Nomograms for Predicting Survival Outcomes in Patients with Neuroendocrine Neoplasms of the Gallbladder Undergoing Primary Tumor Resection: A Population-Based Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval and Informed Consent
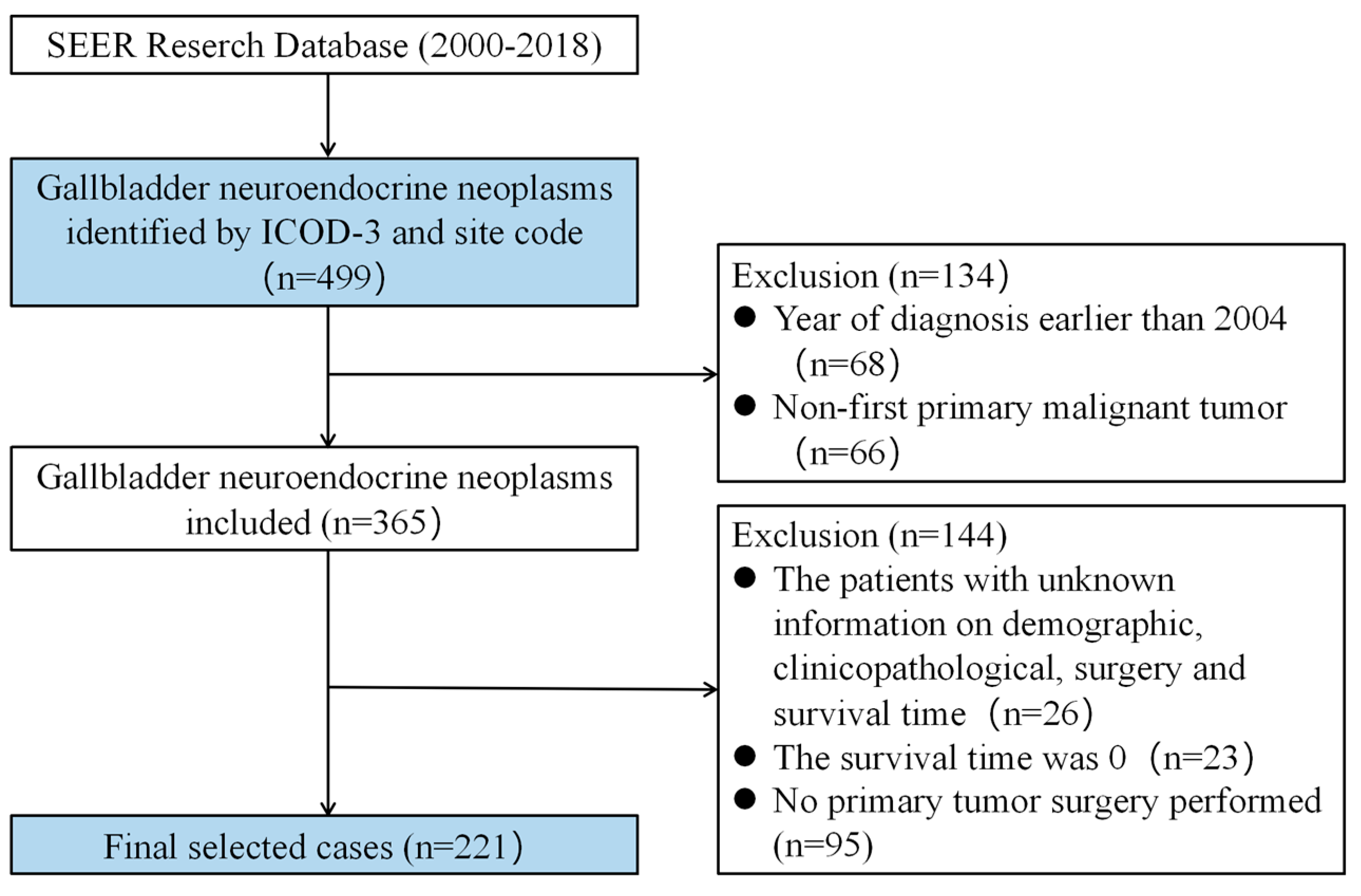
2.2. Source Data and Screening Criteria
2.3. Construction and Validation of the Nomogram Models
3. Results
3.1. Patient Characteristics
3.2. Identification of Prognostic Factors
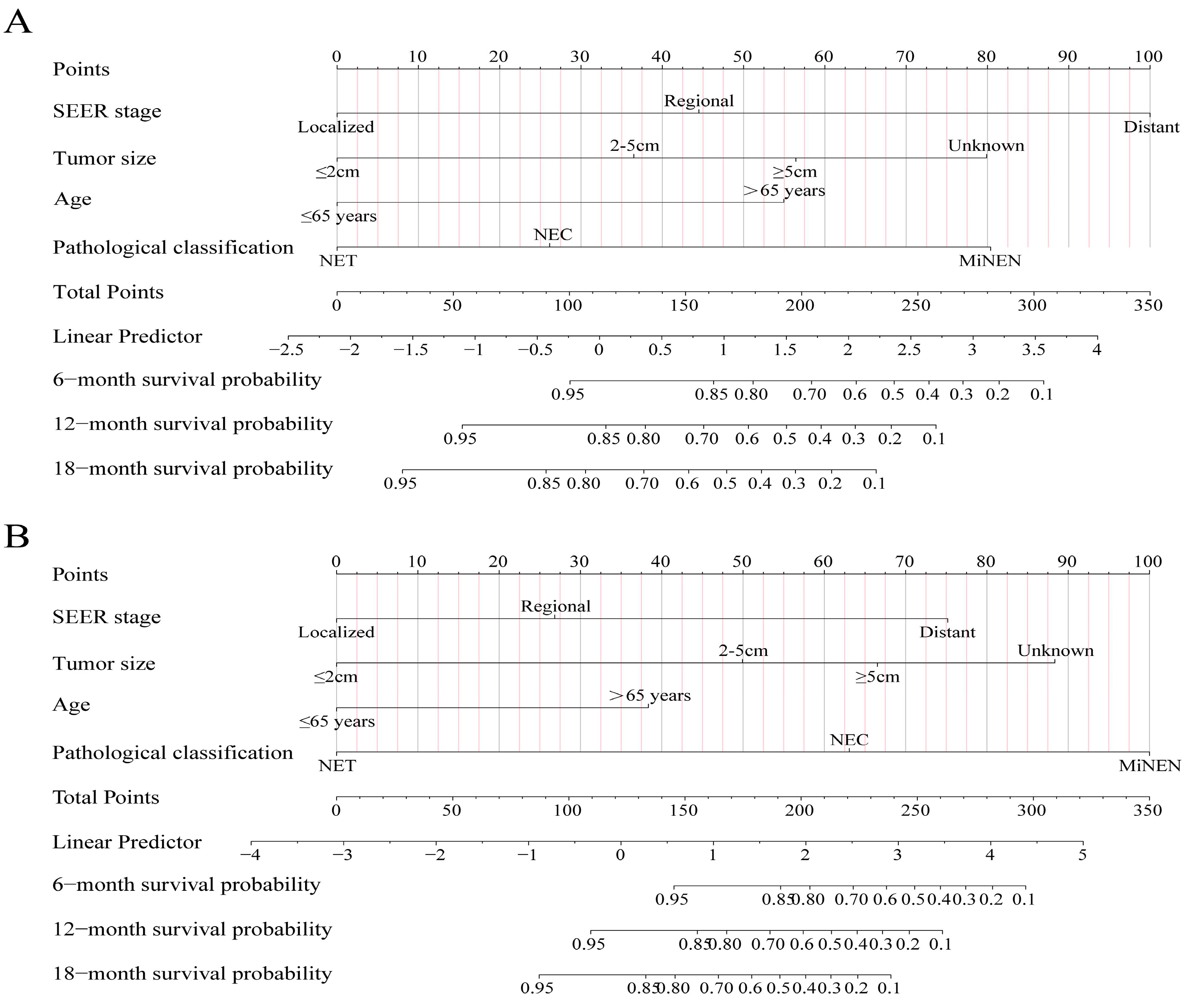
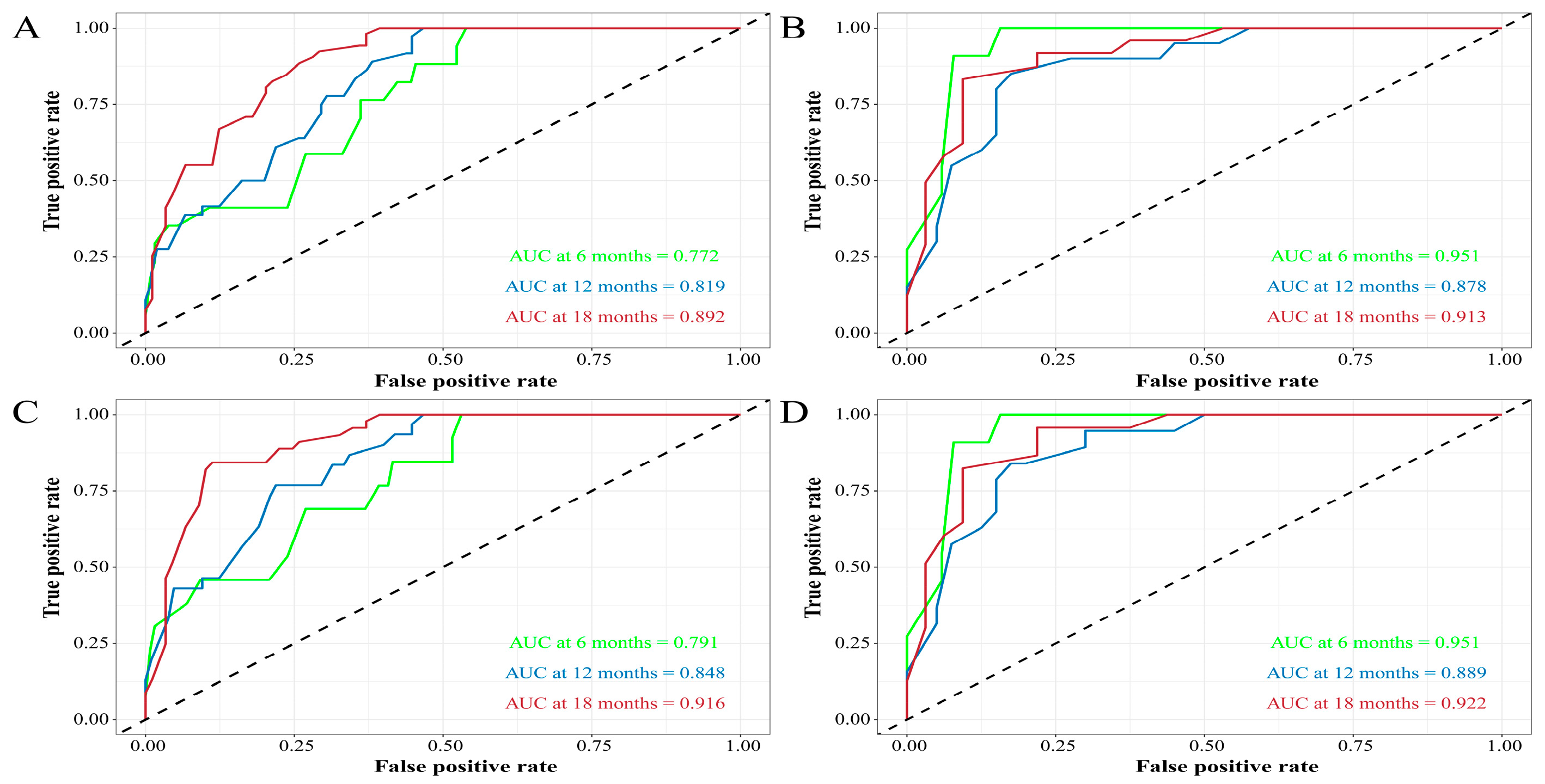
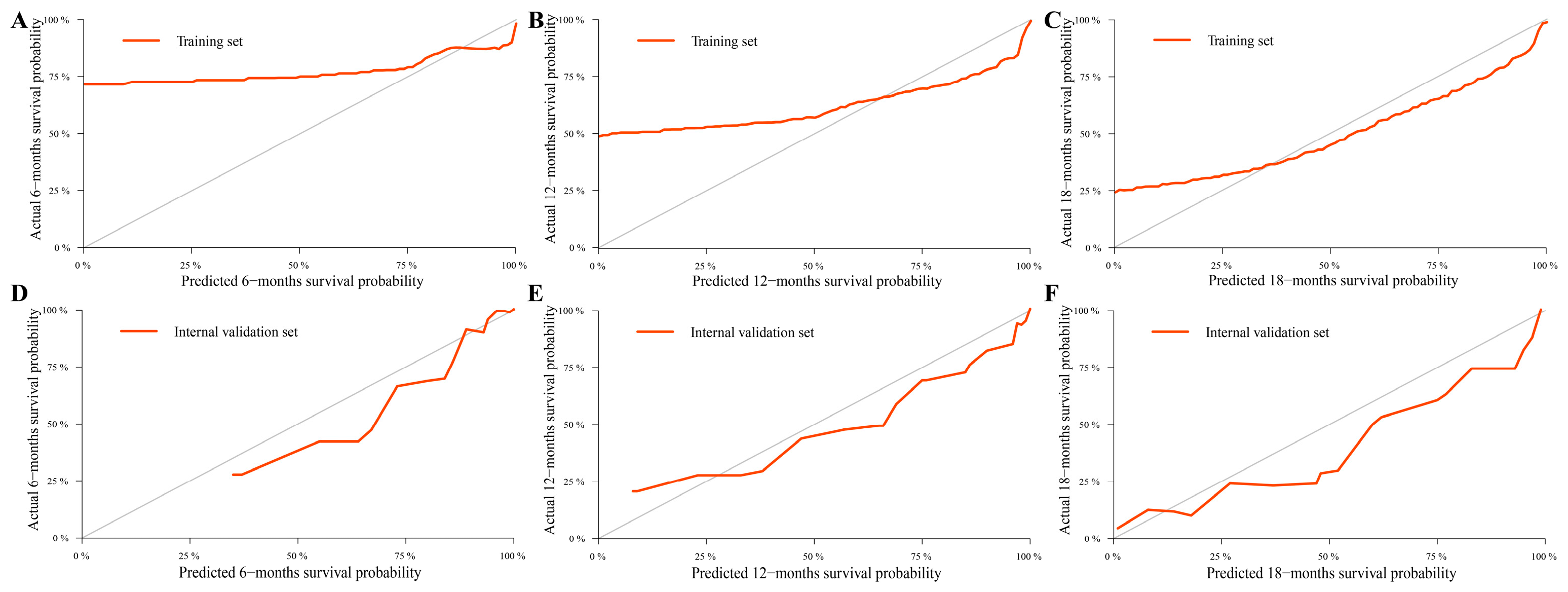
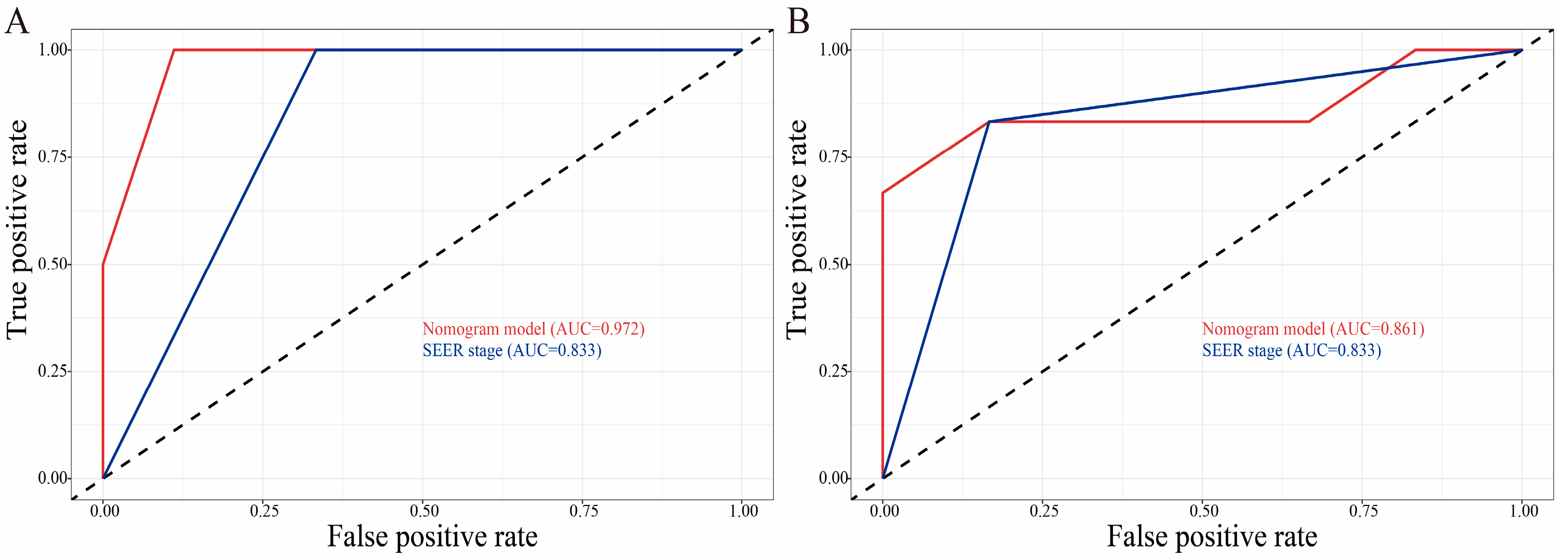
3.3. Construction and Validation of the Nomograms
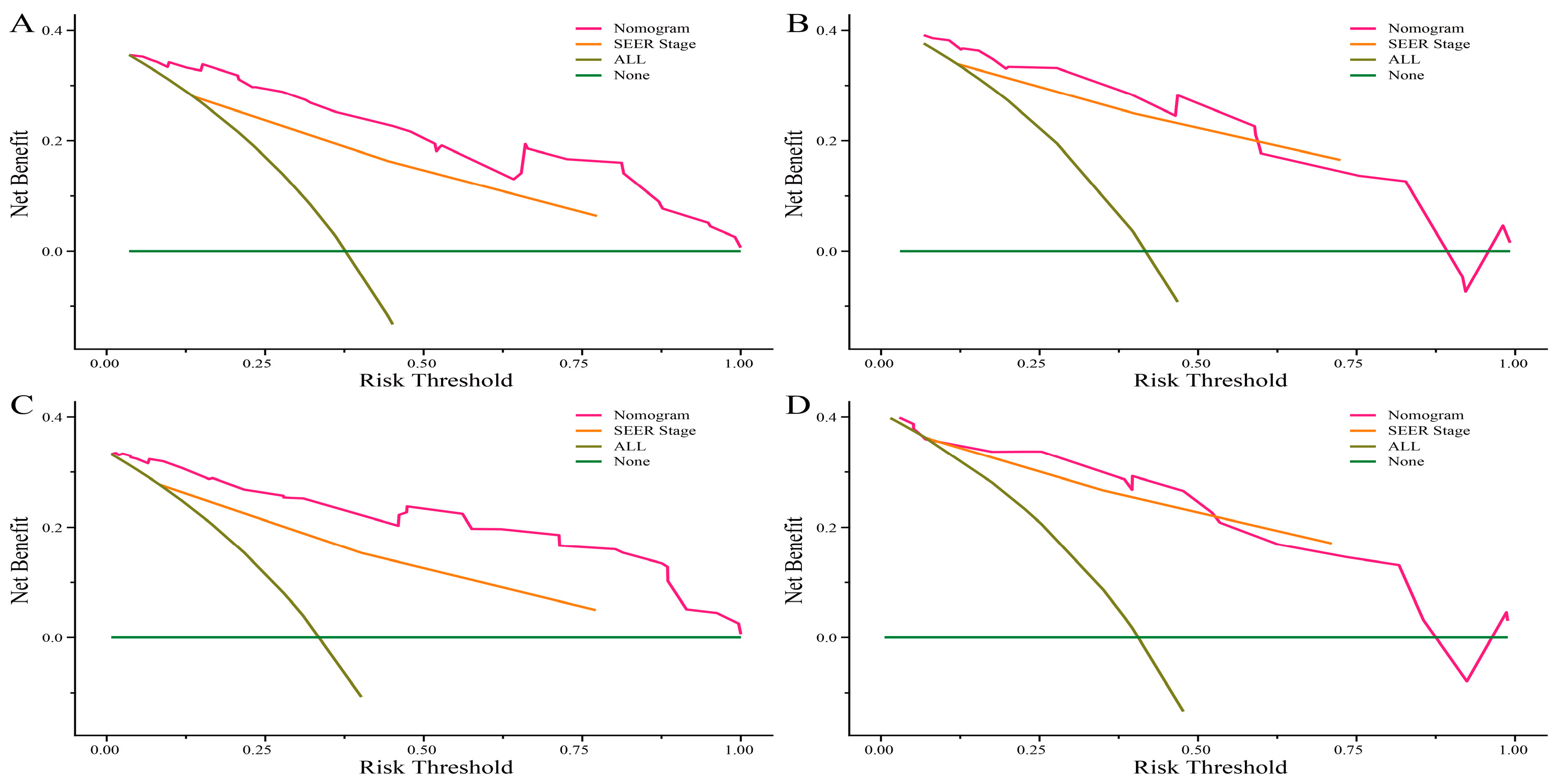
3.4. Clinical Application of the Nomograms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cives, M.; Strosberg, J.R. Gastroenteropancreatic Neuroendocrine Tumors. CA Cancer J. Clin. 2018, 68, 471–487. [Google Scholar] [CrossRef]
- Mafficini, A.; Scarpa, A. Genetics and Epigenetics of Gastroenteropancreatic Neuroendocrine Neoplasms. Endocr. Rev. 2019, 40, 506–536. [Google Scholar] [CrossRef] [PubMed]
- Dasari, A.; Shen, C.; Halperin, D.; Zhao, B.; Zhou, S.; Xu, Y.; Shih, T.; Yao, J.C. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA Oncol. 2017, 3, 1335–1342. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A.; Dagohoy, C.; Leary, C.; Mares, J.E.; Abdalla, E.K.; Fleming, J.B.; Vauthey, J.N.; Rashid, A.; et al. One hundred years after “carcinoid”: Epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J. Clin. Oncol. 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- O’Toole, D.; Kianmanesh, R.; Caplin, M. ENETS 2016 Consensus Guidelines for the Management of Patients with Digestive Neuroendocrine Tumors: An Update. Neuroendocrinology 2016, 103, 117–118. [Google Scholar] [CrossRef]
