Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives
Abstract
:1. Introduction
2. Estrogens and ER-Mediated Signaling Pathways
3. ER-Low-Positive BC
3.1. Epidemiology and Clinicopathological Characteristics
3.2. Prognosis and (Neo)adjuvant Therapy
3.3. Immune Microenvironment and Immunotherapy
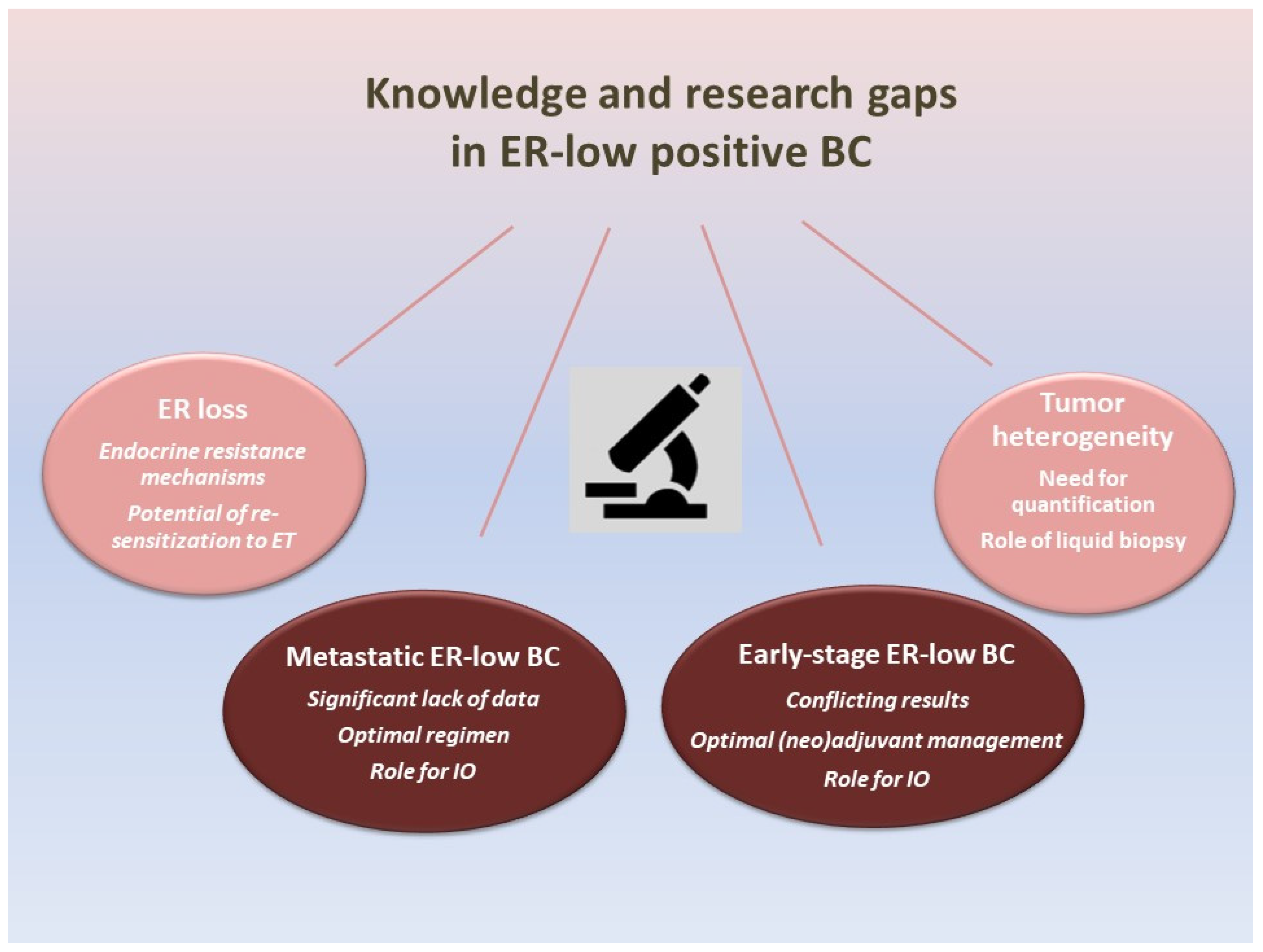
4. Knowledge and Research Gaps in ER-Low-Positive BC (Figure 1)
4.1. Early-Stage ER-Low-Positive BC
4.2. Metastatic ER-Low-Positive BC
4.3. ER Expression Heterogeneity
4.4. ER Loss Due to Endocrine Resistance
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
References
- Cardoso, F.; Kyriakides, S.; Ohno, S.; Penault-Llorca, F.; Poortmans, P.; Rubio, I.T.; Zackrisson, S.; Senkus, E.; ESMO Guidelines Committee. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-updagger. Ann. Oncol. 2019, 30, 1194–1220. [Google Scholar] [CrossRef]
- Gennari, A.; Andre, F.; Barrios, C.H.; Cortes, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.A.; et al. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Makhlouf, S.; Althobiti, M.; Toss, M.; Muftah, A.A.; Mongan, N.P.; Lee, A.H.S.; Green, A.R.; Rakha, E.A. The Clinical and Biological Significance of Estrogen Receptor-Low Positive Breast Cancer. Mod. Pathol. 2023, 36, 100284. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.D.; Cai, Y.W.; Wu, S.Y.; Shui, R.H.; Shao, Z.M. Estrogen receptor-low breast cancer: Biology chaos and treatment paradox. Cancer Commun. 2021, 41, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Gomatou, G.; Syrigos, N.; Vathiotis, I.A.; Kotteas, E.A. Tumor Dormancy: Implications for Invasion and Metastasis. Int. J. Mol. Sci. 2021, 22, 4862. [Google Scholar] [CrossRef]
- Heldring, N.; Pike, A.; Andersson, S.; Matthews, J.; Cheng, G.; Hartman, J.; Tujague, M.; Strom, A.; Treuter, E.; Warner, M.; et al. Estrogen receptors: How do they signal and what are their targets. Physiol. Rev. 2007, 87, 905–931. [Google Scholar] [CrossRef]
- Tsagkaraki, I.M.; Kourouniotis, C.D.; Gomatou, G.L.; Syrigos, N.K.; Kotteas, E.A. Orbital metastases of invasive lobular breast carcinoma. Breast Dis. 2019, 38, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, N.; Silveyra, P. Estrogen receptor signaling mechanisms. Adv. Protein Chem. Struct. Biol. 2019, 116, 135–170. [Google Scholar] [CrossRef]
- Vrtacnik, P.; Ostanek, B.; Mencej-Bedrac, S.; Marc, J. The many faces of estrogen signaling. Biochem. Med. 2014, 24, 329–342. [Google Scholar] [CrossRef] [PubMed]
- Bjornstrom, L.; Sjoberg, M. Mechanisms of estrogen receptor signaling: Convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 2005, 19, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Cheskis, B.J.; Greger, J.G.; Nagpal, S.; Freedman, L.P. Signaling by estrogens. J. Cell. Physiol. 2007, 213, 610–617. [Google Scholar] [CrossRef] [PubMed]
