Understanding Characteristics, Treatment Patterns, and Clinical Outcomes for Individuals with Advanced or Recurrent Endometrial Cancer in Alberta, Canada: A Retrospective, Population-Based Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Design
2.2. Data Sources
2.3. Cohort Creation
2.4. Patient Characteristics
2.5. Treatments and Lines of Therapy
2.6. Outcomes
2.7. Subgroup Analysis
2.8. Statistical Analysis
3. Results
3.1. Patient Cohort Characteristics
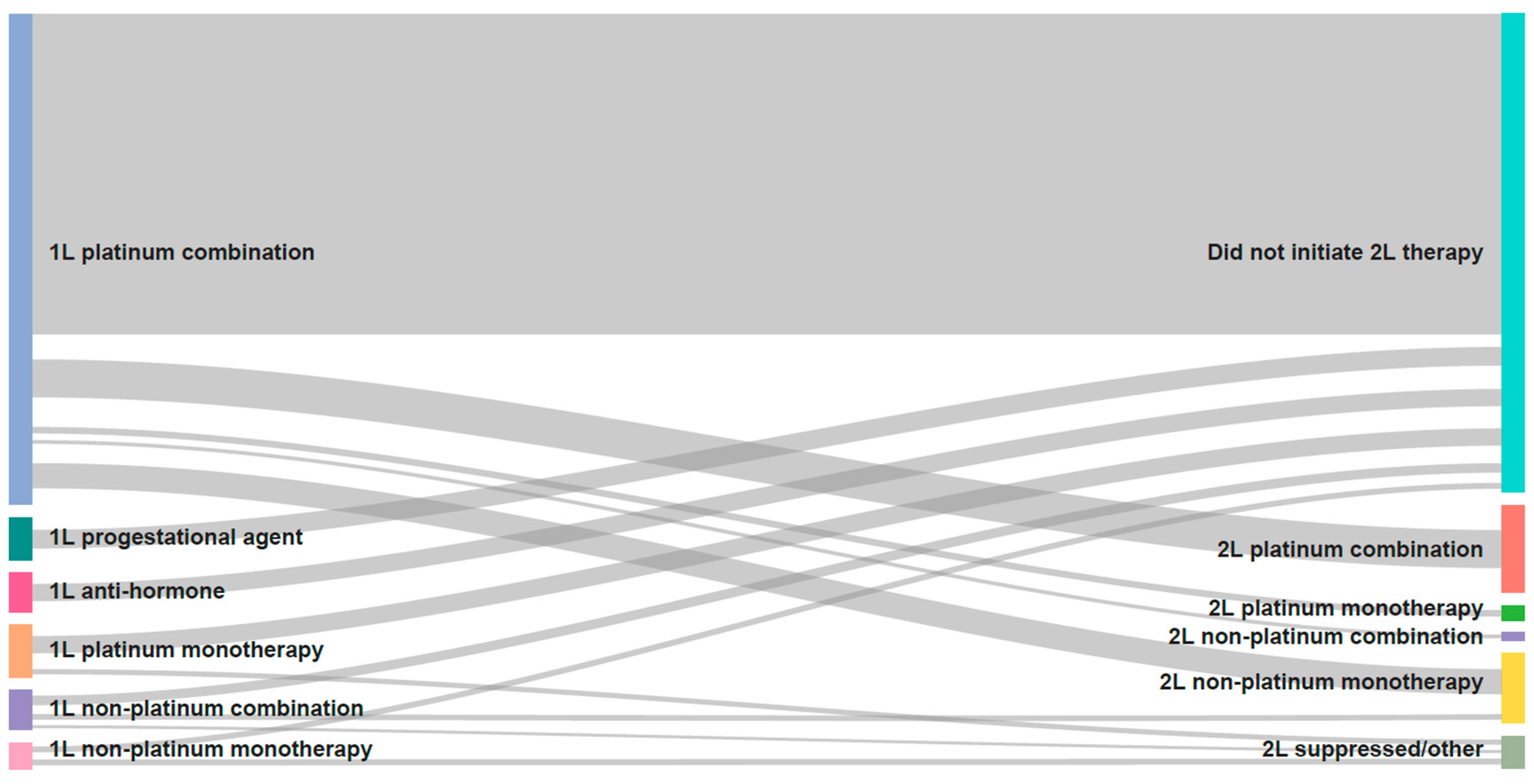
3.2. Treatment Patterns
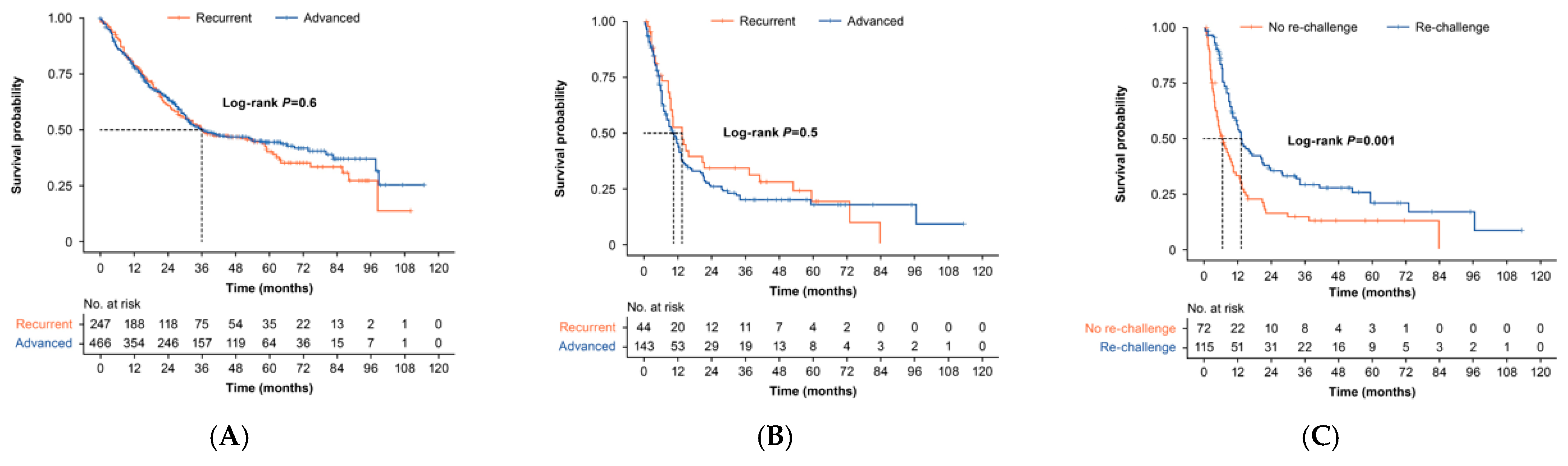
3.3. Overall Survival and Time to Next Treatment
3.3.1. First-Line Therapy
3.3.2. Second-Line Therapy
3.3.3. Third-Line Therapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Poirier, A.; Woods, R.R.; Ellison, L.F.; Billette, J.-M.; Demers, A.A.; Zhang, S.X.; Yao, C.; Finley, C.; Fitzgerald, N.; et al. Projected estimates of cancer in Canada in 2022. Can. Med. Assoc. J. 2022, 194, E601–E607. [Google Scholar] [CrossRef]
- Gu, B.; Shang, X.; Yan, M.; Li, X.; Wang, W.; Wang, Q.; Zhang, C. Variations in incidence and mortality rates of endometrial cancer at the global, regional, and national levels, 1990–2019. Gynecol. Oncol. 2021, 161, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Sobel, M.; Simpson, A.N.; Ferguson, S.E. Endometrial cancer. CMAJ 2021, 193, E1423. [Google Scholar] [CrossRef]
- National Cancer Institute. SEER Cancer Statistics. Cancer Stat Facts: Uterine Cancer. Available online: https://seer.cancer.gov/statfacts/html/corp.html (accessed on 30 June 2022).