- Eltawil, K.M.; Gustafsson, B.I.; Kidd, M.; Modlin, I.M. Neuroendocrine tumors of the gallbladder: An evaluation and reassessment of management strategy. J. Clin. Gastroenterol. 2010, 44, 687–695. [Google Scholar] [CrossRef]
- Lee, S.M.; Sung, C.O. Neuroendocrine Carcinomas of the Gallbladder: A Clinicopathologic and Immunohistochemical Analysis of 34 Resected Cases. Am. J. Surg. Pathol. 2020, 44, 1308–1321. [Google Scholar] [CrossRef] [PubMed]
- Ayabe, R.I.; Wach, M.; Ruff, S.; Martin, S.; Diggs, L.; Wiemken, T.; Hinyard, L.; Davis, J.L.; Luu, C.; Hernandez, J.M. Primary Gallbladder Neuroendocrine Tumors: Insights into a Rare Histology Using a Large National Database. Ann. Surg. Oncol. 2019, 26, 3577–3585. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef]
- Wang, Y.; Li, J.; Xia, Y.; Gong, R.; Wang, K.; Yan, Z.; Wan, X.; Liu, G.; Wu, D.; Shi, L.; et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J. Clin. Oncol. 2013, 31, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Duggan, M.A.; Anderson, W.F.; Altekruse, S.; Penberthy, L.; Sherman, M.E. The Surveillance, Epidemiology, and End Results (SEER) Program and Pathology: Toward Strengthening the Critical Relationship. Am. J. Surg. Pathol. 2016, 40, e94–e102. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A.; the WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yang, L.; Ci, B.; Maclean, M.; Gerber, D.E.; Xiao, G.; Xie, Y. Development and Validation of a Nomogram Prognostic Model for SCLC Patients. J. Thorac. Oncol. 2018, 13, 1338–1348. [Google Scholar] [CrossRef]
- Vickers, A.J.; Elkin, E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Jiang, S.; Chen, W.; Yin, H.; Dong, L.; Zhao, H.; Han, S.; He, X. Biliary Neuroendocrine Neoplasms: Analysis of Prognostic Factors and Development and Validation of a Nomogram. Front. Oncol. 2021, 11, 654439. [Google Scholar] [CrossRef]