- Gomatou, G.; Trontzas, I.; Ioannou, S.; Drizou, M.; Syrigos, N.; Kotteas, E. Mechanisms of resistance to cyclin-dependent kinase 4/6 inhibitors. Mol. Biol. Rep. 2021, 48, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Obr, A.E.; Edwards, D.P. The biology of progesterone receptor in the normal mammary gland and in breast cancer. Mol. Cell. Endocrinol. 2012, 357, 4–17. [Google Scholar] [CrossRef]
- Lashen, A.G.; Toss, M.S.; Mongan, N.P.; Green, A.R.; Rakha, E.A. The clinical value of progesterone receptor expression in luminal breast cancer: A study of a large cohort with long-term follow-up. Cancer 2023, 129, 1183–1194. [Google Scholar] [CrossRef]
- Cui, X.; Schiff, R.; Arpino, G.; Osborne, C.K.; Lee, A.V. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J. Clin. Oncol. 2005, 23, 7721–7735. [Google Scholar] [CrossRef]
- Moldoveanu, D.; Hoskin, T.L.; Day, C.N.; Schulze, A.K.; Goetz, M.P.; Boughey, J.C. Clinical Behavior, Management, and Treatment Response of Estrogen Receptor Low (1–10%) Breast Cancer. Ann. Surg. Oncol. 2023, 30, 6475–6483. [Google Scholar] [CrossRef]
- Li, M.; Zhou, S.; Lv, H.; Cai, M.; Wan, X.; Lu, H.; Shui, R.; Yang, W. FOXC1 and SOX10 in Estrogen Receptor-Low Positive/HER2-Negative Breast Cancer: Potential Biomarkers for the Basal-like Phenotype Prediction. Arch. Pathol. Lab. Med. 2023. [Google Scholar] [CrossRef]
- Luo, C.; Zhong, X.; Fan, Y.; Wu, Y.; Zheng, H.; Luo, T. Clinical characteristics and survival outcome of patients with estrogen receptor low positive breast cancer. Breast 2022, 63, 24–28. [Google Scholar] [CrossRef]
- Yoon, K.H.; Park, Y.; Kang, E.; Kim, E.K.; Kim, J.H.; Kim, S.H.; Suh, K.J.; Kim, S.M.; Jang, M.; Yun, B.; et al. Effect of Estrogen Receptor Expression Level and Hormonal Therapy on Prognosis of Early Breast Cancer. Cancer Res. Treat. 2022, 54, 1081–1090. [Google Scholar] [CrossRef]
- Park, Y.H.; Karantza, V.; Calhoun, S.R.; Park, S.; Lee, S.; Kim, J.Y.; Yu, J.H.; Kim, S.W.; Lee, J.E.; Nam, S.J.; et al. Prevalence, treatment patterns, and prognosis of low estrogen receptor-positive (1% to 10%) breast cancer: A single institution’s experience in Korea. Breast Cancer Res. Treat. 2021, 189, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Schrodi, S.; Braun, M.; Andrulat, A.; Harbeck, N.; Mahner, S.; Kiechle, M.; Klein, E.; Schnelzer, A.; Schindlbeck, C.; Bauerfeind, I.; et al. Outcome of breast cancer patients with low hormone receptor positivity: Analysis of a 15-year population-based cohort. Ann. Oncol. 2021, 32, 1410–1424. [Google Scholar] [CrossRef] [PubMed]
- Fei, F.; Siegal, G.P.; Wei, S. Characterization of estrogen receptor-low-positive breast cancer. Breast Cancer Res. Treat. 2021, 188, 225–235. [Google Scholar] [CrossRef]
