Systematic Review of Nomograms Used for Predicting Pathological Complete Response in Early Breast Cancer
Abstract
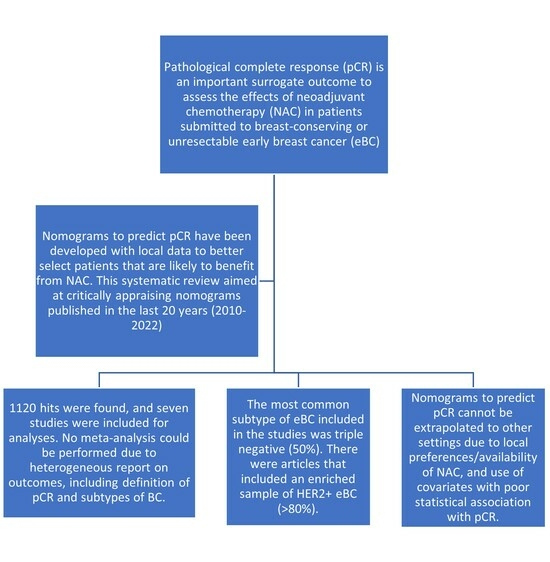
:1. Introduction
2. Methods
2.1. Protocol Registration and Rationale of Review
- What nomograms are available in the literature for predicting pathological complete response (PCR) in early-stage breast cancer?
- What are the clinical characteristics from the studied population?
- How were such nomograms validated?
- Did nomograms use an established database (administrative data of procedures) or were they validated with a cohort built for the purpose of developing a nomogram with clear inclusion and exclusion criteria?
- Are the nomograms still valid for clinical use? If so, what are the current gaps to be addressed in future studies?
2.2. Data Sources and Searches
2.3. Study Selection and Data Extraction
2.4. Data Synthesis and Analysis
3. Results
3.1. Overall and BC Characteristics from Studies
3.2. Nomogram Development and Assessment of How They Were Developed
| Author–Year | Country | Inclusion Criteria | Exclusion Criteria | Other Relevant Information |
|---|---|---|---|---|
| Kim et al., 2021 [11] | Republic of Korea | Stage 2 and 3 BC submitted to neoadjuvant chemotherapy, consecutive patients from January 2011 to December 2017 | Lack of imaging (magnetic resonance) or mammography (before or after neoadjuvant scheme); bilateral cancer; previous history of cancer. | Use of anti-HER2 therapies increased from 2014 |
| Hwang et al., 2019 [12] | Republic of Korea | Not described. It is suggested that patients with BC treated with NAC and followed by surgical resection and that had histologic evaluation were included | Not described. | A significant part of the cohort (248) had BC treated with NAC and followed by surgery between 2004 to 2013. Additional 60 patients treated between 2016 and 2017 were included because they received double anti-HER2 therapy |
| Li et al.,2021 [8] | China | Unilateral primary invasive breast cancer diagnosed by biopsy in patients with age between 18 and 70 years, clinical stage: 2 or 3, which met the requirements of the 2019 NCCN guidelines for NAC of breast cancer | Patients in the process of treatment, incomplete pathological results, receiving nonstandard neoadjuvant chemotherapy or surgery. | Does not apply |
| Hou et al., 2020 [13] | China | Not clearly described | 1. Bilateral BCs or meta-synchronous primary malignancies; Stage 0 and Stage 4 BC. 2. Patients already treated outside our hospital; unavailability of variables we wanted to include. | Does not apply |
| Fujii et al., 2017 [14] | USA | Partially described Stages 1–3 HER2-positive invasive breast cancer patients who had definitive surgery in 1999–2015 and received NST | Patients for whom continuous ER and PR levels or HER2/CEP17 ratios were not available were excluded from analysis. | Does not apply |
| Zhang et al., 2019 [15] | China | Female, histologically and molecularly confirmed to have TNBC before NAC, and received four cycles of anthracycline (epirubicin or adriamycin) and cyclophosphamide followed by four cycles of taxane every 3 weeks before surgery | Previous or concurrent cancer, bilateral breast cancer, or distant metastases. | Does not apply |
| Jin et al., 2016 [16] | China | Patients diagnosed with primary breast cancer and who received neoadjuvant chemotherapy followed by standard surgery were enrolled | Patients with HER2-positive core needle biopsy samples, with metastatic disease, with missing data or with previous endocrine therapy were not eligible for this study. Patients missing relevant information, who were HER2-positive or who had received neoadjuvant chemotherapy regimens other than cyclophosphamide, epirubicin and 5-fluorouracil, cyclophosphamide, epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin, navelbine and epirubicin or paclitaxel and carboplatin or paclitaxel and cisplatin were excluded from our study. | Does not apply |