- Yadav, S.; Tella, S.H.; Kommalapati, A.; Mara, K.; Prasai, K.; Mady, M.H.; Hassan, M.; Smoot, R.L.; Cleary, S.P.; Truty, M.J.; et al. A Novel Clinically Based Staging System for Gallbladder Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 151–159. [Google Scholar] [CrossRef]
- Hallet, J.; Law, C.; Singh, S.; Mahar, A.; Myrehaug, S.; Zuk, V.; Zhao, H.; Chan, W.; Assal, A.; Coburn, N. Risk of Cancer-Specific Death for Patients Diagnosed With Neuroendocrine Tumors: A Population-Based Analysis. J. Natl. Compr. Cancer Netw. 2021, 19, 935–944. [Google Scholar] [CrossRef]
- Hu, P.; Bai, J.; Liu, M.; Xue, J.; Chen, T.; Li, R.; Kuai, X.; Zhao, H.; Li, X.; Tian, Y.; et al. Trends of incidence and prognosis of gastric neuroendocrine neoplasms: A study based on SEER and our multicenter research. Gastric Cancer 2020, 23, 591–599. [Google Scholar] [CrossRef]
- Wang, Z.J.; An, K.; Li, R.; Shen, W.; Bao, M.D.; Tao, J.H.; Chen, J.N.; Mei, S.W.; Shen, H.Y.; Ma, Y.B.; et al. Analysis of 72 patients with colorectal high-grade neuroendocrine neoplasms from three Chinese hospitals. World J. Gastroenterol. 2019, 25, 5197–5209. [Google Scholar] [CrossRef]
- Miao, D.L.; Song, W.; Qian, J.; Zhu, Z.G.; Wu, Q.; Lv, C.G.; Chen, L. Development and Validation of a Nomogram for Predicting Overall Survival in Pancreatic NeuroendocrineTumors. Transl. Oncol. 2018, 11, 1097–1103. [Google Scholar] [CrossRef]
- Lin, Q.; Wagner, W. Epigenetic Aging Signatures Are Coherently Modified in Cancer. PLoS Genet. 2015, 11, e1005334. [Google Scholar] [CrossRef] [PubMed]