- Kralickova, M.; Vetvicka, V.; Lagana, A.S. Endometrial cancer-is our knowledge changing? Transl. Cancer Res. 2020, 9, 7734–7745. [Google Scholar] [CrossRef] [PubMed]
- Alberta Health Services. Cancer Guidelines: Endometrial Cancer. Available online: https://www.albertahealthservices.ca/assets/info/hp/cancer/if-hp-cancer-guide-gyne002-endometrial.pdf (accessed on 21 March 2022).
- Brooks, R.A.; Fleming, G.F.; Lastra, R.R.; Lee, N.K.; Moroney, J.W.; Son, C.H.; Tatebe, K.; Veneris, J.L. Current recommendations and recent progress in endometrial cancer. CA Cancer J. Clin. 2019, 69, 258–279. [Google Scholar] [CrossRef]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef]
- Ontario Health (Cancer Care Ontario). Endometrial Cancer Treatment and Follow up Pathway Map. Available online: https://www.cancercareontario.ca/sites/ccocancercare/files/assets/EndometrialCancerTreatmentPathwayMap.pdf (accessed on 21 March 2022).
- Miller, D.S.; Filiaci, V.L.; Mannel, R.S.; Cohn, D.E.; Matsumoto, T.; Tewari, K.S.; DiSilvestro, P.; Pearl, M.L.; Argenta, P.A.; Powell, M.A.; et al. Carboplatin and Paclitaxel for Advanced Endometrial Cancer: Final Overall Survival and Adverse Event Analysis of a Phase III Trial (NRG Oncology/GOG0209). J. Clin. Oncol. 2020, 38, 3841–3850. [Google Scholar] [CrossRef]
- Vale, C.L.; Tierney, J.; Bull, S.J.; Symonds, P.R. Chemotherapy for advanced, recurrent or metastatic endometrial carcinoma. Cochrane Database Syst. Rev. 2012, 2012, CD003915. [Google Scholar] [CrossRef]
- Li, T.; Mizrahi, D.; Goldstein, D.; Kiernan, M.C.; Park, S.B. Chemotherapy and peripheral neuropathy. Neurol. Sci. 2021, 42, 4109–4121. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.; Halpenny, D.; Makker, V.; Grisham, R.N.; Aghajanian, C.; Cadoo, K. Retreatment with carboplatin and paclitaxel for recurrent endometrial cancer: A retrospective study of the Memorial Sloan Kettering Cancer Center experience. Gynecol. Oncol. Rep. 2019, 28, 120–123. [Google Scholar] [CrossRef]
- Nagao, S.; Nishio, S.; Michimae, H.; Tanabe, H.; Okada, S.; Otsuki, T.; Tanioka, M.; Fujiwara, K.; Suzuki, M.; Kigawa, J. Applicability of the concept of “platinum sensitivity” to recurrent endometrial cancer: The SGSG-012/GOTIC-004/Intergroup study. Gynecol. Oncol. 2013, 131, 567–573. [Google Scholar] [CrossRef] [PubMed]
- Nagao, S.; Nishio, S.; Okada, S.; Otsuki, T.; Fujiwara, K.; Tanabe, H.; Takano, M.; Hasumi, Y.; Takei, Y.; Hasegawa, T.; et al. What is an appropriate second-line regimen for recurrent endometrial cancer? Ancillary analysis of the SGSG012/GOTIC004/Intergroup study. Cancer Chemother. Pharmacol. 2015, 76, 335–342. [Google Scholar] [CrossRef]
- Giudice, E.; Salutari, V.; Ricci, C.; Nero, C.; Carbone, M.V.; Musacchio, L.; Ghizzoni, V.; Perri, M.T.; Camarda, F.; Tronconi, F.; et al. Recent progress in the use of pharmacotherapy for endometrial cancer. Expert Opin. Pharmacother. 2022, 24, 83–94. [Google Scholar] [CrossRef]
- Giustozzi, A.; Salutari, V.; Giudice, E.; Musacchio, L.; Ricci, C.; Landolfo, C.; Perri, M.T.; Scambia, G.; Lorusso, D. Refining Adjuvant Therapy for Endometrial Cancer: New Standards and Perspectives. Biology 2021, 10, 845. [Google Scholar] [CrossRef] [PubMed]
- Amant, F.; Moerman, P.; Neven, P.; Timmerman, D.; Van Limbergen, E.; Vergote, I. Endometrial cancer. Lancet 2005, 366, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.A.; Kim, J.E.; Ruzevick, J.; Lim, M. Present and future of immune checkpoint blockade: Monotherapy to adjuvant approaches. World J. Immunol. 2015, 5, 1–15. [Google Scholar] [CrossRef]
- GSK. Jemperli Product Monograph. Available online: https://ca.gsk.com/media/6620/jemperli_pm.pdf (accessed on 21 March 2022).