| Author–Year | Demographics | Clinical Staging | Neoadjuvant Schemes | Histologic Grade/Type | Receptor Status | Other Relevant Information |
|---|---|---|---|---|---|---|
| Kim et al., 2021 [11] | 49 ± 10 years old | T1/T2 60% N0-N1 45% | 97% taxane-based | High grade: 46% | HER2: 34% | Anti-HER2 therapy was higher in developing cohort (37%) than validation cohort (94%). Ki-67: 15 (1–90) |
| Hwang et al., 2019 [12] | ~73% were <50 years old | ~38% cT1-2 ~62% cT3-4 ~21% cN0-1 ~79% cN2-3 | 60% taxane-based 40% anthracycline-based 60% > 4 cycles of NAC | ~48% Grade 1–2 ~52% Grade 3 91% Ductal type ~9% others | ~19% Lu A ~28% HER2− Lu B ~10% HER2+ Lu B ~14% HER2+ ~30% TN | The 60 patients who received double anti-HER2 block, but that population was not described anywhere except from methods. Ki-67 < 20 was 30% |
| Li et al., 2021 [8] | 47 ± 10 years old | 2 77% and 3 23% 87% cT0-2 and 13% cT2 > 2 79% N0-1 and 21% N2-3 | 100% contained taxanes 53% contained anthracycline 46% contained anti-HER2 3% contained double anti-HER2 block 83% > 5 NAC cycles | Not included | 32% HER2− Lu B 7% HER2+ Lu B 44% HER2+ 17% TN | Ki-67 < 65 was 76% |
| Hou et al., 2020 [13] | More than 40% were >49 years old | 90% T1-2 | 26% taxane and anthracycline-based schemes | Not included | 76% HER2-negative | Ki-67 > 20 was 69% |
| Fujii et al., 2017 [14] | 49 years old (range 19–84) | 2 50% and 3 47% | Cytotoxic agents alone 15% Anti-HER2 based 10% Double anti-HER2 75% | Ductal 94% Lobular 2% | Unclear, but it is suggestive that based on anti-HER2 therapies, the sample was largely composed of HER2+ | Does not apply |
| Zhang et al., 2019 [15] | 49.5 (33.1–64.0, IC95%) | cT1-2 80% cN0-1 62.5% | Eight cycles of thrice-weekly standard NAC (anthracycline and cyclophosphamide followed by taxane) | Not described | The sample was composed of TN | The sample was composed of TN |
| Jin et al., 2016 [16] | 80% were >40 years old | T1-2: 53% T3-4: 47% N0-1: 94% N2-3: 6% | Median of 4 cycles (range, 1–6 cycles): navelbine and epirubicin, cyclophosphamide, epirubicin and 5-fluorouracil, paclitaxel with carboplatin/paclitaxel with cisplatin or epirubicin and 5-fluorouracil followed by paclitaxel or docetaxel and epirubicin | Not described | Not described | Does not apply |
| Author–Year | Predictors from Nomogram | Outcome from Nomogram | Other Relevant Outcomes | Other Relevant Information |
|---|---|---|---|---|
| Kim et al., 2021 [11] | Not described | pCR. Manner of assessment not described in detail | Not applicable | Not applicable |
| Hwang et al., 2019 [12] | Pre-NAC TIL level, age, menopausal status, tumor size, clinical nodal stage (cN), histologic grade, NAC regimen and cycle number, expression level of ER, PR, HER2, and Ki-67 | pCR 15% and non-PCR 85% | Disease free survival and breast cancer-specific survival, as means to assess the prognostic value of post-NAC TILSs. Five-year BCSS rate was 45.6%, and 5-year DFS rate was 38.3% | High post-NAC TILs and low Ki-67 index were significant predictors of BCSS and DFS in the multivariable model. DFS and BCSS had undetailed definitions about censoring, follow-up and criteria for “disease-free” or “breast cancer-related mortality” |
| Li et al., 2021 [8] | Body mass index, Carbohydrate antigen 125, Total protein, Blood urea nitrogen, Cystatin C, Potassium, Phosphorus, platelet distribution width, activated partial thromboplastin time, thrombin time, antibody of hepatitis B surface | pCR 35.4% and non-pCR 64.6% | Not applicable | Not applicable |
| Hou et al., 2020 [13] | Menopause status, family history of BC, initial tumor size, estrogen receptor status, HER2/neu status, and Ki67 expression | Training set pCR 30% Validation set pCR 23% | Not applicable | Not applicable |