- Cen, D.; Liu, H.; Wan, Z.; Lin, Z.; Wang, Y.; Xu, J.; Liang, Y. Clinicopathological features and survival for gallbladder NEN: A population-based study. Endocr. Connect. 2019, 8, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Shapera, E.; Bitting, C. Survival: A rare outcome in large cell neuroendocrine carcinoma of the gallbladder. Acta Gastro-Enterol. Belg. 2019, 82, 433–436. [Google Scholar]
- Zheng, Z.; Chen, C.; Li, B.; Liu, H.; Zhou, L.; Zhang, H.; Zheng, C.; He, X.; Liu, W.; Hong, T.; et al. Biliary Neuroendocrine Neoplasms: Clinical Profiles, Management, and Analysis of Prognostic Factors. Front. Oncol. 2019, 9, 38. [Google Scholar] [CrossRef]
- Wang, W.Q.; Zhang, W.H.; Gao, H.L.; Huang, D.; Xu, H.X.; Li, S.; Li, T.J.; Xu, S.S.; Li, H.; Long, J.; et al. A novel risk factor panel predicts early recurrence in resected pancreatic neuroendocrine tumors. J. Gastroenterol. 2021, 56, 395–405. [Google Scholar] [CrossRef]
- Zhang, X.F.; Xue, F.; Wu, Z.; Lopez-Aguiar, A.G.; Poultsides, G.; Makris, E.; Rocha, F.; Kanji, Z.; Weber, S.; Fisher, A.; et al. Development and Validation of a Modified Eighth AJCC Staging System for Primary Pancreatic Neuroendocrine Tumors. Ann. Surg. 2022, 275, e773–e780. [Google Scholar] [CrossRef]
- Iype, S.; Mirza, T.A.; Propper, D.J.; Bhattacharya, S.; Feakins, R.M.; Kocher, H.M. Neuroendocrine tumours of the gallbladder: Three cases and a review of the literature. Postgrad. Med. J. 2009, 85, 213–218. [Google Scholar] [CrossRef]
- Chorath, J.; Placencio-Hickok, V.R.; Guan, M.; Nissen, N.; Kamrava, M.; Klempner, S.J.; Nassir, Y.; Hendifar, A.; Gong, J. Durable Response to Carboplatin, Etoposide, Nivolumab, and Ipilimumab in Metastatic High-Grade Neuroendocrine Carcinoma of the Gallbladder. Pancreas 2020, 49, e19–e20. [Google Scholar] [CrossRef]
- Hofland, J.; Zandee, W.T.; de Herder, W.W. Role of biomarker tests for diagnosis of neuroendocrine tumours. Nat. Rev. Endocrinol. 2018, 14, 656–669. [Google Scholar] [CrossRef]
