Long-Term Follow-Up of Tamoxifen Treatment and the Use of Imaging in Psammocarcinoma: A Case Report, Review of the Literature and Discussion of Diagnostic and Therapeutic Challenges
Abstract
1. Introduction
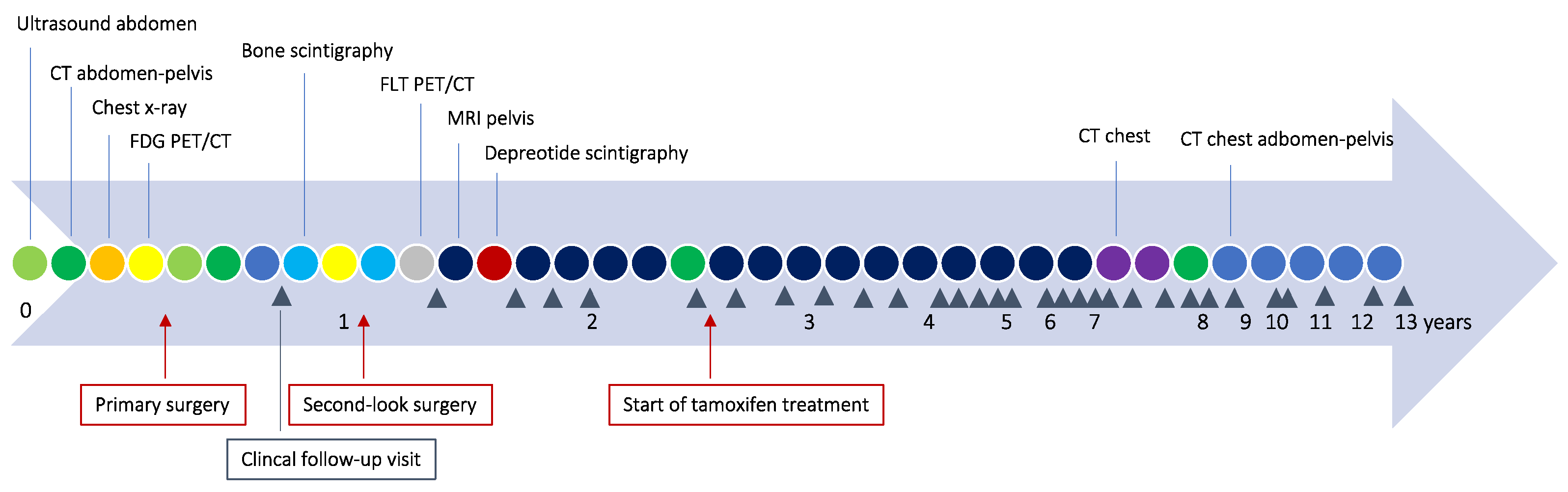
2. Detailed Case Description
3. Method
4. Results
5. Discussion
5.1. Clinical Characteristics and Pathological Evaluation
5.2. Radiology
5.3. Therapy
6. Conclusions
7. Patient Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vimplis, S.; Williamson, K.M.; Chaudry, Z.; Nuuns, D. Psammocarcinoma of the ovary: A case report and review of the literature. Gynecol. Surg. 2006, 3, 55–57. [Google Scholar] [CrossRef]
- Gilks, C.B.; Bell, D.A.; Scully, R.E. Serous psammocarcinoma of the ovary and peritoneum. Int. J. Gynecol. Pathol. 1990, 9, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Giordano, G.; Gnetti, L.; Milione, M.; Piccolo, D.; Soliani, P. Serous psammocarcinoma of the ovary: A case report and review of literature. Gynecol. Oncol. 2005, 96, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Jaindl, M.; Wenzl, R.; Jirecek, S.G.; Stengg, K.; Sevelda, P. Psammocarcinoma of the peritoneum diagnosed during operative laparoscopy. Acta Obstet. Gynecol. Scand. 1998, 77, 870–871. [Google Scholar] [PubMed]
- Zhen-Zhong, D.; Zheng, N.; Liu, H.; Liao, D.Y.; Ye, X.Q.; Zhou, Q.M.; Ma, J.; Li, H.H.; Liu, Q.; Lu, K.M. Elevated CA125 in primary peritoneal serous psammocarcinoma: A case report and review of the literature. BMJ Case Rep. 2009, 2009, bcr1020081063. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.T. Psammocarcinoma of the peritoneum. Diagn. Cytopathol. 1994, 10, 224–228. [Google Scholar] [CrossRef]
- Powell, J.L.; McDonald, T.J.; White, W.C. Serous psammocarcinoma of the ovary. South. Med. J. 1998, 91, 477–480. [Google Scholar] [CrossRef]
- Molpus, K.L.; Wu, H.; Fuller, A.F., Jr. Recurrent psammocarcinoma of the peritoneum with complete response to tamoxifen therapy. Gynecol. Oncol. 1998, 68, 206–209. [Google Scholar] [CrossRef]
- Munkarah, A.R.; Jacques, S.M.; Qureshi, F.; Deppe, G. Conservative surgery in a young patient with peritoneal psammocarcinoma. Gynecol. Oncol. 1999, 73, 312–314. [Google Scholar] [CrossRef]
- Whitcomb, B.P.; Kost, E.R.; Hines, J.F.; Zahn, C.M.; Hall, K.L. Primary peritoneal psammocarcinoma: A case presenting with an upper abdominal mass and elevated CA-125. Gynecol. Oncol. 1999, 73, 331–334. [Google Scholar] [CrossRef]
- Radin, A.I.; Youssef, I.M.; Quimbo, R.D.; Perone, R.W.; Guerrieri, C.; Abdel-Dayem, H.M. Technetium-99m diphosphonate imaging of psammocarcinoma of probable ovarian origin: Case report and literature review. Clin. Nucl. Med. 2005, 30, 395–399. [Google Scholar] [CrossRef] [PubMed]