- Merck & Co Inc. Keytruda Product Monograph. Available online: https://pdf.hres.ca/dpd_pm/00065368.PDF (accessed on 14 April 2022).
- O’Malley, D.M.; Bariani, G.M.; Cassier, P.A.; Marabelle, A.; Hansen, A.R.; Acosta, A.D.J.; Miller, W.H., Jr.; Safra, T.; Italiano, A.; Mileshkin, L.; et al. Pembrolizumab in Patients with Microsatellite Instability–High Advanced Endometrial Cancer: Results From the KEYNOTE-158 Study. J. Clin. Oncol. 2022, 40, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Oaknin, A.; Gilbert, L.; Tinker, A.V.; Brown, J.; Mathews, C.; Press, J.; Sabatier, R.; O’Malley, D.M.; Samouelian, V.; Boni, V.; et al. Safety and antitumor activity of dostarlimab in patients with advanced or recurrent DNA mismatch repair deficient/microsatellite instability-high (dMMR/MSI-H) or proficient/stable (MMRp/MSS) endometrial cancer: Interim results from GARNET-a phase I, single-arm study. J. Immunother. Cancer 2022, 10, e003777. [Google Scholar] [PubMed]
- Eisai Ltd. LENVIMA Product Monograph. Available online: https://ca.eisai.com/-/media/Files/CanadaEisai/LENVIMA-Product-Monograph-EN.pdf?hash=f43eb602-ffb4-469b-910b-6253e91084bc#:~:text=Recommended%20Dose%20for%20Endometrial%20Carcinoma,unacceptable%20toxicity%20or%20disease%20progression (accessed on 2 November 2022).
- Makker, V.; Colombo, N.; Herráez, A.C.; Santin, A.D.; Colomba, S.E.; Miller, D.S.; Fujiwara, K.; Pignata, S.; Baron-Hay, S.; Ray-Coquard, I.; et al. Lenvatinib plus Pembrolizumab for Advanced Endometrial Cancer. N. Engl. J. Med. 2022, 386, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Vanderpuye-Orgle, J.; Erim, D.; Qian, Y.; Boyne, D.J.; Cheung, W.Y.; Bebb, G.; Shah, A.; Pericleous, L.; Maruszczak, M.; Brenner, D.R. Estimating the Impact of Delayed Access to Oncology Drugs on Patient Outcomes in Canada. Oncol. Ther. 2022, 10, 195–210. [Google Scholar] [CrossRef]
- Gotfrit, J.; Shin, J.J.; Mallick, R.; Stewart, D.J.; Wheatley-Price, P. Potential Life-Years Lost: The Impact of the Cancer Drug Regulatory and Funding Process in Canada. Oncologist 2019, 25, e130–e137. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, S.; Cheung, W.Y.; Bouchard-Fortier, A.; Dort, J.C.; Quan, H.; Buie, E.M.; McKinnon, G.; Quan, M.L. Development and validation of case-finding algorithms for recurrence of breast cancer using routinely collected administrative data. BMC Cancer 2019, 19, 210. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lheureux, S.; Oza, A.M. Treatment strategies for endometrial cancer: Current practice and perspective. Curr. Opin. Obstet. Gynecol. 2017, 29, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- O’Sullivan, D.E.; Cheung, W.Y.; Syed, I.A.; Moldaver, D.; Shanahan, M.K.; Bebb, D.G.; Sit, C.; Brenner, D.R.; Boyne, D.J. Real-World Treatment Patterns, Clinical Outcomes, and Health Care Resource Utilization in Extensive-Stage Small Cell Lung Cancer in Canada. Curr. Oncol. 2021, 28, 3091–3103. [Google Scholar] [CrossRef] [PubMed]