- Clay, V.; Papaxoinis, G.; Sanderson, B.; Valle, J.W.; Howell, M.; Lamarca, A.; Krysiak, P.; Bishop, P.; Nonaka, D.; Mansoor, W. Evaluation of diagnostic and prognostic significance of Ki-67 index in pulmonary carcinoid tumours. Clin. Transl. Oncol. 2017, 19, 579–586. [Google Scholar] [CrossRef]

| Variables | SEER Database (n = 221) | Training Dataset (n = 156) | Internal Validation Dataset (n = 65) | External Validation Dataset (n = 12) |
|---|---|---|---|---|
| Gender | ||||
| Female | 143 (64.7) | 102 (65.4) | 41 (63.1) | 6 (50.0) |
| Male | 78 (35.3) | 54 (34.6) | 24 (36.9) | 6 (50.0) |
| Race | ||||
| White | 175 (79.2) | 121 (77.6) | 54 (83.1) | 0 (0) |
| Black | 30 (13.6) | 21 (13.4) | 9 (13.8) | 0 (0) |
| Other | 16 (7.2) | 14 (9.0) | 2 (3.1) | 12 (100.0) |
| Age at diagnosis | ||||
| ≤65 years | 119 (53.8) | 87 (55.8) | 32 (49.2) | 7 (58.3) |
| >65 years | 102 (46.2) | 69 (44.2) | 33 (50.8) | 5 (41.7) |
| Marital status | ||||
| Married | 146 (66.1) | 97 (62.2) | 49 (75.4) | 12 (100.0) |
| Unmarried | 75 (33.9) | 59 (37.8) | 16 (24.6) | 0 (0) |
| Pathological classification | ||||
| NET | 86 (38.9) | 61 (39.1) | 25 (38.5) | 0 (0) |
| NEC | 126 (57.0) | 89 (57.1) | 37 (56.9) | 12 (100.0) |
| MiNEN | 9 (4.1) | 6 (3.8) | 3 (4.6) | 0 (0) |
| N stage | ||||
| N0 | 150 (67.9) | 110 (70.5) | 40 (61.5) | 7 (58.3) |
| N1 | 49 (22.2) | 32 (20.5) | 17 (26.2) | 5 (41.7) |
| Unknown | 22 (10.0) | 14 (9.0) | 8 (12.3) | 0 (0) |
| M Stage | ||||
| M0 | 161 (72.9) | 115 (73.7) | 46 (70.8) | 6 (50.0) |
| M1 | 42 (19.0) | 29 (18.6) | 13 (20.0) | 6 (50.0) |
| Unknown | 18 (8.1) | 12 (7.7) | 6 (9.2) | 0 (0) |
| Tumor size | ||||
| ≤2 cm | 92 (41.6) | 66 (42.3) | 26 (40.0) | 2 (16.7) |
| 2–5 cm | 50 (22.6) | 33 (21.2) | 17 (26.1) | 8 (66.6) |