- Jena, S.K.; Mishra, P.; Mohapatra, V.; Singh, S. Bilateral Serous Psammocarcinoma of Ovary: Rare Variant Low Grade Serous Carcinoma. Case Rep. Obstet. Gynecol. 2015, 2015, 531242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kelley, J.L.; Capelle, S.C.; Kanbour-Shakir, A. Serous psammocarcinoma of the ovary in an adolescent female. Gynecol. Oncol. 1995, 59, 309–311. [Google Scholar] [CrossRef] [PubMed]
- Weir, M.M.; Bell, D.A.; Young, R.H. Grade 1 peritoneal serous carcinomas: A report of 14 cases and comparison with 7 peritoneal serous psammocarcinomas and 19 peritoneal serous borderline tumors. Am. J. Surg. Pathol. 1998, 22, 849–862. [Google Scholar] [CrossRef]
- Chase, D.M.; Sparks, D.A.; Gener, M.; Smith, J. A unique case of ovarian psammocarcinoma with mediastinal, pulmonary, subcutaneous, and omental metastases. Arch. Gynecol. Obstet. 2009, 280, 283–286. [Google Scholar] [CrossRef]
- Bodnar, L.; Wcislo, G.; Górnas, M.; Gasowska-Bodnar, A.; Wiśniewski, P.; Stec, R.; Baranowski, W.; Szczylik, C. Serous peritoneal psammocarcinoma with an aggressive course: A case and review of the literature. Ginekol. Pol. 2009, 80, 632–636. [Google Scholar]
- Akbulut, M.; Kelten, C.; Bir, F.; Soysal, M.E.; Duzcan, S.E. Primary peritoneal serous psammocarcinoma with recurrent disease and metastasis: A case report and review of the literature. Gynecol. Oncol. 2007, 105, 248–251. [Google Scholar] [CrossRef]
- Poggi, S.H.; Bristow, R.E.; Nieberg, R.K.; Berek, J.S. Psammocarcinoma with an aggressive course. Obstet. Gynecol. 1998, 92, 659–661. [Google Scholar] [CrossRef]
- Alanbay, I.; Dede, M.; Ustün, Y.; Karaşahin, E.; Deveci, S.; Günhan, O.; Yenen, M.C. Serous psammocarcinoma of the ovary and peritoneum: Two case reports and review of the literature. Arch. Gynecol. Obstet. 2009, 279, 931–936. [Google Scholar] [CrossRef]
- Delhorme, J.B.; Ohayon, J.; Gouy, S.; Averous, G.; Genestie, C.; Gaichies, L.; Glehen, O.; Guilloit, J.M.; Pezet, D.; Quenet, F.; et al. Ovarian and peritoneal psammocarcinoma: Results of a multicenter study on 25 patients. Eur. J. Surg. Oncol. 2020, 46, 862–867. [Google Scholar] [CrossRef]
- Kurman, R.J. Blaustein’s Pathology of the Female Genital Tract; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2019. [Google Scholar]
- Piura, B.; Rabinovich, A.; Yanai-Inbar, I. Psammomacarcinoma of the peritoneum. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 97, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Cobellis, L.; Pezzani, I.; Cataldi, P.; Bome, A.; Santopietro, R.; Petraglia, F. Ovarian psammocarcinoma with peritoneal implants. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 107, 217–219. [Google Scholar] [CrossRef] [PubMed]