| Fujii et al., 2017 [14] | Variables of interest included age, race, BMI prior to NST, menopausal status, histologic findings, clinical stage, IBC or nonIBC, ER expression, PR expression, HER2/CEP17 ratio, and NST regimen (containing TmAb, TmAb plus pertuzumab (PmAb), or cytotoxic agents only) | pCR 56% and non-pCR 44% pCR after NST was defined as no evidence of residual invasive cancer in the breast and no residual cancer in the axilla at the time of definitive surgery | Not applicable | Not applicable |
| Zhang et al., 2019 [15] | Clinical tumor stage, lymphocyte to monocyte radio, fibrinogen level, D-dimer level | pCR 48.8% and non-pCR 51.2% | Not applicable | The sample was composed of TN |
| Jin et al., 2016 [16] | Tumor size, hormone receptor status, neoadjuvant chemotherapy regimens, cycles used, age, menopausal status, nodal status | pCR was defined as complete disappearance of invasive carcinoma in the breast and regional lymph nodes pCR 13% and non-pCR 77% | Not applicable | Not applicable |
| Author–Year | Cohort for Development and Validation | Sample (n, Adequate Report of Participants, Covariates and Outcomes) | Missing Data | Statistical Methods | Final Prediction Model Specified, Including 95% CI? |
|---|---|---|---|---|---|
| Kim et al., 2021 [11] | Yes | Development (n = 359) and validation (n = 351) There was not sample report for covariates and outcomes | Not described; however, according to baseline characteristics, there were no missing data | Uni- and multivariate analyses based on logistic regression. Predictors included based on p < 0.1. Calibration based on slope (1 = perfect, <1 overfitted). Interobserver independent validation (kappa and interclass correlation). | Yes. AUC 0.9 (IC95% de 0.86 a 0.94) |
| Hwang et al., 2019 [12] | Not described | The sample was 248 pair-matched pre-NAC biopsy and post-NAC resection | Not described; however, according to baseline characteristics, there were no missing data | Univariable logistic regression model and backward stepwise selection for final multivariable model were conducted. Calibration was assessed graphically. | Unreported, although it was described in methods |
| Li et al., 2021 [8] | Unclear | The sample consisted of 130 patients. All covariates and predictors had their respective number of patients | Not described; however, according to baseline characteristics, there were no missing data | Univariable analysis and multivariable binary logistic regression were used to determine independent predictors of bpCR after NAC. The nomogram was developed using a multivariable logistic regression model. Calibration of the nomogram was carried out by the 1000 bootstrap resampling internal verification and was displayed by the calibration curve. GiViTI calibration band: agreement between predicted and observed probability. Brier score: prediction accuracy. | Undescribed. AUC was 0.941 (95% confidence interval: 0.900–0.982) |
| Hou et al., 2020 [13] | Yes | Development (n = 689) and validation (n = 357). All covariates and predictors had their respective number of patients | Tumor grade was missing, and that is why it was not included as covariate | Univariate logistic regression analysis was conducted on variables in the training set, and variables with p < 0.05 were included in multivariate logistic regression. External validation was performed on the nomogram. The unbiased prediction of pCR by the nomogram was ensured by drawing the calibration curve. | Undescribed; AUC was 0.753 (95% confidence interval: 0.718–0.788) |