| ≥5 cm | 32 (14.5) | 20 (12.8) | 12 (18.5) | 2 (16.7) |
| Unknown | 47 (21.3) | 37 (23.7) | 10 (15.4) | 0 (0) |
| Stage | ||||
| Localized | 95 (43.0) | 72 (46.2) | 23 (35.4) | 0 (0) |
| Regional | 72 (32.6) | 48 (30.8) | 24 (36.9) | 6 (50.0) |
| Distant | 54 (24.4) | 36 (23.0) | 18 (27.7) | 6 (50.0) |
| Surgery at other sites | ||||
| No | 195 (88.2) | 134 (85.9) | 61 (93.8) | 12 (100.0) |
| Yes | 26 (11.8) | 22 (14.1) | 4 (6.2) | 0 (0) |
| LN surgery | ||||
| No | 147 (66.5) | 102 (65.4) | 45 (69.2) | 10 (83.3) |
| Yes | 74 (33.5) | 54 (34.6) | 20 (30.8) | 2 (16.7) |
| Chemotherapy | ||||
| No | 148 (67.0) | 103 (66.0) | 45 (69.2) | 7 (58.3) |
| Yes | 73 (33.0) | 53 (34.0) | 20 (30.8) | 5 (41.7) |
| Radiotherapy | ||||
| No | 191 (86.4) | 135 (86.5) | 56 (86.2) | 11 (91.7) |
| Yes | 30 (13.6) | 21 (13.5) | 9 (13.8) | 1 (8.3) |
| Variables | OS | p-Value | Multivariate Analysis | p-Value | CSS | p-Value | Multivariate Analysis | p-Value |
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Univariate Analysis | |||||||
| HR (95% CI) | HR (95% CI) | HR (95% CI) | HR (95% CI) | |||||
| Gender | ||||||||
| Female | Ref. | - | Ref. | - | ||||
| Male | 1.26 (0.80, 2.01) | 0.321 | 0.95 (0.54, 1.66) | 0.857 | ||||
| Race | ||||||||
| White | Ref. | - | Ref. | - | ||||
| Black | 0.93 (0.46, 1.89) | 0.849 | 0.85 (0.36, 2.01) | 0.710 | ||||
| Others | 1.48 (0.73, 3.01) | 0.272 | 1.41 (0.64, 3.14) | 0.396 | ||||
| Age at diagnosis | ||||||||
| ≤65 years | Ref. | Ref. | ||||||
| >65 years | 2.77 (1.74, 4.42) | <0.001 | 2.49 (1.42, 4.36) | 0.001 | 2.34 (1.37, 3.99) | 0.002 | 2.32 (1.16, 4.62) | 0.017 |
| Marital status | ||||||||
| Married | Ref. | - | Ref. | |||||
| Unmarried | 0.783 (0.49, 1.26) | 0.313 | 0.60 (0.33, 1.07) | 0.082 | - | |||
| Pathological classification | ||||||||