- Poon, I.K.; Tsang, J.Y.; Li, J.; Chan, S.K.; Shea, K.H.; Tse, G.M. The significance of highlighting the oestrogen receptor low category in breast cancer. Br. J. Cancer 2020, 123, 1223–1227. [Google Scholar] [CrossRef] [PubMed]
- Landmann, A.; Farrugia, D.J.; Zhu, L.; Diego, E.J.; Johnson, R.R.; Soran, A.; Dabbs, D.J.; Clark, B.Z.; Puhalla, S.L.; Jankowitz, R.C.; et al. Low Estrogen Receptor (ER)-Positive Breast Cancer and Neoadjuvant Systemic Chemotherapy: Is Response Similar to Typical ER-Positive or ER-Negative Disease? Am. J. Clin. Pathol. 2018, 150, 34–42. [Google Scholar] [CrossRef]
- Yoder, R.; Kimler, B.F.; Staley, J.M.; Schwensen, K.; Wang, Y.Y.; Finke, K.; O’Dea, A.; Nye, L.; Elia, M.; Crane, G.; et al. Impact of low versus negative estrogen/progesterone receptor status on clinico-pathologic characteristics and survival outcomes in HER2-negative breast cancer. NPJ Breast Cancer 2022, 8, 80. [Google Scholar] [CrossRef]
- He, A.; Zhou, T. Clinicopathological Features and Survival for Low ER-positive Breast-cancer Patients. Altern. Ther. Health Med. 2022, 28, 36–41. [Google Scholar]
- Gloyeske, N.C.; Dabbs, D.J.; Bhargava, R. Low ER+ breast cancer: Is this a distinct group? Am. J. Clin. Pathol. 2014, 141, 697–701. [Google Scholar] [CrossRef]
- Deyarmin, B.; Kane, J.L.; Valente, A.L.; van Laar, R.; Gallagher, C.; Shriver, C.D.; Ellsworth, R.E. Effect of ASCO/CAP guidelines for determining ER status on molecular subtype. Ann. Surg. Oncol. 2013, 20, 87–93. [Google Scholar] [CrossRef]
- Tarantino, P.; Jin, Q.; Tayob, N.; Jeselsohn, R.M.; Schnitt, S.J.; Vincuilla, J.; Parker, T.; Tyekucheva, S.; Li, T.; Lin, N.U.; et al. Prognostic and Biologic Significance of ERBB2-Low Expression in Early-Stage Breast Cancer. JAMA Oncol. 2022, 8, 1177–1183. [Google Scholar] [CrossRef]
- Domagala, W.; Lasota, J.; Bartkowiak, J.; Weber, K.; Osborn, M. Vimentin is preferentially expressed in human breast carcinomas with low estrogen receptor and high Ki-67 growth fraction. Am. J. Pathol. 1990, 136, 219–227. [Google Scholar]
- Iwamoto, T.; Booser, D.; Valero, V.; Murray, J.L.; Koenig, K.; Esteva, F.J.; Ueno, N.T.; Zhang, J.; Shi, W.; Qi, Y.; et al. Estrogen receptor (ER) mRNA and ER-related gene expression in breast cancers that are 1% to 10% ER-positive by immunohistochemistry. J. Clin. Oncol. 2012, 30, 729–734. [Google Scholar] [CrossRef] [PubMed]