- Rettenmaier, M.A.; Goldstein, B.H.; Epstein, H.D.; Brown, J.V., 3rd; Micha, J.P. Serous psammocarcinoma of the ovary: An unusual finding. Gynecol. Oncol. 2005, 99, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, T.; Ozuysal, S.; Cankiliç, H. Primary psammocarcinoma of the peritoneum. Int. J. Gynecol. Cancer 2006, 16 (Suppl. S1), 129–131. [Google Scholar] [CrossRef] [PubMed]
- Koumoundourou, D.; Michail, G.; Kourounis, G.; Ravazoula, P. Primary peritoneal psammocarcinoma: A case presenting synchronously with bowel carcinoma. Gynecol. Oncol. 2006, 102, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Hiromura, T.; Tanaka, Y.O.; Nishioka, T.; Tomita, K. Serous psammocarcinoma of the ovary: CT and MR findings. J. Comput. Assist. Tomogr. 2007, 31, 490–492. [Google Scholar] [CrossRef] [PubMed]
- Riboni, F.; Giana, M.; Piantanida, P.; Vigone, A.; Surico, N.; Boldorini, R. Peritoneal psammocarcinoma diagnosed by a Papanicolau smear: A case report. Acta Cytol. 2010, 54, 311–313. [Google Scholar] [CrossRef]
- Pusiol, T.; Parolari, A.M.; Piscioli, I.; Morelli, L.; Del Nonno, F.; Licci, S. Prevalence and significance of psammoma bodies in cervicovaginal smears in a cervical cancer screening program with emphasis on a case of primary bilateral ovarian psammocarcinoma. Cytojournal 2008, 5, 7. [Google Scholar] [CrossRef]
- Sanguedolce, F.; Indraccolo, U.; Tortorella, S.; Nappi, L.; Rosenberg, P.; Greco, P.; Bufo, P. A case of ovarian psammocarcinoma associated with endometrioid cysts: A morphological and immunohistochemical study. Tumori 2009, 95, 112–114. [Google Scholar] [CrossRef]
- Tiro, A.V.; Talukdar, R.; Lewis, M.G. A unique case of serous psammocarcinoma of the ovary presenting with pleural effusion and manifesting psammoma body implants in the pleural cavity and pericardium. Gynecol. Oncol. 2009, 113, 402–404. [Google Scholar] [CrossRef]
- Poujade, O.; Uzan, C.; Gouy, S.; Pautier, P.; Duvillard, P.; Morice, P. Primary psammocarcinoma of the ovary or peritoneum. Int. J. Gynecol. Cancer 2009, 19, 844–846. [Google Scholar] [CrossRef]
- Jain, D.; Akhila, L.; Kawatra, V.; Aggarwal, P.; Khurana, N. Psammocarcinoma of ovary with serous cystadenofibroma of contralateral ovary: A case report. J. Med. Case Rep. 2009, 3, 9330. [Google Scholar] [CrossRef]
- Zakkouri, F.A.; Berrada, N.; Kettani, F.; Mrabti, H.; Jalil, A.; Errihani, H. Aggressive ovarian psammocarcinoma: A case report. Eur. J. Gynaecol. Oncol. 2011, 32, 214–215. [Google Scholar]