- Akada, K.; Koyama, N.; Miura, T.; Fukunaga, E.; Miura, Y.; Aoshima, K.; Fujiwara, K. Real-world database analysis of the characteristics and treatment patterns of patients with endometrial cancer in Japan. Curr. Med. Res. Opin. 2021, 37, 1171–1178. [Google Scholar] [CrossRef]
- Makker, V.; Hensley, M.L.; Zhou, Q.; Iasonos, A.; Aghajanian, C.A. Treatment of Advanced or Recurrent Endometrial Carcinoma with Doxorubicin in Patients Progressing After Paclitaxel/Carboplatin: Memorial Sloan-Kettering Cancer Center Experience From 1995 to 2009. Int. J. Gynecol. Cancer 2013, 23, 929–934. [Google Scholar] [CrossRef] [PubMed]
- McMeekin, S.; Dizon, D.; Barter, J.; Scambia, G.; Manzyuk, L.; Lisyanskaya, A.; Oaknin, A.; Ringuette, S.; Mukhopadhyay, P.; Rosenberg, J.; et al. Phase III randomized trial of second-line ixabepilone versus paclitaxel or doxorubicin in women with ad-vanced endometrial cancer. Gynecol. Oncol. 2015, 138, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.S.; Scambia, G.; Bondarenko, I.; Westermann, A.M.; Oaknin, A.; Oza, A.M.; Lisyanskaya, A.S.; Vergote, I.; Wenham, R.M.; Temkin, S.M.; et al. ZoptEC: Phase III randomized controlled study comparing zoptarelin with doxorubicin as second line therapy for locally advanced, recurrent, or metastatic endometrial cancer (NCT01767155). J. Clin. Oncol. 2018, 36 (Suppl. S15), 5503. [Google Scholar] [CrossRef]
- Lincoln, S.; Blessing, J.A.; Lee, R.B.; Rocereto, T.F. Activity of paclitaxel as second-line chemotherapy in endometrial carcinoma: A gynecologic oncology group study. Gynecol. Oncol. 2003, 88, 277–281. [Google Scholar] [CrossRef]
- Ueda, Y.; Miyake, T.; Egawa-Takata, T.; Miyatake, T.; Matsuzaki, S.; Yokoyama, T.; Yoshino, K.; Fujita, M.; Enomoto, T.; Kimura, T. Second-line chemotherapy for advanced or recurrent endometrial carcinoma previously treated with paclitaxel and carboplatin, with or without epirubicin. Cancer Chemother. Pharmacol. 2010, 67, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Heffernan, K.; Nikitas, F.S.; Shukla, U.; Camejo, H.S.; Knott, C. Previously treated recurrent or advanced endometrial cancer in England: A real-world observational analysis. Gynecol. Oncol. 2022, 166, 317–325. [Google Scholar] [CrossRef]
- Mazgani, M.; Le, N.; Hoskins, P.J. Reuse of carboplatin and paclitaxel in patients with relapsed endometrial cancer—The British Columbia Cancer Agency experience. Gynecol. Oncol. 2008, 111, 474–477. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Testing the Addition of the Immunotherapy Drug Pembrolizumab to the Usual Chemotherapy Treatment (Paclitaxel and Carboplatin) in Stage III-IV Recurrent Endometrial Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03914612 (accessed on 1 July 2022).
