Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force
Abstract
1. Background
2. Questions to Address
3. Summary and Recommendations
- It is recommended that testing upon medical oncologist request only is indicated.
- Testing must be performed on treatment-naïve tumor tissue. Testing on the core biopsy is preferred; however, a well-fixed resection specimen is an acceptable alternative.
- Adhering to ASCO/CAP fixation guidelines for breast biomarkers is advised, including cold ischemic times of <1 h, duration of fixation of 6 to 72 h and using neutral buffered formalin only as a fixative.
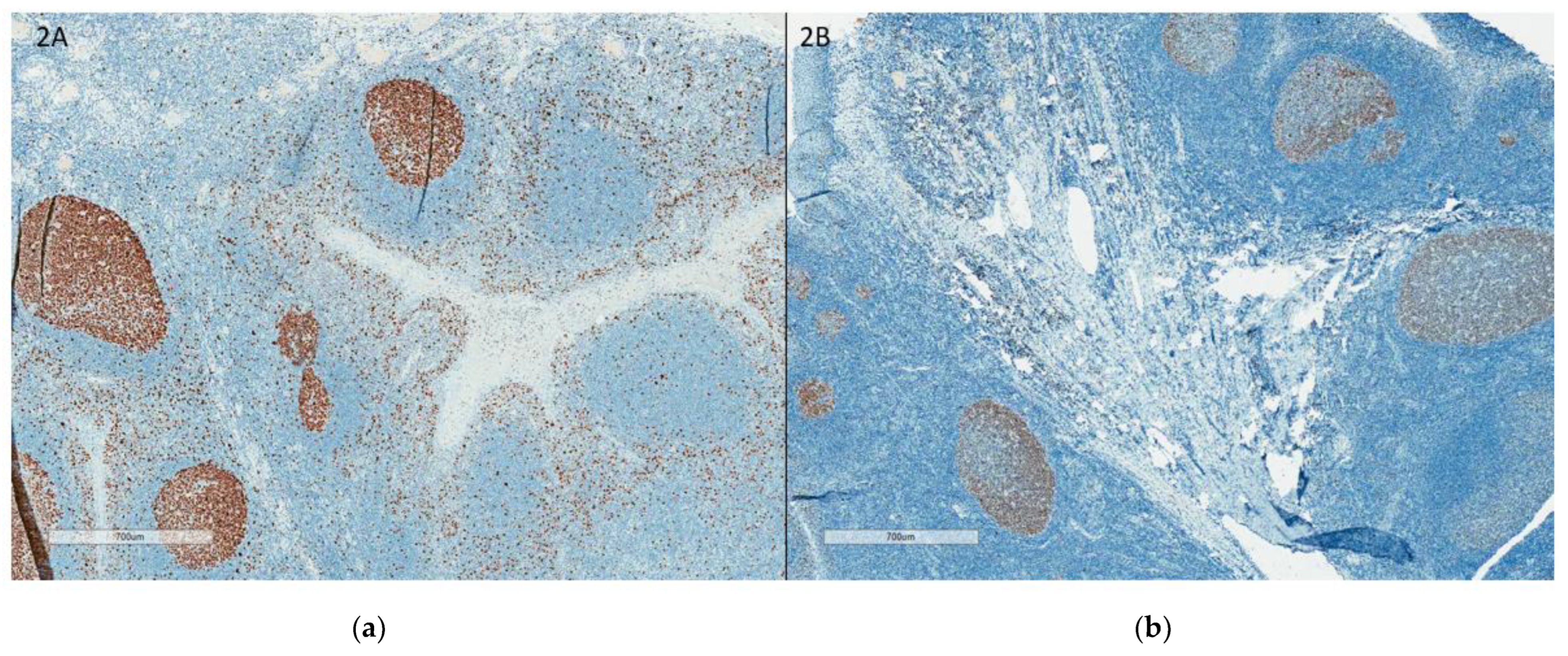
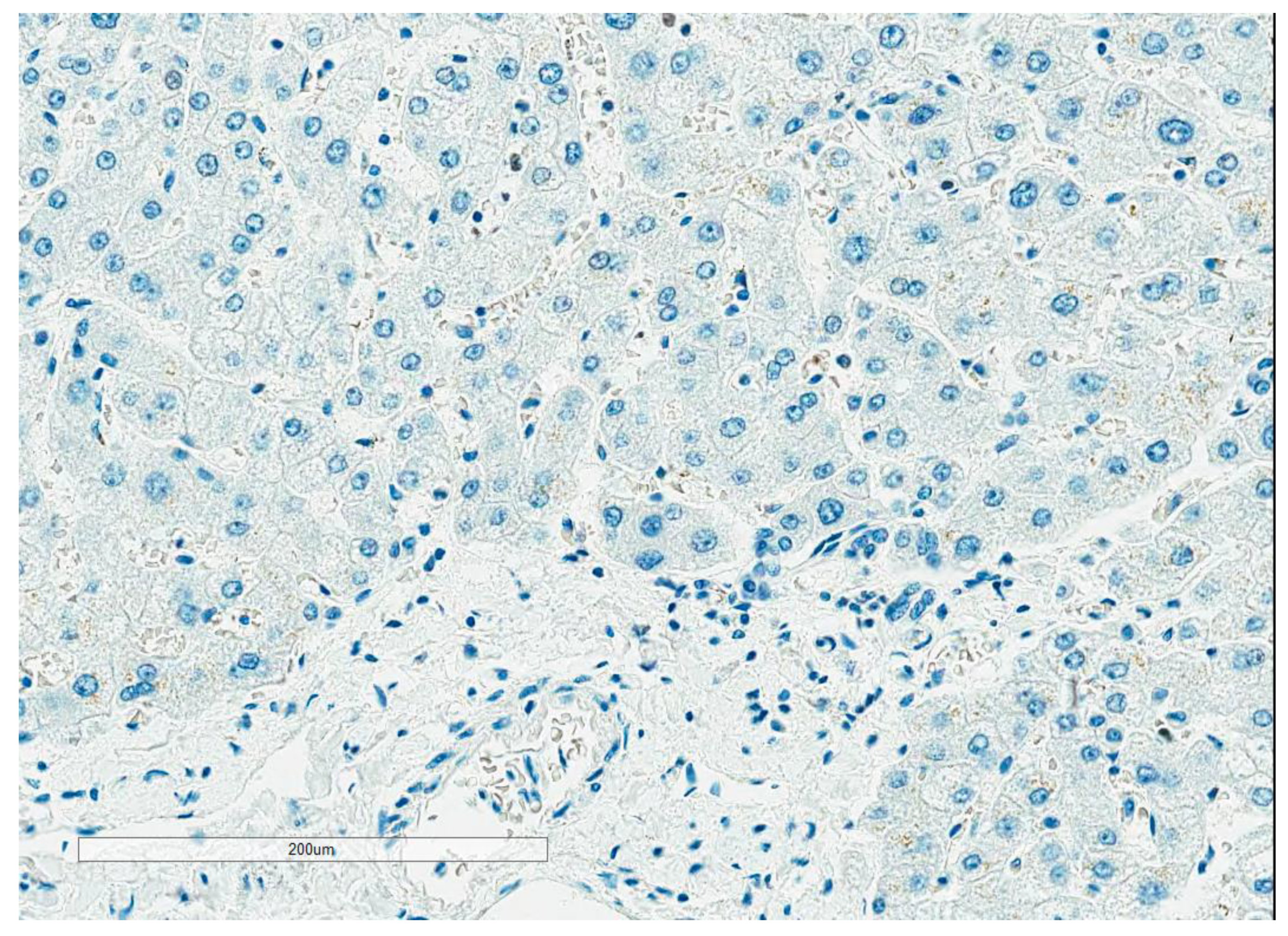
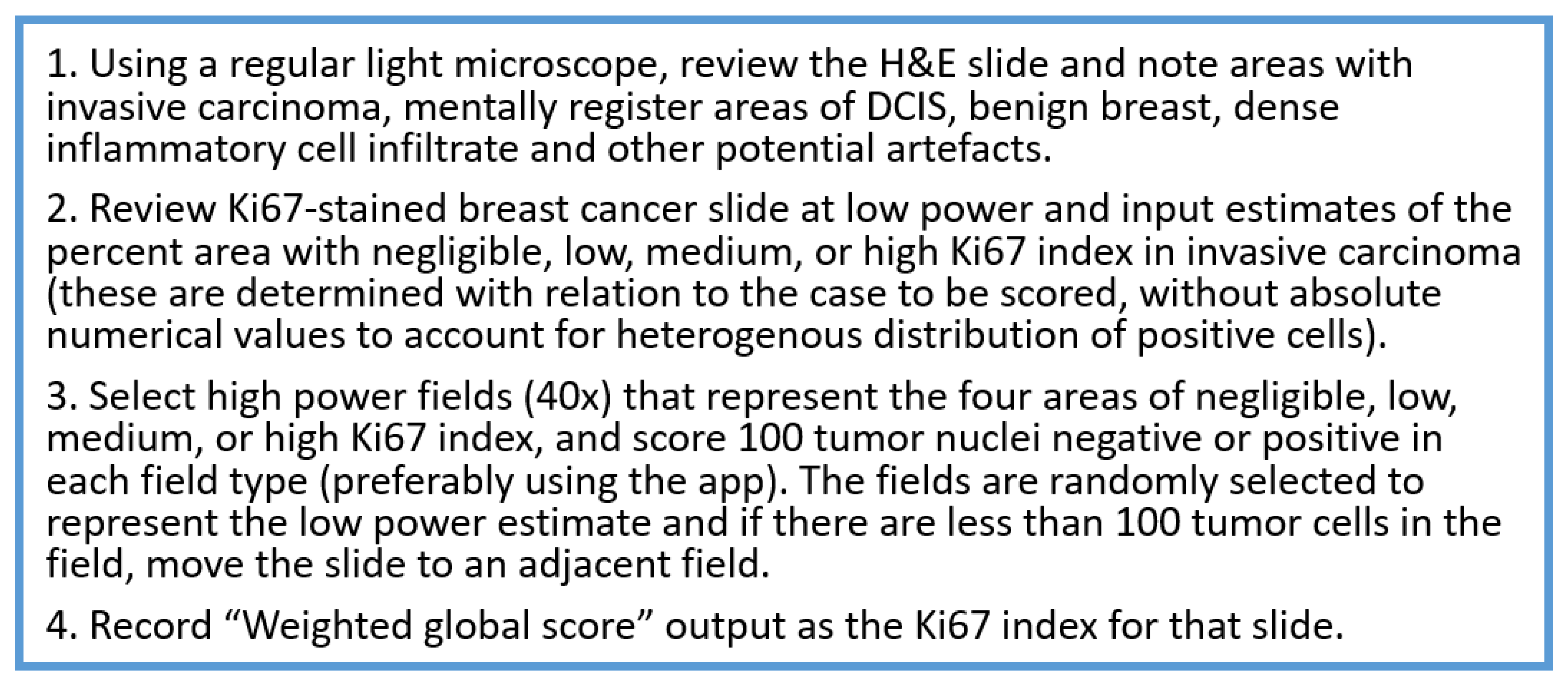
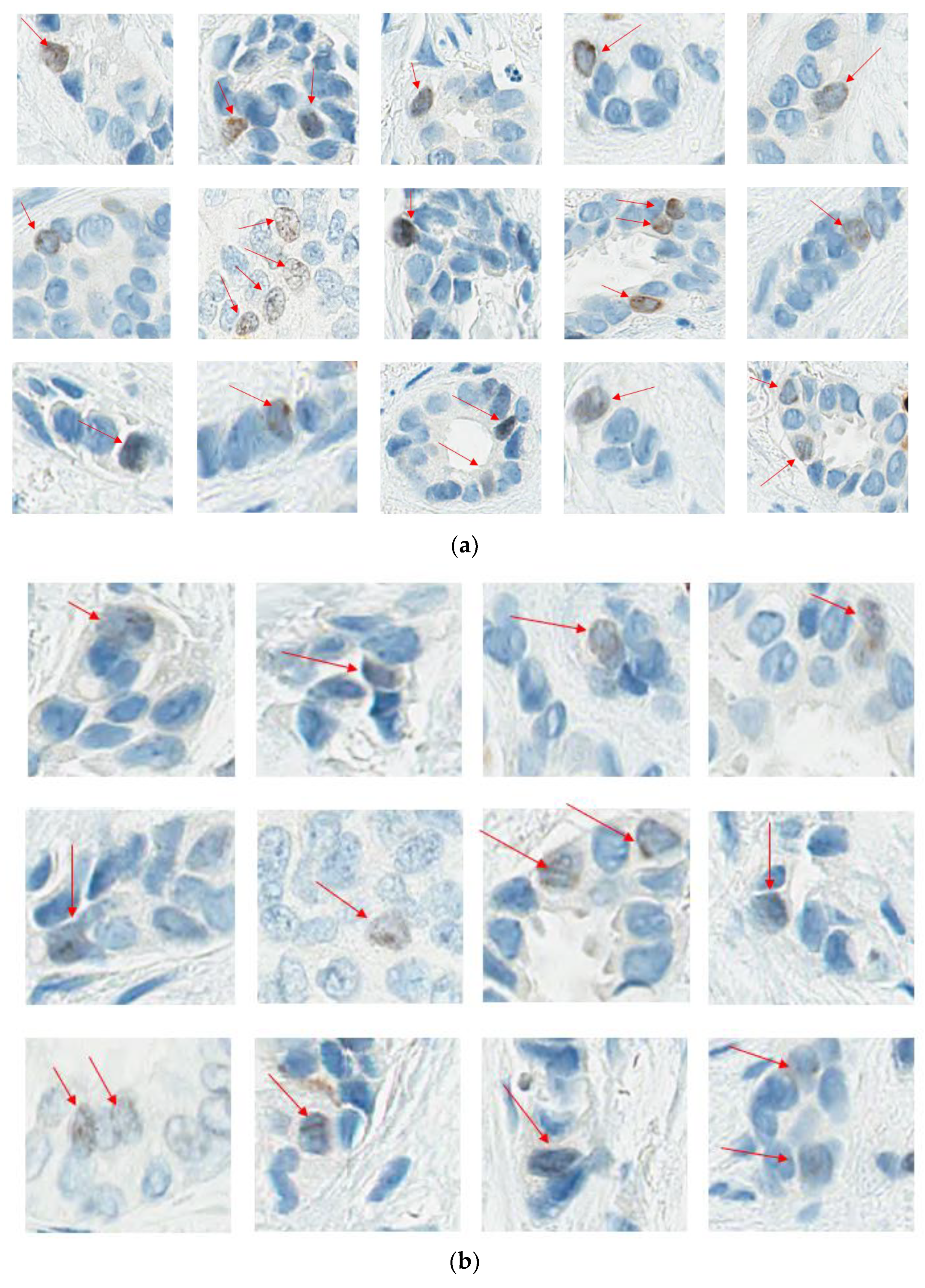
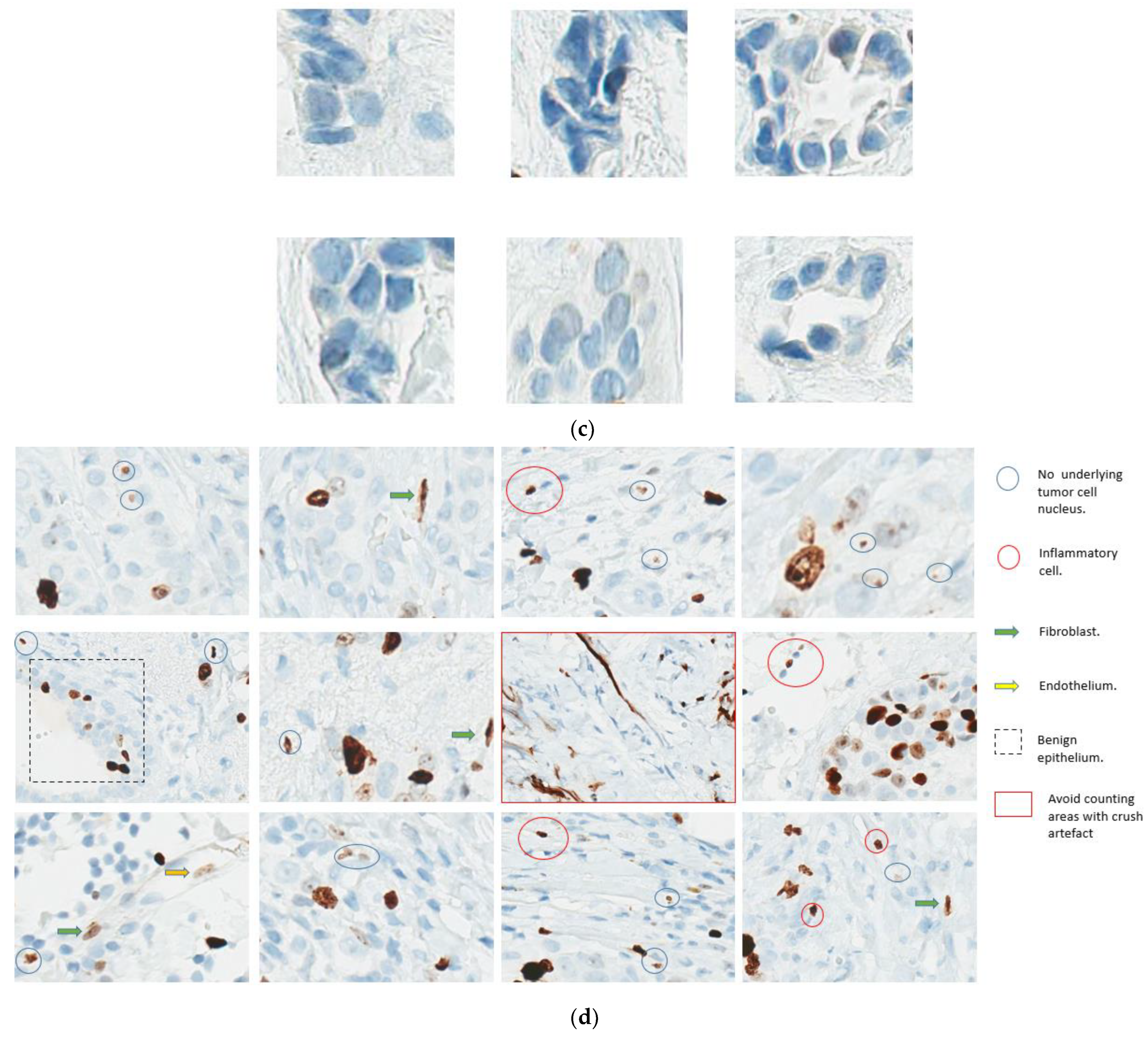
- Readout training is strongly recommended. Visual counting methods, other than eyeballing, should be used, with global rather than hot spot assessment preferred. Counting 100 cells in at least four areas of the tumor is recommended. It is suggested to evaluate Ki67 at 40× magnification to capture weakly staining nuclei. The Ki67scoring app developed to assist pathologists with scoring Ki67 using the standardized scoring method proposed by the IKWG, available for free download, may be used (https://play.google.com/store/apps/details?id=ca.ubc.gpec.ki67counter&hl=en_CA&gl=US, accessed on 15 February 2023). Automated image analysis is very promising and can be easily standardized, and laboratories with such technology are encouraged to use it as an adjunct to visual counting.
- A score of <5 or >30 is more robust. In cases within the 5–30% range, consider counting another set of 400 cells, have a second reader count, or refer to an image analysis system.
- The task force recommends that the results are best expressed as a continuous variable and reported in a synoptic format using the CAP checklist or the suggested format (Appendix A).
- The appropriate antibody clone and staining protocols to be used may take time to address. For the time being, the task force recommends having tonsils/+pancreas on-slide control and enrollment in at least one national/international EQA program.