| Fujii et al., 2017 [14] | No | The sample was 793 | Not described; however, according to baseline characteristics, there were no missing data | Associations between categorical variables were examined using the χ2 test or Fisher exact test when appropriate. The Wilcoxon rank-sum test or Kruskal–Wallis test was used to examine differences in continuous variables between or among patient groups. Multivariate logistic regression models were applied to assess the effect of variables of interest on pCR status. Backward stepwise selection was applied to determine which variables were included in the final multivariable model. A nomogram was built to estimate the probability of pCR given the risk factors in the final multivariable model. A bootstrap validation method based on 200 bootstrap samples was employed to estimate the bias-corrected or overfitting-corrected predictive discriminative ability of the model, which was presented as the concordance index. | Not reported |
| Zhang et al., 2019 [15] | No | The sample was 80 | Not described; however, according to baseline characteristics, there were no missing data | The optimal cut-off values of the laboratory indexes were determined by the Youden index using receiver operating characteristic (ROC) curve analyses. Forward stepwise logistic regression (likelihood ratio) analysis was applied to identify predictive factors for a pCR of NAC. A nomogram was then developed according to the logistic model, and internally validated using the bootstrap resampling method. | AUC was 0.803 (95% confidence interval 0.706–0.899 |
| Jin et al., 2016 [16] | Yes | The 815 were randomized to a training (500) and validation set (315) | Missing data on chemotherapy was an exclusion criterion. | Chi-square test was used to evaluate the relationship between neoadjuvant chemotherapy regimens and other characteristics. Fisher’s exact test was performed when necessary. All reported p-values are two-sided. | AUC from validation set: 0.703 (95% CI: 0.624–0.782) |
4. Discussion
4.1. Low pCR Prediction Variables in the Nomogram
4.2. Clinical Applicability for 2022
4.3. Quality of the Validation Process
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- World Health Organization. Breast Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 14 May 2022).
- American Society of Clinical Oncology. Breast Cancer: Statistics. Available online: https://www.cancer.net/cancer-types/breast-cancer/statistics#:~:text=Sixty%2Dfive%20percent%20(65%25),cases%20in%20this%20age%20group (accessed on 22 April 2023).
- Mauri, D.; Pavlidis, N.; Ioannidis, J.P.A. Neoadjuvant versus adjuvant systemic treatment in breast cancer: A meta-analysis. J. Natl. Cancer Institut. 2005, 97, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, P.; Anderson, S.J.; Bear, H.D.; Geyer, C.E.; Kahlenberg, M.S.; Robidoux, A.; Margolese, R.G.; Hoehn, J.L.; Vogel, V.G.; Dakhil, S.R.; et al. Preoperative chemotherapy: Updates of national surgical adjuvant breast and bowel project protocols B-18 and B-27. J. Clin. Oncol. 2008, 26, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Spring, L.M.; Fell, G.; Arfe, A.; Sharma, C.; Greenup, R.; Reynolds, K.L.; Smith, B.L.; Alexander, B.; Moy, B.; Isakoff, S.J.; et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: A comprehensive meta-analysis. Clin. Cancer Res. 2020, 26, 2838–2848. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, e173–e180. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, X.; Ling, H.; Gong, Y.; Guo, L.; He, M.; Sun, H.; Hu, X. Nomogram for predicting breast cancer-specific mortality of elderly women with breast cancer. Med. Sci. Monit. 2020, 26, e925210. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, J.; Wang, B.; Zhang, H.; He, J.; Wang, K. A nomogram based on clinicopathological features and serological indicators predicting breast pathologic complete response of neoadjuvant chemotherapy in breast cancer. Sci. Rep. 2021, 11, 11348. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Moons, K.G.M.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.A.