A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Clinical Study
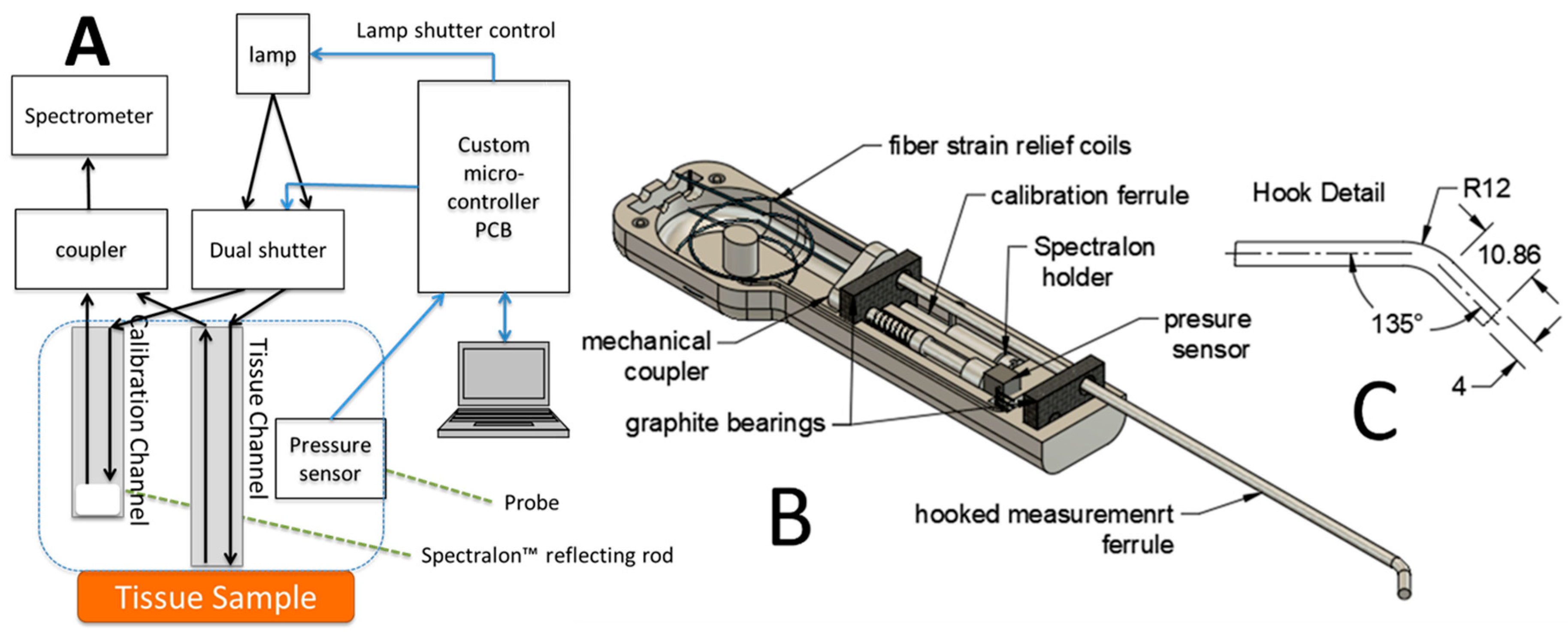
2.2. Optical Spectroscopy
2.3. Self-Calibration
2.4. Pressure-Sensing Probe
2.5. Spectral Data Analysis
3. Results
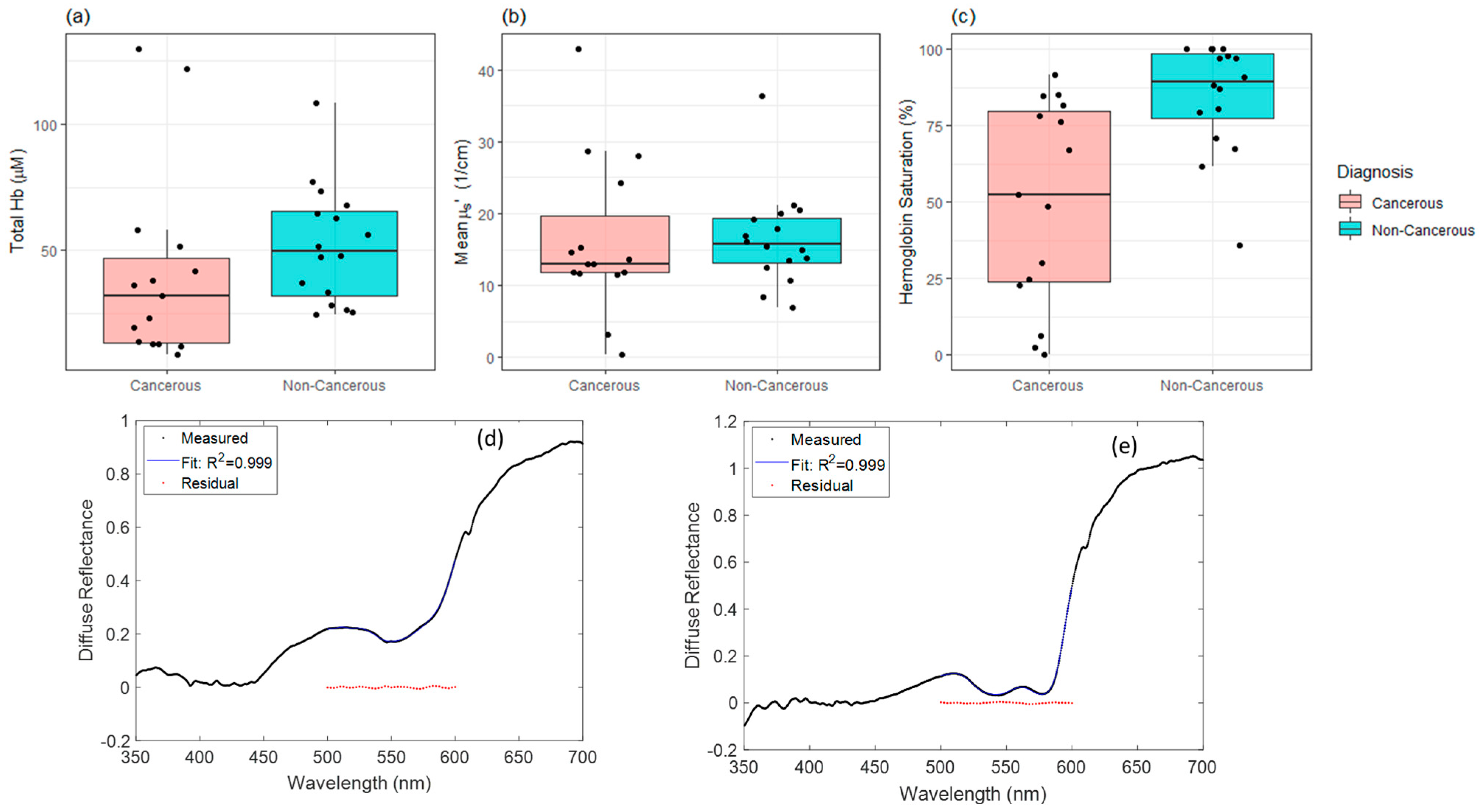
3.1. Hemoglobin Saturation Significantly Lower in Cancerous Tissues
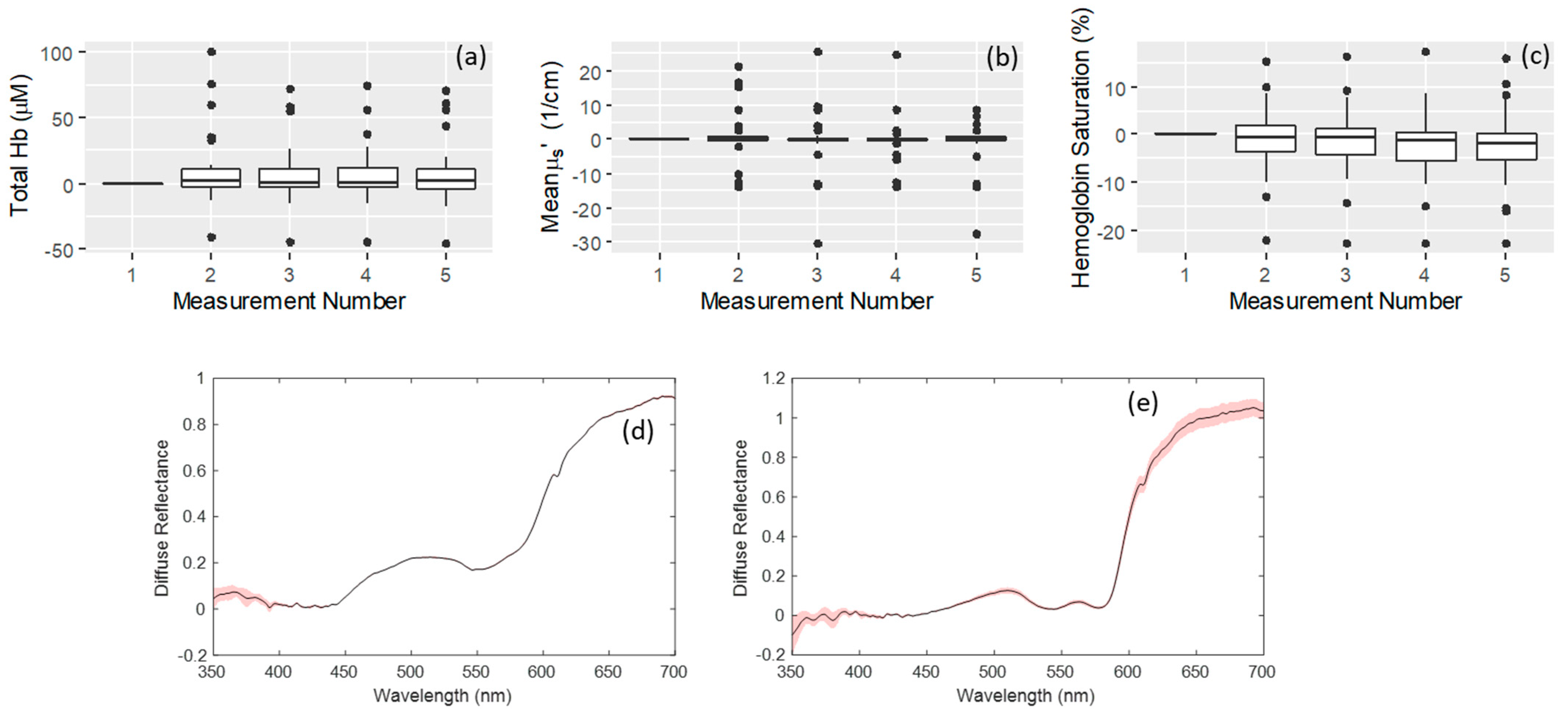
3.2. Effects of Probe Placement upon Repeated Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Head and Neck Cancer: Statistics. Available online: www.cancer.net (accessed on 23 June 2022).
- Argiris, A.; Karamouzis, M.V.; Raben, D.; Ferris, R.L. Head and neck cancer. Lancet 2008, 371, 1695–1709. [Google Scholar] [CrossRef] [PubMed]
- Brockstein, B.; Haraf, D.J.; Rademaker, A.W.; Kies, M.S.; Stenson, K.M.; Rosen, F.; Mittal, B.B.; Pelzer, H.; Fung, B.B.; Witt, M.E.; et al. Patterns of failure, prognostic factors and survival in locoregionally advanced head and neck cancer treated with concomitant chemoradiotherapy: A 9-year, 337-patient, multi-institutional experience. Ann. Oncol. 2004, 15, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Budach, V.; Tinhofer, I. Novel prognostic clinical factors and biomarkers for outcome prediction in head and neck cancer: A systematic review. Lancet Oncol. 2019, 20, e313–e326. [Google Scholar] [CrossRef] [PubMed]
- Palmer, G.M.; Zhang, H.; Lee, C.T.; Mikati, H.; Herbert, J.A.; Krieger, M.; von Windheim, J.; Koester, D.; Stevenson, D.; Rocke, D.J.; et al. Assessing effects of pressure on tumor and normal tissue physiology using an automated self-calibrated, pressure-sensing probe for diffuse reflectance spectroscopy. J. Biomed. Opt. 2018, 23, 057004. [Google Scholar] [CrossRef]