- Analytical validation remains a pending goal. Until the data become available, using local Ki67 protocols is acceptable. The task force recommends participation in upcoming calibration and technical validation initiatives. When reporting Ki67, consider adding a disclaimer indicating that the assay is currently not validated for prognostic/predictive purposes in breast cancer, and calibration/validation will be pursued once they become available.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A. Synoptic Report Template
References
- de Azambuja, E.; Cardoso, F.; De Castro, G.; Colozza, M.; Mano, M.S.; Durbecq, V.; Sotiriou, C.; Larsimont, D.; Piccart-Gebhart, M.; Paesmans, M. Ki-67 as prognostic marker in early breast cancer: A meta-analysis of published studies involving 12,155 patients. Br. J. Cancer 2007, 96, 1504–1513. [Google Scholar] [CrossRef]
- Inwald, E.C.; Klinkhammer-Schalke, M.; Hofstädter, F.; Zeman, F.; Koller, M.; Gerstenhauer, M.; Ortmann, O. Ki-67 is a prognostic parameter in breast cancer patients: Results of a large population-based cohort of a cancer registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef]
- Petrelli, F.; Viale, G.; Cabiddu, M.; Barni, S. Prognostic value of different cut-off levels of Ki-67 in breast cancer: A systematic review and meta-analysis of 64,196 patients. Breast Cancer Res. Treat. 2015, 153, 477–491. [Google Scholar] [CrossRef]
- Ács, B.; Zámbó, V.; Vízkeleti, L.; Szász, A.M.; Madaras, L.; Szentmártoni, G.; Tőkés, T.; Molnár, B.; Molnár, I.A.; Vári-Kakas, S.; et al. Ki-67 as a controversial predictive and prognostic marker in breast cancer patients treated with neoadjuvant chemotherapy. Diagn. Pathol. 2017, 12, 20. [Google Scholar] [CrossRef]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef] [PubMed]
- Sahebjam, S.; Aloyz, R.; Pilavdzic, D.; Brisson, M.-L.; Ferrario, C.; Bouganim, N.; Cohen, V.; Miller, W.H.; Panasci, L.C. Ki 67 is a major, but not the sole determinant of Oncotype Dx recurrence score. Br. J. Cancer 2011, 105, 1342–1345. [Google Scholar] [CrossRef] [PubMed]
- Polley, M.Y.C.; Leung, S.C.Y.; McShane, L.M.; Gao, D.; Hugh, J.C.; Mastropasqua, M.G.; Viale, G.; Zabaglo, L.A.; Penault-Llorca, F.; Bartlett, J.M.; et al. An International Ki67 Reproducibility Study. J Natl Cancer Inst. 2013, 105, 1897–1906. [Google Scholar] [CrossRef]
- Polley, M.Y.C.; Leung, S.C.Y.; Gao, D.; Mastropasqua, M.G.; Zabaglo, L.A.; Bartlett, J.M.S.; McShane, L.M.; Enos, R.A.; Badve, S.S.; Bane, A.L.; et al. An international study to increase concordance in Ki67 scoring. Mod. Pathol. 2015, 28, 778–786. [Google Scholar] [CrossRef] [PubMed]
- Focke, C.M.; Decker, T.; van Diest, P.J. Reliability of the Ki67-Labelling Index in Core Needle Biopsies of Luminal Breast Cancers is Unaffected by Biopsy Volume. Ann. Surg. Oncol. 2017, 24, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Turner, N.C.; Bondarenko, I.; Ro, J.; Im, S.A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Fulvestrant plus palbociclib versus fulvestrant plus placebo for treatment of hormone-receptor-positive, HER2-negative metastatic breast cancer that progressed on previous endocrine therapy (PALOMA-3): Final analysis of the multicentre, double-blind, phase 3 randomised controlled trial. Lancet Oncol. 2016, 17, 425–439. [Google Scholar] [CrossRef]
- Harbeck, N.; Rastogi, P.; Martin, M.; Tolaney, S.; Shao, Z.; Fasching, P.; Huang, C.; Jaliffe, G.; Tryakin, A.; Goetz, M.; et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study. Ann. Oncol. 2021, 32, 1571–1581. [Google Scholar] [CrossRef] [PubMed]