- ClinicalTrials.gov. Atezolizumab Trial in Endometrial Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT03603184 (accessed on 25 May 2022).
- ClinicalTrials.gov. Durvalumab with or without Olaparib as Maintenance Therapy after First-Line Treatment of Advanced and Recurrent Endometrial Cancer (DUO-E). Available online: https://www.clinicaltrials.gov/ct2/show/NCT04269200 (accessed on 25 May 2022).
- ClinicalTrials.gov. Study of Pembrolizumab (MK-3475) Versus Chemotherapy in Mismatch Repair Deficient (dMMR) Ad-vanced or Recurrent Endometrial Carcinoma (MK-3475-C93/KEYNOTE-C93/GOG-3064/ENGOT-en15). Available online: https://clinicaltrials.gov/ct2/show/NCT05173987 (accessed on 25 May 2022).
- ClinicalTrials.gov. A Study to Evaluate Dostarlimab Plus Carboplatin-Paclitaxel Versus Placebo Plus Carboplatin-Paclitaxel in Participants with Recurrent or Primary Advanced Endometrial Cancer (RUBY). Available online: https://clinicaltrials.gov/ct2/show/NCT03981796 (accessed on 25 May 2022).
- ClinicalTrials.gov. Pembrolizumab (MK-3475) Plus Lenvatinib (E7080/MK-7902) Versus Chemotherapy for Endometrial Carcinoma (ENGOT-en9/MK-7902-001) (LEAP-001). Available online: https://clinicaltrials.gov/ct2/show/NCT03884101 (accessed on 25 May 2022).
- Havrilesky, L.J.; Cragun, J.M.; Calingaert, B.; Synan, I.; Secord, A.A.; Soper, J.T.; Clarke-Pearson, D.L.; Berchuck, A. Resection of lymph node metastases influences survival in stage IIIC endometrial cancer. Gynecol. Oncol. 2005, 99, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Lamont, E.B.; Herndon, J.E., II; Weeks, J.C.; Henderson, I.C.; Earle, C.C.; Schilsky, R.L.; Christakis, N.A. Measuring disease-free survival and cancer relapse using Medicare claims from CALGB breast cancer trial participants (companion to 9344). J. Natl. Cancer Inst. 2006, 98, 1335–1338. [Google Scholar] [CrossRef] [PubMed]
| Variable | Overall (n = 620) | Chemo 1 (n = 451) | No Chemo 2 (n = 169) | p-Value |
|---|---|---|---|---|
| Age, years (mean ± SD, range) | 65 ± 11 31–98 | 64 ± 10 31–92 | 69 ± 13 35–98 | <0.001 3 |
| <60 years, n (%) | 185 (29.8) | 145 (32.2) | 40 (23.7) | <0.001 4 |
| 60–<70 years, n (%) | 224 (36.1) | 181 (40.1) | 43 (25.4) | –– |
| 70+, n (%) | 211 (34.0) | 125 (27.7) | 86 (50.9) | –– |
| Weight, kg (mean ± SD) 5 | 81.4 ± 23.1 | 81.7 ± 22.6 | 80.5 ± 24.8 | 0.66 3 |
| <60 kg, n (%) | 82 (13.2) | 64 (14.2) | 18 (10.6) | 0.89 4 |
| 60+ kg, n (%) | 423 (68.2) | 336 (74.5) | 87 (51.5) | –– |
| Missing information, n (%) | 115 (18.5) | 51 (11.3) | 64 (37.9) | –– |
| Charlson Comorbidity Index, n (%) | <0.001 4 | |||
| 0 | 433 (69.8) | 333 (73.8) | 100 (59.2) | –– |