- Prabhu, J.S.; Korlimarla, A.; Desai, K.; Alexander, A.; Raghavan, R.; Anupama, C.; Dendukuri, N.; Manjunath, S.; Correa, M.; Raman, N.; et al. A Majority of Low (1–10%) ER Positive Breast Cancers Behave Like Hormone Receptor Negative Tumors. J. Cancer 2014, 5, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Sanford, R.A.; Song, J.; Gutierrez-Barrera, A.M.; Profato, J.; Woodson, A.; Litton, J.K.; Bedrosian, I.; Albarracin, C.T.; Valero, V.; Arun, B. High incidence of germline BRCA mutation in patients with ER low-positive/PR low-positive/HER-2 neu negative tumors. Cancer 2015, 121, 3422–3427. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Yan, J.Q.; Chen, Q.X.; Yang, Y.Z.; Li, Y.L.; Ren, Y.X.; Weng, Z.J.; Zhang, X.F.; Guan, J.X.; Tang, L.Y.; et al. Association of H3K9me3 with breast cancer prognosis by estrogen receptor status. Clin. Epigenetics 2022, 14, 135. [Google Scholar] [CrossRef] [PubMed]
- Paakkola, N.M.; Karakatsanis, A.; Mauri, D.; Foukakis, T.; Valachis, A. The prognostic and predictive impact of low estrogen receptor expression in early breast cancer: A systematic review and meta-analysis. ESMO Open 2021, 6, 100289. [Google Scholar] [CrossRef] [PubMed]
- Skjervold, A.H.; Valla, M.; Bofin, A.M. Oestrogen receptor low positive breast cancer: Associations with prognosis. Breast Cancer Res. Treat. 2023, 201, 535–545. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group; Davies, C.; Godwin, J.; Gray, R.; Clarke, M.; Cutter, D.; Darby, S.; McGale, P.; Pan, H.C.; Taylor, C.; et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Yi, M.; Huo, L.; Koenig, K.B.; Mittendorf, E.A.; Meric-Bernstam, F.; Kuerer, H.M.; Bedrosian, I.; Buzdar, A.U.; Symmans, W.F.; Crow, J.R.; et al. Which threshold for ER positivity? a retrospective study based on 9639 patients. Ann. Oncol. 2014, 25, 1004–1011. [Google Scholar] [CrossRef]
- Balduzzi, A.; Bagnardi, V.; Rotmensz, N.; Dellapasqua, S.; Montagna, E.; Cardillo, A.; Viale, G.; Veronesi, P.; Intra, M.; Luini, A.; et al. Survival outcomes in breast cancer patients with low estrogen/progesterone receptor expression. Clin. Breast Cancer 2014, 14, 258–264. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, N.; Moran, M.S.; Su, P.; Haffty, B.G.; Yang, Q. Borderline ER-Positive Primary Breast Cancer Gains No Significant Survival Benefit From Endocrine Therapy: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2018, 18, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Yang, L.; Wu, Y.; Zheng, H.; Gou, Q. Adjuvant endocrine therapy in patients with estrogen receptor-low positive breast cancer: A prospective cohort study. Breast 2022, 66, 89–96. [Google Scholar] [CrossRef]
- Cai, Y.W.; Shao, Z.M.; Yu, K.D. De-escalation of five-year adjuvant endocrine therapy in patients with estrogen receptor-low positive (immunohistochemistry staining 1%–10%) breast cancer: Propensity-matched analysis from a prospectively maintained cohort. Cancer 2022, 128, 1748–1756. [Google Scholar] [CrossRef] [PubMed]
- Reinert, T.; Cascelli, F.; de Resende, C.A.A.; Goncalves, A.C.; Godo, V.S.P.; Barrios, C.H. Clinical implication of low estrogen receptor (ER-low) expression in breast cancer. Front. Endocrinol. 2022, 13, 1015388. [Google Scholar] [CrossRef] [PubMed]