- Harbeck, N.; Rastogi, P.; Shahir, A.; Johnston, S.; O’Shaughnessy, J. Letter to the Editor for ‘Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: Updated efficacy and Ki-67 analysis from the monarchE study’. Ann. Oncol. 2022, 33, 227–228. [Google Scholar] [CrossRef]
- Toi, M.; Boyle, F.; Im, Y.-H.; Reinisch, M.; Molthrop, D.; Jiang, Z.; Wei, R.; Sapunar, F.; Grimes, B.; Nabinger, S.; et al. Adjuvant Abemaciclib Combined with Endocrine Therapy: Efficacy Results in monarchE Cohort 1. Ann. Oncol. 2022, 33, S149. [Google Scholar] [CrossRef]
- Andre, F.; Ismaila, N.; Allison, K.H.; Barlow, W.E.; Collyar, D.E.; Damodaran, S.; Henry, N.L.; Jhaveri, K.; Kalinsky, K.; Kuderer, N.M.; et al. Biomarkers for Adjuvant Endocrine and Chemotherapy in Early-Stage Breast Cancer: ASCO Guideline Update. J. Clin. Oncol. 2022, 40, 1816–1837. [Google Scholar] [CrossRef]
- Leung, S.C.Y.; O Nielsen, T.; A Zabaglo, L.; Arun, I.; Badve, S.S.; Bane, A.L.; Bartlett, J.M.S.; Borgquist, S.; Chang, M.C.; Dodson, A.; et al. Analytical validation of a standardised scoring protocol for Ki67 immunohistochemistry on breast cancer excision whole sections: An international multicentre collaboration. Histopathology 2019, 75, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, T.O.; Leung, S.C.Y.; Rimm, D.L.; Dodson, A.; Acs, B.; Badve, S.; Denkert, C.; Ellis, M.J.; Fineberg, S.; Flowers, M.; et al. Assessment of Ki67 in Breast Cancer: Updated Recommendations From the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst. 2020, 113, 808–819. [Google Scholar] [CrossRef]
- Dowsett, M.; Nielsen, T.O.; A’hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in breast cancer: Recommendations from the International Ki67 in breast cancer working group. JNCI J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Leung, S.C.Y.; Nielsen, T.O.; Zabaglo, L.; Arun, I.; Badve, S.S.; Bane, A.L.; Bartlett, J.M.S.; Borgquist, S.; Chang, M.C.; Dodson, A.; et al. Analytical validation of a standardized scoring protocol for Ki67: Phase 3 of an international multicenter collaboration. npj Breast Cancer 2016, 2, 16014. [Google Scholar] [CrossRef]
- Raap, M.; Ließem, S.; Rüschoff, J.; Fisseler-Eckhoff, A.; Reiner, A.; Dirnhofer, S.; von Wasielewski, R.; Kreipe, H. Quality assurance trials for Ki67 assessment in pathology. Virchows Arch. 2017, 471, 501–508. [Google Scholar] [CrossRef]
- Varga, Z.; Diebold, J.; Dommann-Scherrer, C.; Frick, H.; Kaup, D.; Noske, A.; Obermann, E.; Ohlschlegel, C.; Padberg, B.; Rakozy, C.; et al. How Reliable Is Ki-67 Immunohistochemistry in Grade 2 Breast Carcinomas? A QA Study of the Swiss Working Group of Breast- and Gynecopathologists. PLoS ONE 2012, 7, e37379. [Google Scholar] [CrossRef]
- Polewski, M.D.; Nielsen, G.B.; Gu, Y.; Weaver, A.T.B.; Gegg, G.B.; Tabuena-Frolli, S.B.; Cajaiba, M.; Hanks, D.; Method, M.M.; Press, M.F.M.; et al. A Standardized Investigational Ki-67 Immunohistochemistry Assay Used to Assess High-Risk Early Breast Cancer Patients in the monarchE Phase 3 Clinical Study Identifies a Population With Greater Risk of Disease Recurrence When Treated With Endocrine Therapy Alone. Appl. Immunohistochem. Mol. Morphol. 2022, 30, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Kalvala, J.; Parks, R.M.; Green, A.R.; Cheung, K.L. Concordance between core needle biopsy and surgical excision specimens for Ki-67 in breast cancer—A systematic review of the literature. Histopathology 2022, 80, 468–484. [Google Scholar] [CrossRef]
- Ahn, S.; Lee, J.; Cho, M.S.; Park, S.; Sung, S.H. Evaluation of Ki-67 Index in Core Needle Biopsies and Matched Breast Cancer Surgical Specimens. Arch. Pathol. Lab. Med. 2018, 142, 364–368. [Google Scholar] [CrossRef]