| NET | Ref. | Ref. | ||||||
| NEC | 6.26 (3.34, 11.70) | <0.001 | 1.78 (0.74, 4.31) | 0.200 | 19.1 (5.93, 61.50) | <0.001 | 5.30 (1.33, 21.21) | 0.018 |
| MiNEN | 12.60 (4.33, 36.40) | <0.001 | 5.57 (1.52, 20.46) | 0.010 | 33.9 (7.49, 153) | <0.001 | 15.44 (2.71, 87.94) | 0.002 |
| N stage | ||||||||
| N0 | Ref. | Ref. | ||||||
| N1 | 1.78 (1.03, 3.09) | 0.038 | 0.65 (0.34, 1.24) | 0.194 | 2.06 (1.14, 3.75) | 0.017 | 0.67 (0.33, 1.35) | 0.266 |
| Unknown | 1.19 (0.47, 3.04) | 0.710 | 2.31 (0.56, 9.58) | 0.248 | 1.50 (0.58, 3.87) | 0.402 | 2.87 (0.66, 12.56) | 0.162 |
| M stage | ||||||||
| M0 | Ref. | Ref. | ||||||
| M1 | 5.64 (3.34, 9.53) | <0.001 | 1.91 (0.66, 5.47) | 0.231 | 6.45 (3.62, 11.5) | <0.001 | 1.29 (0.42, 3.96) | 0.655 |
| Unknown | 0.99 (0.30, 3.23) | 0.984 | 0.31 (0.05, 1.95) | 0.212 | 1.24 (0.37, 4.11) | 0.726 | 0.25 (0.04, 1.76) | 0.163 |
| Tumor size | ||||||||
| ≤2 cm | Ref. | Ref. | ||||||
| 2–5 cm | 3.74 (1.95, 7.18) | <0.001 | 1.75 (0.81, 3.79) | 0.156 | 8.30 (3.26, 21.10) | <0.001 | 3.29 (1.13, 9.53) | 0.028 |
| ≥5 cm | 5.45 (2.69, 11.0) | <0.001 | 2.88 (1.31, 6.33) | 0.009 | 12.6 (4.73, 33.50) | <0.001 | 5.55 (1.92, 16.04) | 0.002 |
| Unknown | 4.28 (2.29, 7.99) | <0.001 | 4.15 (2.07, 8.30) | <0.001 | 9.50 (3.81, 23.70) | <0.001 | 9.04 (3.19, 25.64) | <0.001 |
| SEER Stage | ||||||||
| Localized | Ref. | Ref. | ||||||
| Regional | 4.01 (2.20, 7.32) | <0.001 | 2.50 (1.09, 5.72) | 0.030 | 5.92 (2.62, 13.40) | <0.001 | 2.17 (0.78, 6.00) | 0.136 |
| Distant | 10.10 (5.43, 18.90) | <0.001 | 4.50 (1.39, 14.62) | 0.012 | 17.00 (7.52, 38.50) | <0.001 | 6.18 (1.52, 25.08) | 0.011 |
| Surgery at other sites | ||||||||
| No | Ref. | - | Ref. | - | ||||
| Yes | 1.56 (0.85, 2.84) | 0.149 | 1.69 (0.87, 3.27) | 0.120 | ||||
| LN surgery | ||||||||
| No | Ref. | - | Ref. | - | ||||