- Yu, B.; Fu, H.; Bydlon, T.; Bender, J.; Ramanujam, N. A Self-Calibrating Fiber Optic Probe for Tissue Optical Spectroscopy. In Proceedings of the 2008 Conference on Lasers and Electro-Optics and 2008 Conference on Quantum Electronics and Laser Science, San Jose, CA, USA, 4–9 May 2008. [Google Scholar] [CrossRef]
- Chang, V.T.; Merisier, D.; Yu, B.; Walmer, D.K.; Ramanujam, N. Towards a field-compatible optical spectroscopic device for cervical cancer screening in resource-limited settings: Effects of calibration and pressure. Opt. Express 2011, 19, 17908–17924. [Google Scholar] [CrossRef]
- Jayanthi, J.L.; Nisha, G.U.; Manju, S.; Philip, E.K.; Jeemon, P.; Baiju, K.V.; Beena, V.T.; Subhash, N. Diffuse reflectance spectroscopy: Diagnostic accuracy of a non-invasive screening technique for early detection of malignant changes in the oral cavity. BMJ Open 2011, 1, e000071. [Google Scholar] [CrossRef]
- Arimoto, H.; Egawa, M.; Yamada, Y. Depth profile of diffuse reflectance near-infrared spectroscopy for measurement of water content in skin. Skin Res. Technol. 2005, 11, 27–35. [Google Scholar] [CrossRef]
- Palmer, G.M.; Vishwanath, K.; Dewhirst, M.W. Application of optical imaging and spectroscopy to radiation biology. Radiat. Res. 2012, 177, 365–375. [Google Scholar] [CrossRef]
- Nichols, M.G.; Hull, E.L.; Foster, T.H. Design and testing of a white-light, steady-state diffuse reflectance spectrometer for determination of optical properties of highly scattering systems. Appl. Opt. 1997, 36, 93–104. [Google Scholar] [CrossRef]
- Chae, E.Y.; Kim, H.H.; Sabir, S.; Kim, Y.; Kim, H.; Yoon, S.; Ye, J.C.; Cho, S.; Heo, D.; Kim, K.H.; et al. Development of digital breast tomosynthesis and diffuse optical tomography fusion imaging for breast cancer detection. Sci. Rep. 2020, 10, 13127. [Google Scholar] [CrossRef]