- Acs, B.; Leung, S.C.Y.; Kidwell, K.M.; Arun, I.; Augulis, R.; Badve, S.S.; Bai, Y.; Bane, A.L.; Bartlett, J.M.S.; Bayani, J.; et al. Systematically higher Ki67 scores on core biopsy samples compared to corresponding resection specimen in breast cancer: A multi-operator and multi-institutional study. Mod. Pathol. 2022, 35, 1362–1369. [Google Scholar] [CrossRef]
- Arima, N.; Nishimura, R.; Osako, T.; Nishiyama, Y.; Fujisue, M.; Okumura, Y.; Nakano, M.; Tashima, R.; Toyozumi, Y. The importance of tissue handling of surgically removed breast cancer for an accurate assessment of the Ki-67 index. J. Clin. Pathol. 2016, 69, 255–259. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Aung, T.N.; Acs, B.; Jonathan, W; Bai, Y; Gaule, P. A New tool for Technical Standarization of Ki67 Immunohistochemical Assay. Mod. Pathology 2021, 34, 1261–1270. [Google Scholar] [CrossRef]
- Parry, S.; Dowsett, M.; Dodson, A. UK NEQAS ICC & ISH Ki-67 Data Reveal Differences in Performance of Primary Antibody Clones. Appl. Immunohistochem. Mol. Morphol. 2021, 29, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Gonen, M.; Hedvat, C.; Modlin, I.M.; Klimstra, D.S. Objective Quantification of the Ki67 Proliferative Index in Neuroendocrine Tumors of the Gastroenteropancreatic System: A comparison of digital image analysis with manual methods. Am. J. Surg. Pathol. 2012, 36, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Benjamin Chun-Kit Tong. Ki67 Immunohistochemistry quantitation in breast carcinoma: A comparison of visual estimation, counting and Immunoratio. Physiol. Behav. 2021, 29, 105–111. [Google Scholar] [CrossRef]
- Arun, I.; Venkatesh, S.; Ahmed, R.; Agrawal, S.K.; Leung, S.C.Y. Reliability of Ki67 visual scoring app compared to eyeball estimate and digital image analysis and its prognostic significance in hormone receptor-positive breast cancer. Apmis 2021, 129, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Acs, B.; Pelekanou, V.; Bai, Y.; Martinez-Morilla, S.; Toki, M.; Leung, S.C.Y.; Nielsen, T.O.; Rimm, D.L. Ki67 reproducibility using digital image analysis: An inter-platform and inter-operator study. Lab. Investig. 2019, 99, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Rimm, D.L.; Leung, S.C.Y.; McShane, L.M.; Bai, Y.; Bane, A.L.; Bartlett, J.M.S.; Bayani, J.; Chang, M.C.; Dean, M.; Denkert, C.; et al. An international multicenter study to evaluate reproducibility of automated scoring for assessment of Ki67 in breast cancer. Mod. Pathol. 2019, 32, 59–69. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faragalla, H.; Plotkin, A.; Barnes, P.; Lu, F.-I.; Kos, Z.; Mulligan, A.M.; Bane, A.; Nofech Mozes, S. Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force. Curr. Oncol. 2023, 30, 3079-3090. https://doi.org/10.3390/curroncol30030233
Faragalla H, Plotkin A, Barnes P, Lu F-I, Kos Z, Mulligan AM, Bane A, Nofech Mozes S. Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force. Current Oncology. 2023; 30(3):3079-3090. https://doi.org/10.3390/curroncol30030233
Chicago/Turabian StyleFaragalla, Hala, Anna Plotkin, Penny Barnes, Fang-I Lu, Zuzana Kos, Anna Marie Mulligan, Anita Bane, and Sharon Nofech Mozes. 2023. "Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force" Current Oncology 30, no. 3: 3079-3090. https://doi.org/10.3390/curroncol30030233
APA StyleFaragalla, H., Plotkin, A., Barnes, P., Lu, F.-I., Kos, Z., Mulligan, A. M., Bane, A., & Nofech Mozes, S. (2023). Ki67 in Breast Cancer Assay: An Ad Hoc Testing Recommendation from the Canadian Association of Pathologists Task Force. Current Oncology, 30(3), 3079-3090. https://doi.org/10.3390/curroncol30030233