| 1 | 118 (19.0) | 81 (18.0) | 37 (21.9) | –– |
| 2+ | 69 (11.1) | 37 (8.2) | 32 (18.9) | –– |
| Tumor characteristics and metastatic sites, n (%) | ||||
| Type | 0.02 4 | |||
| I | 272 (43.9) | 184 (40.8) | 88 (52.1) | –– |
| II | 348 (56.1) | 267 (59.2) | 81 (47.9) | –– |
| AJCC Stage | <0.001 4 | |||
| IIIB | 42 (6.8) | 19 (4.2) | 23 (13.6) | –– |
| IIIC | 329 (53.1) | 263 (58.3) | 66 (39.1) | –– |
| IV | 249 (40.2) | 169 (37.5) | 80 (47.3) | –– |
| Number of metastatic sites at diagnosis | 0.11 4 | |||
| 0 | 371 (59.8) | 282 (62.5) | 89 (52.7) | –– |
| 1 | 149 (24.0) | 106 (23.5) | 43 (25.4) | –– |
| 2 | 54 (8.7) | 36 (8.0) | 18 (10.7) | –– |
| 3+ | 35 (5.6) | 21 (4.7) | 14 (8.3) | –– |
| Missing | 11 (1.8) | <10 (<2.2) | <10 (<5.9) | –– |
| Treatment characteristics, n (%) | ||||
| Surgery | 475 (76.6) | 382 (84.7) | 93 (55.0) | <0.001 4 |
| Radiotherapy | 287 (46.3) | 224 (49.7) | 63 (37.3) | 0.01 4 |
| Patients Who Received: | Overall | Advanced EC | Recurrent EC |
|---|---|---|---|
| (N = 1053) | (N = 620; 58.9%) | (N = 433; 41.1%) | |
| No systemic therapy | N = 340 (32.3%) | N = 154 (24.8%) | N = 186 (43.0%) |
| 1L systemic therapy, n (%) | N = 713 (67.7%) | N = 466 (75.2%) | N = 247 (57.0%) |
| Platinum combination | 506 (71.0) | 410 (88.0) | 96 (38.9) |
| Platinum monotherapy | 54 (7.6) | 38 (8.2) | 16 (6.5) |
| Non-platinum combination | 41 (5.8) | <10 (<2.1) | 66 (26.7) |
| Non-platinum monotherapy | 28 (3.9) | ||
| Progestational agent/hormone therapy | 84 (11.8) | 15 (3.2) | 69 (27.9) |
| 2L systemic therapy, n (%) | N = 257 (24.4%) | N = 169 (27.3%) | N = 88 (20.32%) |
| Platinum combination | 97 (37.7) | 79 (46.7) | 18 (20.5) |
| Platinum monotherapy | 20 (7.8) | >10 (>5.9) | <10 (<11.4) |
| Non-platinum combination | 18 (7.0) | <10 (<5.9) | >8 (>9.1) |
| Non-platinum monotherapy | 85 (33.1) | 45 (26.6) | 40 (45.5) |
| Progestational agent/hormone therapy | 37 (14.4) | 25 (14.8) | 12 (13.6) |
| 2L systemic therapy following 1L PBCT, n (%) | N = 187 (17.8%) | N = 144 (23.2%) | N = 43 (9.9) |
| Platinum combination | 96 (51.3) | 79 (54.9) | 17 (39.5) |
| Platinum monotherapy | 19 (10.2) | >9 (6.3) | <10 (<23.0) |
| Non-platinum combination | <10 (<5.3) | <10 (<6.9) | <10 (<23.0) |
| Non-platinum monotherapy | 65 (34.8) | 44 (30.6) | 21 (48.8) |
| 3L systemic therapy, n (%) | N = 90 (8.5%) | N = 39–57 (<9.2%) | N = 33–41 (<9.5%) |
| Non-platinum monotherapy | 40 (44.4) | 22 | 18 |
| Platinum combination | 22 (24.4) | >13 | <10 |
| Other chemotherapy | <10 (<11.1) | <10 | <10 |
| Progestational agent/hormone therapy | 19 (21.1) | >10 | <10 |
| Median Survival, Months (95% CI) | 2-Year Survival Probability (95% CI) | 5-Year Survival Probability (95% CI) | ||