- Uncu, Y.A.; Sevim, G.; Mercan, T.; Vural, V.; Durmaz, E.; Canpolat, M. Differentiation of tumoral and non-tumoral breast lesions using back reflection diffuse optical tomography: A pilot clinical study. Int. J. Imaging Syst. Technol. 2021, 31, 2023–2031. [Google Scholar] [CrossRef]
- Uddin, K.M.S.; Zhang, M.H.; Anastasio, M.; Zhu, Q. Optimal breast cancer diagnostic strategy using combined ultrasound and diffuse optical tomography. Biomed. Opt. Express 2020, 11, 2722–2737. [Google Scholar] [CrossRef] [PubMed]
- Marin, N.M.; MacKinnon, N.; MacAulay, C.; Chang, S.K.; Atkinson, E.N.; Cox, D.; Serachitopol, D.; Pikkula, B.; Follen, M.; Richards-Kortum, R. Calibration standards for multicenter clinical trials of fluorescence spectroscopy for in vivo diagnosis. J. Biomed. Opt. 2006, 11, 014010. [Google Scholar] [CrossRef] [PubMed]
- Ti, Y.; Lin, W.C. Effects of probe contact pressure on in vivo optical spectroscopy. Opt. Express 2008, 16, 4250–4262. [Google Scholar] [CrossRef] [PubMed]
- Vishwanath, K.; Klein, D.; Chang, K.; Schroeder, T.; Dewhirst, M.W.; Ramanujam, N. Quantitative optical spectroscopy can identify long-term local tumor control in irradiated murine head and neck xenografts. J. Biomed. Opt. 2009, 14, 054051. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Vishwanath, K.; Lo, J.; Erkanli, A.; Mulvey, C.; Lee, W.T.; Ramanujam, N. Rapid determination of oxygen saturation and vascularity for cancer detection. PLoS ONE 2013, 8, e82977. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.Q.; Wilke, L.G.; Geradts, J.; Kennedy, S.A.; Palmer, G.M.; Ramanujam, N. Quantitative optical spectroscopy: A robust tool for direct measurement of breast cancer vascular oxygenation and total hemoglobin content in vivo. Cancer Res. 2009, 69, 2919–2926. [Google Scholar] [CrossRef]