| Yes | 0.89 (0.55, 1.43) | 0.620 | 1.17 (0.69, 1.99) | 0.564 | ||||
| Chemotherapy | ||||||||
| No | Ref. | Ref. | ||||||
| Yes | 2.41 (1.52, 3.80) | <0.001 | 0.96 (0.52, 1.76) | 0.900 | 3.20 (1.89, 5.43) | <0.001 | 0.96 (0.47, 1.96) | 0.918 |
| Radiotherapy | ||||||||
| No | Ref. | - | Ref. | |||||
| Yes | 1.68 (0.94, 3.02) | 0.079 | 2.00 (1.05, 3.80) | 0.034 | 0.92 (0.44, 1.89) | 0.812 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.-R.; Hu, G.-C.; Fan, M.-K.; Yao, H.-L.; Jiang, C.; Shi, H.-Y.; Lin, R. Nomograms for Predicting Survival Outcomes in Patients with Neuroendocrine Neoplasms of the Gallbladder Undergoing Primary Tumor Resection: A Population-Based Study. Curr. Oncol. 2023, 30, 2889-2899. https://doi.org/10.3390/curroncol30030221
Zhang Y-R, Hu G-C, Fan M-K, Yao H-L, Jiang C, Shi H-Y, Lin R. Nomograms for Predicting Survival Outcomes in Patients with Neuroendocrine Neoplasms of the Gallbladder Undergoing Primary Tumor Resection: A Population-Based Study. Current Oncology. 2023; 30(3):2889-2899. https://doi.org/10.3390/curroncol30030221
Chicago/Turabian StyleZhang, Yu-Rui, Geng-Cheng Hu, Meng-Ke Fan, Hai-Ling Yao, Chen Jiang, Hui-Ying Shi, and Rong Lin. 2023. "Nomograms for Predicting Survival Outcomes in Patients with Neuroendocrine Neoplasms of the Gallbladder Undergoing Primary Tumor Resection: A Population-Based Study" Current Oncology 30, no. 3: 2889-2899. https://doi.org/10.3390/curroncol30030221
APA StyleZhang, Y.-R., Hu, G.-C., Fan, M.-K., Yao, H.-L., Jiang, C., Shi, H.-Y., & Lin, R. (2023). Nomograms for Predicting Survival Outcomes in Patients with Neuroendocrine Neoplasms of the Gallbladder Undergoing Primary Tumor Resection: A Population-Based Study. Current Oncology, 30(3), 2889-2899. https://doi.org/10.3390/curroncol30030221