|---|---|---|---|---|
| OS from 1L, by disease type | All (N = 713) | 35.9 (31.5–53.5) | 0.63 (0.59–0.67) | 0.43 (0.39–0.47) |
| Recurrent (N = 247) | 35.9 (29.0–58.9) | 0.61 (0.55–0.68) | 0.40 (0.33–0.49) | |
| Advanced (N = 466) | 35.4 (30.9–57.5) | 0.64 (0.60–0.69) | 0.44 (0.39–0.50) | |
| OS from 2L among patients treated with 1L PBCT, by disease | All (N = 187) | 10.4 (8.9–13.3) | 0.28 (0.22–0.35) | 0.17 (0.12–0.26) |
| Recurrent (N = 43) | 13.4 (9.3–37.4) | 0.34 (0.22–0.53) | 0.19 (0.09–0.40) | |
| Advanced (N = 144) | 10.3 (8.0–13.2) | 0.26 (0.19–0.35) | 0.18 (0.11–0.27) | |
| OS from 2L among patients treated with 1L PBCT, by therapy * | All (N = 187) | 10.4 (8.9–13.3) | 0.28 (0.22–0.35) | 0.17 (0.12–0.26) |
| Rechallenged (N = 115) | 13.3 (11.2–20.9) | 0.35 (0.27–0.46) | 0.21 (0.13–0.33) | |
| Not Rechallenged (N = 72) | 6.4 (4.6–10.4) | 0.16 (0.09–0.28) | 0.13 (0.07–0.24) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, D.; O’Sullivan, D.E.; Boyne, D.J.; Cheung, W.Y.; Allonby, O.; Habash, M.; Brenner, D.R.; Riemer, J.; McGee, J. Understanding Characteristics, Treatment Patterns, and Clinical Outcomes for Individuals with Advanced or Recurrent Endometrial Cancer in Alberta, Canada: A Retrospective, Population-Based Cohort Study. Curr. Oncol. 2023, 30, 2277-2289. https://doi.org/10.3390/curroncol30020176
Martins D, O’Sullivan DE, Boyne DJ, Cheung WY, Allonby O, Habash M, Brenner DR, Riemer J, McGee J. Understanding Characteristics, Treatment Patterns, and Clinical Outcomes for Individuals with Advanced or Recurrent Endometrial Cancer in Alberta, Canada: A Retrospective, Population-Based Cohort Study. Current Oncology. 2023; 30(2):2277-2289. https://doi.org/10.3390/curroncol30020176
Chicago/Turabian StyleMartins, Diana, Dylan E. O’Sullivan, Devon J. Boyne, Winson Y. Cheung, Odette Allonby, Mara Habash, Darren R. Brenner, Justin Riemer, and Jacob McGee. 2023. "Understanding Characteristics, Treatment Patterns, and Clinical Outcomes for Individuals with Advanced or Recurrent Endometrial Cancer in Alberta, Canada: A Retrospective, Population-Based Cohort Study" Current Oncology 30, no. 2: 2277-2289. https://doi.org/10.3390/curroncol30020176
APA StyleMartins, D., O’Sullivan, D. E., Boyne, D. J., Cheung, W. Y., Allonby, O., Habash, M., Brenner, D. R., Riemer, J., & McGee, J. (2023). Understanding Characteristics, Treatment Patterns, and Clinical Outcomes for Individuals with Advanced or Recurrent Endometrial Cancer in Alberta, Canada: A Retrospective, Population-Based Cohort Study. Current Oncology, 30(2), 2277-2289. https://doi.org/10.3390/curroncol30020176