- Beumer, H.W.; Vishwanath, K.; Puscas, L.; Afshari, H.R.; Ramanujam, N.; Lee, W.T. Detection of squamous cell carcinoma and corresponding biomarkers using optical spectroscopy. Otolaryngol. Head Neck Surg. 2011, 144, 390–394. [Google Scholar] [CrossRef]
- van Veen, R.L.; Verkruysse, W.; Sterenborg, H.J. Diffuse-reflectance spectroscopy from 500 to 1060 nm by correction for inhomogeneously distributed absorbers. Opt. Lett. 2002, 27, 246–248. [Google Scholar] [CrossRef]
- Jacques, S.L. Skin Optics Summary. Oregon Medical Laser Center News, 1 June 1998. [Google Scholar]
- Virtanen, P.; Gommers, R.; Oliphant, T.E.; Haberland, M.; Reddy, T.; Cournapeau, D.; Burovski, E.; Peterson, P.; Weckesser, W.; Bright, J.; et al. SciPy 1.0: Fundamental algorithms for scientific computing in Python. Nat Methods. 2020, 17, 261–272. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing [Computer Software Manual]; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Reif, R.; Amorosino, M.S.; Calabro, K.W.; A’Amar, O.; Singh, S.K.; Bigio, I.J. Analysis of changes in reflectance measurements on biological tissues subjected to different probe pressures. J. Biomed. Opt. 2008, 13, 010502. [Google Scholar] [CrossRef] [PubMed]
- van Veen, R.L.; Amelink, A.; Menke-Pluymers, M.; van der Pol, C.; Sterenborg, H.J. Optical biopsy of breast tissue using differential path-length spectroscopy. Phys. Med. Biol. 2005, 50, 2573–2581. [Google Scholar] [CrossRef] [PubMed]
- Wendling, P.; Manz, R.; Thews, G.; Vaupel, P. Heterogeneous oxygenation of rectal carcinomas in humans: A critical parameter for preoperative irradiation? Adv. Exp. Med. Biol. 1984, 180, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.; Vishwanath, K.; Beumer, H.W.; Puscas, L.; Afshari, H.R.; Esclamado, R.M.; Scher, R.; Fisher, S.; Lo, J.; Mulvey, C.; et al. Assessment of the sensitivity and specificity of tissue-specific-based and anatomical-based optical biomarkers for rapid detection of human head and neck squamous cell carcinoma. Oral Oncol. 2014, 50, 848–856. [Google Scholar] [CrossRef] [PubMed]