- Giordano, J.; McGrath, M.; Harrison, B.; Kantor, O.; Vora, H.; Burstein, H.J.; Tolaney, S.M.; King, T.A.; Mittendorf, E.A. Is there a role for the oncotype DX breast recurrence score genomic assay in estrogen receptor-low positive breast cancer? J. Clin. Oncol. 2022, 40, 564. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, K.; Yu, K.; Zou, D.; Yang, H.; He, X.; Mo, W.; Yu, X.; Ding, X. Prognosis and endocrine therapy selection for patients with low hormone receptor-positive breast cancer following neoadjuvant chemotherapy: A retrospective study of 570 patients in China. Oncol. Lett. 2019, 18, 6690–6696. [Google Scholar] [CrossRef]
- Dieci, M.V.; Griguolo, G.; Bottosso, M.; Tsvetkova, V.; Giorgi, C.A.; Vernaci, G.; Michieletto, S.; Angelini, S.; Marchet, A.; Tasca, G.; et al. Impact of estrogen receptor levels on outcome in non-metastatic triple negative breast cancer patients treated with neoadjuvant/adjuvant chemotherapy. NPJ Breast Cancer 2021, 7, 101. [Google Scholar] [CrossRef]
- Fujii, T.; Kogawa, T.; Dong, W.; Sahin, A.A.; Moulder, S.; Litton, J.K.; Tripathy, D.; Iwamoto, T.; Hunt, K.K.; Pusztai, L.; et al. Revisiting the definition of estrogen receptor positivity in HER2-negative primary breast cancer. Ann. Oncol. 2017, 28, 2420–2428. [Google Scholar] [CrossRef]
- Villegas, S.L.; Nekljudova, V.; Pfarr, N.; Engel, J.; Untch, M.; Schrodi, S.; Holms, F.; Ulmer, H.U.; Fasching, P.A.; Weber, K.E.; et al. Therapy response and prognosis of patients with early breast cancer with low positivity for hormone receptors—An analysis of 2765 patients from neoadjuvant clinical trials. Eur. J. Cancer 2021, 148, 159–170. [Google Scholar] [CrossRef]
- Tarantino, P.; Corti, C.; Schmid, P.; Cortes, J.; Mittendorf, E.A.; Rugo, H.; Tolaney, S.M.; Bianchini, G.; Andre, F.; Curigliano, G. Immunotherapy for early triple negative breast cancer: Research agenda for the next decade. NPJ Breast Cancer 2022, 8, 23. [Google Scholar] [CrossRef]
- Vathiotis, I.A.; Trontzas, I.; Gavrielatou, N.; Gomatou, G.; Syrigos, N.K.; Kotteas, E.A. Immune Checkpoint Blockade in Hormone Receptor-Positive Breast Cancer: Resistance Mechanisms and Future Perspectives. Clin. Breast Cancer 2022, 22, 642–649. [Google Scholar] [CrossRef]
- O’Meara, T.A.; Tolaney, S.M. Tumor mutational burden as a predictor of immunotherapy response in breast cancer. Oncotarget 2021, 12, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Voorwerk, L.; Sanders, J.; Keusters, M.S.; Balduzzi, S.; Cornelissen, S.; Duijst, M.; Lips, E.H.; Sonke, G.S.; Linn, S.C.; Horlings, H.M.; et al. Immune landscape of breast tumors with low and intermediate estrogen receptor expression. NPJ Breast Cancer 2023, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thurlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann. Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef]