- Takahashi, R.; Ueda, Y.; Shioji, M.; Adachi, S.; Ohashi, H.; Morii, E.; Aozasa, K.; Yoshino, K.; Fujita, M.; Enomoto, T.; et al. Enormous cystic tumor of peritoneal psammocarcinoma exhibiting complete response to Cisplatin and cyclophosphamide after suboptimal cytoreduction: Case report and review of the literature. Gynecol. Obstet. Investig. 2012, 74, 165–170. [Google Scholar] [CrossRef]
- Debbagh, A.; Khmamouche, M.R.; Allaoui, M.; Ichou, M.; Errihani, H. Chemotherapy in the Treatment of Ovarian Psammocarcinoma: A Case Report and Review of the Literature. J. Clin. Case Rep. 2013, 3, 1–3. [Google Scholar] [CrossRef]
- Grimaldi, L.; Danzi, M.; Reggio, S.; Pannullo, M.; Danzi, R.; Lauria, R. Decision making in borderline ovarian tumors: Report of a rare case of mesosigma psammocarcinoma. Ann. Ital. Chir. 2013, 84, S2239253X13021609. [Google Scholar]
- Norese, G.; Moreira, A.; Alessandria, S.; Gorosito, F.; Lange, M.J.; Bermudez, A. Serous Psammocarcinoma of the Ovary and Peritoneum: Long Term Recurrence: IGCS-0080 Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25 (Suppl. S1), 65. [Google Scholar] [CrossRef]
- Togănel, R.D.; Şimon, I.; Zolog, A.; Simescu, R.; Cozma, A.; Muntean, V. Primary peritoneal serous psammocarcinoma: A case report. J. Gastrointestin Liver Dis. 2015, 24, 253–256. [Google Scholar] [CrossRef]
- Arole, V.C.; Deshpande, K.A.; Jadhav, D.B.; Jogi, A. Primary peritoneal psammocarcinoma. J. Obstet. Gynaecol. Res. 2017, 43, 1366–1372. [Google Scholar] [CrossRef]
- Baba, T.; Fukagawa, Y.; Itamochi, H.; Sato, C.; Tomabechi, H.; Nagasawa, T.; Kagabu, M.; Shoji, T.; Sugai, T. Primary ovarian serous psammocarcinoma-a case report with mini literature review. Obstet. Gynecol. Int. J. 2020, 11, 192–195. [Google Scholar] [CrossRef]
- Helal, I.; Khanchel, F.; Jouini, R.; Thayer, M.B.; Mbarki, C.; Bettaieb, H.; Rebii, S.; Ismail, I.B.; Brahim, E.B.; Chedli-Debbiche, A. Case Report: Fortuitous discovery of primary peritoneal psammocarcinoma. F1000Research 2022, 11, 696. [Google Scholar] [CrossRef]
- Kancharla, S.; Alaniz, A.; Kothari, P.; Norton, S. Primary peritoneal serous psammocarcinoma, rare variant: A case report. Gynecol. Oncol. Rep. 2023, 47, 101176. [Google Scholar] [CrossRef]
- Sapna, F.; Cajigas, A.; Perkash, R.S.; Qi, X.; Gera, S. Recurrent Primary Peritoneal Psammocarcinoma: A Case Report on a Diagnostic Challenge in Cytology. Cureus 2023, 15, e41964. [Google Scholar] [CrossRef]
- Dedick, A.P.; Whelan, V.M. Roentgen demonstration of psammoma bodies in cystadenocarcinoma of the ovaries. Radiology 1955, 64, 353–356. [Google Scholar] [CrossRef]