| Study Summary | |
| Subjects in Study | 19 |
| Subjects with cancer sites sampled | 12 |
| Subjects with normal sites sampled | 14 |
| Subjects with benign sites samples | 1 |
| Specifics of Sampled Sites | |
| Squamous Cell Carcinoma | Benign |
| 8 tongue 1 larynx 1 oropharynx 1 tonsil 1 piriform lesion | 1 benign atypical verrucous hyperplasia |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rickard, A.G.; Mikati, H.; Mansourati, A.; Stevenson, D.; Krieger, M.; Rocke, D.; Esclamado, R.; Dewhirst, M.W.; Ramanujam, N.; Lee, W.T.; et al. A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer. Curr. Oncol. 2023, 30, 2751-2760. https://doi.org/10.3390/curroncol30030208
Rickard AG, Mikati H, Mansourati A, Stevenson D, Krieger M, Rocke D, Esclamado R, Dewhirst MW, Ramanujam N, Lee WT, et al. A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer. Current Oncology. 2023; 30(3):2751-2760. https://doi.org/10.3390/curroncol30030208
Chicago/Turabian StyleRickard, Ashlyn G., Husam Mikati, Antoine Mansourati, Daniel Stevenson, Marlee Krieger, Daniel Rocke, Ramon Esclamado, Mark W. Dewhirst, Nirmala Ramanujam, Walter T. Lee, and et al. 2023. "A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer" Current Oncology 30, no. 3: 2751-2760. https://doi.org/10.3390/curroncol30030208
APA StyleRickard, A. G., Mikati, H., Mansourati, A., Stevenson, D., Krieger, M., Rocke, D., Esclamado, R., Dewhirst, M. W., Ramanujam, N., Lee, W. T., & Palmer, G. M. (2023). A Clinical Study to Assess Diffuse Reflectance Spectroscopy with an Auto-Calibrated, Pressure-Sensing Optical Probe in Head and Neck Cancer. Current Oncology, 30(3), 2751-2760. https://doi.org/10.3390/curroncol30030208