- Sharma, P.; Stecklein, S.; Yoder, R.; Staley, J.; Schwensen, K.; O’Dea, A.; Nye, L.; Elia, M.; Satelli, D.; Crane, G.; et al. Clinical and biomarker results of neoadjuvant phase II study of pembrolizumab and carboplatin plus docetaxel in triple-negative breast cancer (TNBC) (NeoPACT). J. Clin. Oncol. 2022, 40, 513. [Google Scholar] [CrossRef]
- Cardoso, F.; Paluch-Shimon, S.; Senkus, E.; Curigliano, G.; Aapro, M.S.; Andre, F.; Barrios, C.H.; Bergh, J.; Bhattacharyya, G.S.; Biganzoli, L.; et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5). Ann. Oncol. 2020, 31, 1623–1649. [Google Scholar] [CrossRef] [PubMed]
- Luond, F.; Tiede, S.; Christofori, G. Breast cancer as an example of tumour heterogeneity and tumour cell plasticity during malignant progression. Br. J. Cancer 2021, 125, 164–175. [Google Scholar] [CrossRef]
- Turashvili, G.; Brogi, E. Tumor Heterogeneity in Breast Cancer. Front. Med. 2017, 4, 227. [Google Scholar] [CrossRef]
- Chen, R.; Qarmali, M.; Siegal, G.P.; Wei, S. Receptor conversion in metastatic breast cancer: Analysis of 390 cases from a single institution. Mod. Pathol. 2020, 33, 2499–2506. [Google Scholar] [CrossRef]
- Shi, Y.J.; Tsang, J.Y.; Ni, Y.B.; Tse, G.M. Intratumoral Heterogeneity in Breast Cancer: A Comparison of Primary and Metastatic Breast Cancers. Oncologist 2017, 22, 487–490. [Google Scholar] [CrossRef]
- Reinhardt, F.; Franken, A.; Fehm, T.; Neubauer, H. Navigation through inter- and intratumoral heterogeneity of endocrine resistance mechanisms in breast cancer: A potential role for Liquid Biopsies? Tumour Biol. 2017, 39, 1010428317731511. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.; Papadaki, A.; Althobiti, M.; Alsaleem, M.; Aleskandarany, M.A.; Rakha, E.A. Breast cancer intratumour heterogeneity: Current status and clinical implications. Histopathology 2018, 73, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Hartkopf, A.D.; Grischke, E.M.; Brucker, S.Y. Endocrine-Resistant Breast Cancer: Mechanisms and Treatment. Breast Care 2020, 15, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Zattarin, E.; Leporati, R.; Ligorio, F.; Lobefaro, R.; Vingiani, A.; Pruneri, G.; Vernieri, C. Hormone Receptor Loss in Breast Cancer: Molecular Mechanisms, Clinical Settings, and Therapeutic Implications. Cells 2020, 9, 2644. [Google Scholar] [CrossRef] [PubMed]
- Rasha, F.; Sharma, M.; Pruitt, K. Mechanisms of endocrine therapy resistance in breast cancer. Mol. Cell. Endocrinol. 2021, 532, 111322. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhou, C.; Jiang, H.; Liang, L.; Shi, W.; Zhang, Q.; Sun, P.; Xiang, R.; Wang, Y.; Yang, S. ZEB1 induces ER-alpha promoter hypermethylation and confers antiestrogen resistance in breast cancer. Cell Death Dis. 2017, 8, e2732. [Google Scholar] [CrossRef]