- Dähnert, W. Radiology Review Manual, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2003. [Google Scholar]
- Dong, A.; Wang, Y.; Zuo, C. FDG PET/CT in serous psammocarcinoma of the ovary. Clin. Nucl. Med. 2014, 39, 453–455. [Google Scholar] [CrossRef]
- Ranner, G.; Ebner, F.; Kullnig, P. Serous cystadenocarcinoma of the ovary: Unusual visualization of calcified intraperitoneal metastases in CT and bone scintigraphy. Rontgenblatter 1987, 40, 260–262. [Google Scholar]
- Mungenast, F.; Thalhammer, T. Estrogen biosynthesis and action in ovarian cancer. Front. Endocrinol. 2014, 5, 192. [Google Scholar] [CrossRef]
- Wong, K.-K.; Lu, K.H.; Malpica, A.; Bodurka, D.C.; Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly Greater Expression of ER, PR, and ECAD in Advanced-Stage Low-Grade Ovarian Serous Carcinoma as Revealed by Immunohistochemical Analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409. [Google Scholar] [CrossRef]
- Zwimpfer, T.A.; Tal, O.; Geissler, F.; Coelho, R.; Rimmer, N.; Jacob, F.; Heinzelmann-Schwarz, V. Low grade serous ovarian cancer—A rare disease with increasing therapeutic options. Cancer Treat. Rev. 2023, 112, 102497. [Google Scholar] [CrossRef]
- Silva, E.G.; Deavers, M.T.; Parlow, A.F.; Gershenson, D.M.; Malpica, A. Calcifications in ovary and endometrium and their neoplasms. Mod. Pathol. 2003, 16, 219–222. [Google Scholar] [CrossRef]


| Author Year | n | Age | Type | FIGO-Stage | Surgery | Res (cm) | m.t. | Follow-Up (Years) |
|---|---|---|---|---|---|---|---|---|
| Gilks, 1990 [2] | 11 | 66 | o | IIIB | SOE | 0 | - | LFU |
| 72 | o | IIIA | SOEB | 0 | - | NED 10.5 | ||
| 55 | o | IIIA | HSOEB | 0 | Ct | NED 6.5 | ||
| 36 | o | IIIB | HSOEB | 0 | - | NED 3 | ||
| 53 | o | IIIB | HSOEB + om | 0 | - | NED 3.6 | ||
| 53 | o | IIIB | HSOEB + om | NS | - | LFU | ||
| 76 | o | IIIB | SOEB + om | res | - | PFS < 1 | ||
| 59 | o | IIIC | SOE | 0 | - | DOD 6.5 | ||
| 55 | p | IIIA | HSOEB + om | 0 | - | NED 10.5 | ||
| 48 | p | IIIB | HSOEB + om | 0 | - | NED < 1 | ||
| 58 | p | IIIB | HSOEB + om | 0 | - | NED < 1 | ||
| Chen, 1994 [6] | 2 | 59 | p | IIIA | HSOEB + om | res < 1 | - | PFS 1.5 |
| 71 | p | IIIB | px (HSOEB prior) | res | Ct | DOD < 1 | ||
| Kelley, 1995 [13] | 1 | 18 | o | IIIC | HSOEB + om + llg + app + bo | 0 | Ct | NED 3.5 |
| Powell, 1998 [7] | 1 | 59 | o | IIIB | HSOEB + om + llg + app | 0 | - | NED > 1 |
| Molpus, 1998 [8] | 1 | 58 | p | IIIC | HSOEB + om | res < 1 | Tx | NED 4 R/CR 8 |
| Poggi, 1998 [18] | 1 | 66 | p | IIIB/C | SOEB + om (H prior) | 0 | - | PFS 1.5 |
| Weir, 1998 [14] | 7 | 48 | p | III | 2 × HSOEB + om | NS | Ct 5/7 | 4 LFU |