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, N.; Choi, Y.; Lee, S.H.; Ha, S.M.; Kim, E.S.; Chang, J.M.; Moon, W.K. Factors affecting pathologic complete response following neoadjuvant chemotherapy in breast cancer: Development and validation of a predictive nomogram. Radiology 2021, 299, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.W.; Jung, H.; Hyeon, J.; Park, Y.H.; Ahn, J.S.; Im, Y.-H.; Nam, S.J.; Kim, S.W.; Lee, J.E.; Yu, J.H.; et al. A nomogram to predict pathologic complete response (pCR) and the value of tumor-infiltrating lymphocytes (TILs) for prediction of response to neoadjuvant chemotherapy (NAC) in breast cancer patients. Breast Cancer Res. Treat. 2019, 173, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Hou, N.; Xiao, J.; Wang, Z.; Wu, Y.; Hou, G.; Guo, L.; Zhang, J.; Ling, R. Development and validation of a nomogram for individually predicting pathologic complete remission after preoperative chemotherapy in Chinese breast cancer: A population-based study. Clin. Breast Cancer 2020, 20, e682–e694. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Kogawa, T.; Wu, J.; Sahin, A.A.; Liu, D.D.; Chavez-MacGregor, M.; Giordano, S.H.; Raghavendra, A.; Murthy, R.K.; Tripathy, D.; et al. Nomogram to predict pathologic complete response in HER2-positive breast cancer treated with neoadjuvant systemic therapy. Br. J. Cancer 2017, 116, 509–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, M.; Zhou, H.; Chen, K.; Jin, J.; Wu, Y.; Ying, L.; Ding, X.; Su, D.; Zou, D. A nomogram to predict the pathologic complete response of neoadjuvant chemotherapy in triple-negative breast cancer based on simple laboratory indicators. Ann. Surg. Oncol. 2019, 26, 3912–3919. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Jiang, Y.Z.; Chen, S.; Yu, K.D.; Ma, D.; Sun, W.; Shao, Z.M.; Di, G.H. A nomogram for predicting pathological complete response in patients with human epidermal growth factor receptor 2 negative breast cancer. BMC Cancer 2016, 16, 606. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Chen, S.; Zhang, X.; Zhou, Q. Ki-67 labeling index is a predictive marker for a pathological complete response to neoadjuvant chemotherapy in breast cancer: A meta-analysis. Medicine 2017, 96, e9384. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J.; Panel, M. Strategies for subtypes—Dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Leong, S.P.L.; Shen, Z.Z.; Liu, T.J.; Agarwal, G.; Tajima, T.; Paik, N.S.; Sandelin, K.; Derossis, A.; Cody, H.; Foulkes, W.D. Is breast cancer the same disease in Asian and Western countries? World J. Surg. 2010, 34, 2308–2324. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonini, M.; Pannain, G.D.; Mattar, A.; Ferraro, O.; Lopes, R.G.C.; Real, J.M.; Okumura, L.M. Systematic Review of Nomograms Used for Predicting Pathological Complete Response in Early Breast Cancer. Curr. Oncol. 2023, 30, 9168-9180. https://doi.org/10.3390/curroncol30100662
Antonini M, Pannain GD, Mattar A, Ferraro O, Lopes RGC, Real JM, Okumura LM. Systematic Review of Nomograms Used for Predicting Pathological Complete Response in Early Breast Cancer. Current Oncology. 2023; 30(10):9168-9180. https://doi.org/10.3390/curroncol30100662
Chicago/Turabian StyleAntonini, Marcelo, Gabriel Duque Pannain, André Mattar, Odair Ferraro, Reginaldo Guedes Coelho Lopes, Juliana Monte Real, and Lucas Miyake Okumura. 2023. "Systematic Review of Nomograms Used for Predicting Pathological Complete Response in Early Breast Cancer" Current Oncology 30, no. 10: 9168-9180. https://doi.org/10.3390/curroncol30100662
APA StyleAntonini, M., Pannain, G. D., Mattar, A., Ferraro, O., Lopes, R. G. C., Real, J. M., & Okumura, L. M. (2023). Systematic Review of Nomograms Used for Predicting Pathological Complete Response in Early Breast Cancer. Current Oncology, 30(10), 9168-9180. https://doi.org/10.3390/curroncol30100662