BC Cancer. Prostate Cancer Managment Manual. Available online: http://www.bccancer.bc.ca/health-professionals/clinical-resources/cancer-management-manual/genitourinary/prostate#High-Ris (accessed on 9 November 2022).
- Ahmadi, H.; Daneshmand, S. Androgen Deprivation Therapy: Evidence-Based Management of Side Effects. BJU Int. 2013, 111, 543–548. [Google Scholar] [CrossRef] [PubMed]
- Higano, C.S. Side Effects of Androgen Deprivation Therapy: Monitoring and Minimizing Toxicity. Urology 2003, 61 (Suppl. S2), 32–38. [Google Scholar] [CrossRef]
- Eastham, J.A.; Auffenberg, G.B.; Barocas, D.A.; Chou, R.; Crispino, T.; Davis, J.W.; Eggener, S.; Horwitz, E.M.; Kane, C.J.; Kirkby, E.; et al. Clinically Localized Prostate Cancer: AUA/ASTRO Guideline, Part I: Introduction, Risk Assessment, Staging, and Risk-Based Management. J. Urol. 2022, 208, 10–18. [Google Scholar] [CrossRef]
- Parker, C.; Castro, E.; Fizazi, K.; Heidenreich, A.; Ost, P.; Procopio, G.; Tombal, B.; Gillessen, S. Prostate Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2020, 31, 1119–1134. [Google Scholar] [CrossRef]
| Variable | Age < 65 (N = 6518) | Age ≥ 65 (N = 16,154) |
|---|---|---|
| Age at diagnosis | ||
| Mean (SD) | 59.5 (3.64) | 74.1 (6.75) |
| Median (IQR) | 60.0 (57.0, 63.0) | 73.0 (69.0, 79.0) |
| Follow-up in years | ||
| Mean (SD) | 5.9 (2.46) | 5.1 (2.54) |
| Median (IQR) | 6.1 (3.7, 8.1) | 4.8 (2.9, 7.2) |
| PCa Stage, n (%) | ||
| Early | 4354 (66.8%) | 10,073 (62.4%) |
| Advanced | 1507 (23.1%) | 4059 (25.1%) |
| Missing | 657 (10.1%) | 2022 (12.5%) |
| Tumor Grade, n (%) | ||
| Low grade | 1924 (29.5%) | 3022 (18.7%) |
| High grade | 4325 (66.4%) | 11,744 (72.7%) |
| Missing | 269 (4.1%) | 1388 (8.6%) |
| Stage and Grade, n (%) | ||
| Early, low grade | 1536 (23.6%) | 2386 (14.8%) |
| Early, high grade | 2673 (41.0%) | 7333 (45.4%) |
| Advanced, low grade | 77 (1.2%) | 106 (0.7%) |
| Advanced, high grade | 1344 (20.6%) | 3304 (20.5%) |
| Missing | 888 (13.6%) | 3025 (18.7%) |
| Primary Treatment, n (%) | ||
| RP or other PCa-related surgeries | 3198 (49.1%) | 3920 (24.3%) |
| RT | 1382 (21.2%) | 4858 (30.1%) |
| ADT and/or chemotherapy | 338 (5.2%) | 3126 (19.4%) |
| No treatment | 1600 (24.5%) | 4250 (26.3%) |
| Early stage: Primary Treatment, n (%) | ||
| RP or other PCa-related surgeries | 1916 (44.0%) | 2169 (21.5%) |
| RT | 1054 (24.2%) | 3562 (35.4%) |
| ADT and/or chemotherapy | 96 (2.2%) | 1164 (11.6%) |
| No treatment | 1288 (29.6%) | 3178 (31.5%) |
| Advanced Stage: Primary Treatment, n (%) | ||
| RP or other PCa-related surgeries | 964 (64.0%) | 1457 (35.9%) |
| RT | 306 (20.3%) | 1162 (28.6%) |
| ADT and/or chemotherapy | 213 (14.1%) | 1309 (32.2%) |
| No treatment | 24 (1.6%) | 131 (3.2%) |
| Low grade: Primary Treatment, n (%) | ||
| RP or other PCa-related surgeries | 585 (30.4%) | 434 (14.4%) |
| RT | 149 (7.7%) | 228 (7.5%) |
| ADT and/or chemotherapy | 18 (0.9%) | 91 (3.0%) |
| No treatment | 1172 (60.9%) | 2269 (75.1%) |
| High grade: Primary Treatment, n (%) | ||
| RP or other PCa-related surgeries | 2469 (57.1%) | 3196 (27.2%) |
| RT | 1192 (27.6%) | 4426 (37.7%) |
| ADT and/or chemotherapy | 294 (6.8%) | 2454 (20.9%) |
| No treatment | 370 (8.6%) | 1668 (14.2%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Guh, D.P.; Mohammadi, T.; Pataky, R.E.; Tam, A.C.T.; Lynd, L.D.; Conklin, A.I. Health Care Costs Attributable to Prostate Cancer in British Columbia, Canada: A Population-Based Cohort Study. Curr. Oncol. 2023, 30, 3176-3188. https://doi.org/10.3390/curroncol30030240
Zhang W, Guh DP, Mohammadi T, Pataky RE, Tam ACT, Lynd LD, Conklin AI. Health Care Costs Attributable to Prostate Cancer in British Columbia, Canada: A Population-Based Cohort Study. Current Oncology. 2023; 30(3):3176-3188. https://doi.org/10.3390/curroncol30030240
Chicago/Turabian StyleZhang, Wei, Daphne P. Guh, Tima Mohammadi, Reka E. Pataky, Alexander C. T. Tam, Larry D. Lynd, and Annalijn I. Conklin. 2023. "Health Care Costs Attributable to Prostate Cancer in British Columbia, Canada: A Population-Based Cohort Study" Current Oncology 30, no. 3: 3176-3188. https://doi.org/10.3390/curroncol30030240
APA StyleZhang, W., Guh, D. P., Mohammadi, T., Pataky, R. E., Tam, A. C. T., Lynd, L. D., & Conklin, A. I. (2023). Health Care Costs Attributable to Prostate Cancer in British Columbia, Canada: A Population-Based Cohort Study. Current Oncology, 30(3), 3176-3188. https://doi.org/10.3390/curroncol30030240