| (27–74) | 3 × SOEB 1 × px, 1 × om | RT 1/7 | 3 NED 1.4, 3.8, 8.3 | |||||
| Lehner, 1998 [4] | 1 | 37 | p | IIIB | HSOEB + om + llg | 0 | Ct | NED 2.4 |
| Munkarah, 1999 [9] | 2 | 27 | p | IIIB | SOE + llg + om + app | res < 1, 0 2:nd | Ct | NED 15 |
| 59 | p | IIIC | NS | 0 | - | NED 2 | ||
| Whitcomb, 1999 [10] | 1 | 59 | p | IIIC | HSOEB + om | 0 | - | NED 2 |
| Piura, 2001 [22] | 1 | 67 | p | IIIC | HSOEB + om + llg + bo | 0 | Ct | NED 1.3 |
| Cobellis, 2003 [23] | 1 | 48 | o | IIIA | HSOEB + om | 0 | - | NED 2 |
| Giordano, 2005 [3] | 1 | 66 | o | IIIB | HSOEB + om | 0 | Ct | NED 1 |
| Radin, 2005 [11] | 1 | 60 | o | IIIC | px | res | Ct | PFS < 1 |
| Rettenmaier, 2005 [24] | 1 | 70 | o | IIC | HSOEB + om | 0 | - | NED < 1 |
| Vimplis, 2006 [1] | 1 | 63 | o | IIIB | HSOEB + om | res < 1 | Ct | NED < 1 |
| Bilgin, 2006 [25] | 1 | 46 | p | IIIB | HSOEB + om + llg | 0 | Ct | NED 5.5 |
| Koumoundourou, 2006 [26] | 1 | 83 | p | IIIB/C | HSOEB + om + bo | 0 | - | NED 4.5 |
| Hiromura, 2007 [27] | 1 | 73 | o | IIIC | HSOEB + om | res | Ct | NED < 1 |
| Akbulut, 2007 [17] | 1 | 67 | p | IIIC | HSOEB + om + llg | 0 | Ct + RT | NED 5.5, AWD 10 |
| Riboni, 2008 [28] | 1 | 70 | p | IIIB | HSOEB + om | 0 | Ct | NED 3 |
| Pusiol, 2008 [29] | 2 | 56 | o | IIIB | HSOEB + om + llg | NS | Ct | NED |
| 50 | o | IIIB | HSOEB + om + llg | 0 | Ct | NED 10.5 | ||
| Bodnar, 2009 [16] | 1 | 52 | IIIC | HSOEB + om + llg + spleen + app | res < 1 | Ct | PFS 0.7, DOD 2.3 | |
| Alanbay, 2009 [19] | 2 | 41 | p | IIIB | HSOEB + om + llg + app | 0 | Ct | NED 6 |
| 50 | o | IIIC | HSOEB + om + llg + app | NS | Ct | NED < 1 | ||
| Zhen-Zhong, 2009 [5] | 1 | 42 | p | IIIC | HSOEB + om + llg | 0 | Ct | NED 1 |
| Sanguedolce, 2009 [30] | 1 | 35 | o | IA | myomectomy + SOE | 0 | - | NED < 1 |
| Tiro, 2009 [31] | 1 | 58 | o | IV | Px | res | Ct | PFS < 1 |
| Chase, 2009 [15] | 1 | 45 | o | IV | SOEB + px (prev H) | res | Tx | PFS < 1 |
| Poujade, 2009 [32] | 4 | 55 | p | IIIC | HSOEB + om | 0 | - | NED 3 |
| 19 | o | IIIC | SOEB + om + llg | 0 | - | NED 2.4 | ||
| 67 | o | IIIC | HSOEB + om + llg + bo | 0 | Ct | NED 3.75 | ||
| 45 | o | IIIC | HSOEB + om + bo | 0 | Ct | NED 2.5, AWD 3 | ||
| Jain, 2009 [33] | 1 | 55 | o | IC | HSOEB | 0 | Ct | NED < 1 |
| Zakkouri, 2010 [34] | 1 | 52 | o | IV | px | res | Ct | AWD < 1 |
| Takahashi, 2012 [35] | 1 | 38 | p | IIC | HSOEB | res 4 | Ct | NED 1.25 |
| Debbagh, 2013 [36] | 1 | 40 | o | px | res | Ct | PFS < 1 | |
| Grimaldi, 2013 [37] | 1 | 44 | p | IA (?) | bo, 2:nd HSOEB + om + llg + app | 0 | - | NED 2, R, NED 4 |