| Author (Year) | N | Prevalence of ER-Low BC | Reference |
|---|---|---|---|
| Makhlouf (2023) | 7559 | 1.6% (123/7559) | [4] |
| Moldoveanu (2023) | 232,762 a | 2.0% (4584/232,762) | [17] |
| Li (2023) | 9082 | 3.29% (299/9082) | [18] |
| Luo (2022) | 5466 b | 5.1% (277/5466) | [19] |
| Yoon (2022) | 2162 b | 2.5% (54/2162) | [20] |
| Park (2021) | 5930 b | 2.0% (117/5930) | [21] |
| Schrodi (2021) | 38,560 b | 2.0% (861/38,560) | [22] |
| Fei (2021) | 4179 | 2.3% (97/4179) | [23] |
| Poon (2020) | 1824 | 3% (54/1824) | [24] |
| Author (Year) | Type of Study | Results | Reference |
|---|---|---|---|
| Schrodi (2021) | Retrospective population-based cohort study | Significantly decreased OS of ER-low/HER2(–) compared to ER-positive/HER2(–) | [22] |
| Park (2021) | Retrospective unicentric cohort | DFS and OS in the ER-low/HER2(–) cohort were more similar to the TNBC cohort than those with ER-high/HER2(–) BC | [21] |
| Paakkola (2021) | Meta-analysis | Significantly worse DFS and OS of ER-low patients compared to patients with ER-positive BC | [36] |
| Skjervold (2023) | Retrospective population-based cohort study | No significant difference in prognosis (risk of death from BC) of patients with ER-low BC compared to those with ER-positive BC for patients diagnosed after 1995 | [37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malainou, C.P.; Stachika, N.; Damianou, A.K.; Anastopoulos, A.; Ploumaki, I.; Triantafyllou, E.; Drougkas, K.; Gomatou, G.; Kotteas, E. Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Curr. Oncol. 2023, 30, 9734-9745. https://doi.org/10.3390/curroncol30110706
Malainou CP, Stachika N, Damianou AK, Anastopoulos A, Ploumaki I, Triantafyllou E, Drougkas K, Gomatou G, Kotteas E. Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Current Oncology. 2023; 30(11):9734-9745. https://doi.org/10.3390/curroncol30110706
Chicago/Turabian StyleMalainou, Christina Panagiotis, Nikolina Stachika, Aikaterini Konstantina Damianou, Aristotelis Anastopoulos, Ioanna Ploumaki, Efthymios Triantafyllou, Konstantinos Drougkas, Georgia Gomatou, and Elias Kotteas. 2023. "Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives" Current Oncology 30, no. 11: 9734-9745. https://doi.org/10.3390/curroncol30110706
APA StyleMalainou, C. P., Stachika, N., Damianou, A. K., Anastopoulos, A., Ploumaki, I., Triantafyllou, E., Drougkas, K., Gomatou, G., & Kotteas, E. (2023). Estrogen-Receptor-Low-Positive Breast Cancer: Pathological and Clinical Perspectives. Current Oncology, 30(11), 9734-9745. https://doi.org/10.3390/curroncol30110706