| Jena, 2015 [12] | 1 | 60 | o | IIIB | HSOEB + om | 0 | - | NED 1 |
| Norese, 2015 [38] | 1 | 44 | o | IIIC | HSOEB + om + llg | res > 1, 0 2:nd | Ct+RT | NED 10, R |
| Toganel, 2015 [39] | 1 | 54 | p | IIIC | SOEB + om + bo | - | NED < 1 | |
| Arole, 2017 [40] | 1 | 60 | p | IIIB | HSOEB + om | 0 | Ct | NED 1 |
| Delhorme, 2020 [20] | 25 | 53 | o+p | 24 III/1 IV | 84% complete surgery | Ct 14 | median 3.5 8 DOD | |
| (17–78) | 8 AWD 7 NED | |||||||
| Fukagawa, 2020 [41] | 1 | 59 | o | IIB | HSOEB + om + llg | 0 | Ct | 8 NED |
| Helal, 2022 [42] | 1 | 74 | p | IIIC | HSOEB + om | NS | - | LFU |
| Kancharla, 2023 [43] | 1 | 49 | p | IIIA2 | HSOEB + om + bo + px | NS | Letr | NED 0.75 |
| Sapna, 2023 [44] | 1 | 51 | p | NS | HSOEB + om + llg | 0 | Ct | NED 5, R, AWD 15 |
| Our case 2023 | 1 | 56 | p | IIIC | SOEB + om + llg (H prior) | res < 1 | Tx | AWD 13 |
| Imaging Modality | Characteristics | Articles |
|---|---|---|
| CT | Less pronounced density, uniform distribution of calcification | Hiromura [27] |
| FDG PET/CT | Increased metabolic activity | Bodnar [16], Dong [47], Chase [15] |
| Bone scintigraphy | Prominent calcification | Ranner [48], Radin [11] |
| MRI | Sandy or coarse granulated | Hiromura [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gideonsson, I.; Israelsson, P.; Strandberg, S.N.; Ottander, U. Long-Term Follow-Up of Tamoxifen Treatment and the Use of Imaging in Psammocarcinoma: A Case Report, Review of the Literature and Discussion of Diagnostic and Therapeutic Challenges. Curr. Oncol. 2023, 30, 10260-10271. https://doi.org/10.3390/curroncol30120747
Gideonsson I, Israelsson P, Strandberg SN, Ottander U. Long-Term Follow-Up of Tamoxifen Treatment and the Use of Imaging in Psammocarcinoma: A Case Report, Review of the Literature and Discussion of Diagnostic and Therapeutic Challenges. Current Oncology. 2023; 30(12):10260-10271. https://doi.org/10.3390/curroncol30120747
Chicago/Turabian StyleGideonsson, Ida, Pernilla Israelsson, Sara N. Strandberg, and Ulrika Ottander. 2023. "Long-Term Follow-Up of Tamoxifen Treatment and the Use of Imaging in Psammocarcinoma: A Case Report, Review of the Literature and Discussion of Diagnostic and Therapeutic Challenges" Current Oncology 30, no. 12: 10260-10271. https://doi.org/10.3390/curroncol30120747
APA StyleGideonsson, I., Israelsson, P., Strandberg, S. N., & Ottander, U. (2023). Long-Term Follow-Up of Tamoxifen Treatment and the Use of Imaging in Psammocarcinoma: A Case Report, Review of the Literature and Discussion of Diagnostic and Therapeutic Challenges. Current Oncology, 30(12), 10260-10271. https://doi.org/10.3390/curroncol30120747





