Safety and Efficacy of Hepatic Artery Embolization in Heavily Treated Patients with Intrahepatic Cholangiocarcinoma: Analysis of Clinicopathological and Radiographic Parameters Associated with Better Overall Survival
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Demographic Characteristics
3.2. Factors Associated with Outcome
- Sex: 68% (n = 23) of the patients were female. The female patients had higher OS compared to male patients (23.67 vs. 16.91 months); however, this difference was not statistically significant (p = 0.47).
- Pathological grading: The majority of the patients had moderately differentiated IHC (n = 21). Ten patients had poorly differentiated IHC, and in three patients the grading was unknown. No significant difference in OS was detected between moderately and poorly differentiated ICC (OS in moderately differentiated was 21.8 months vs. 20.3 months in poorly differentiated vs. 20 months in the unknown patients; p = 0.9).
- Treatment prior to embolization: A group of 13 patients underwent liver tumor resection prior to embolization, while 21 patients did not undergo any liver tumor resection. Patients that were treated with surgical resection prior to embolization demonstrated significantly higher OS compared to patients that were not treated with resection (34 vs. 14.7 months, respectively, p = 0.03).
- Presence of extrahepatic metastatic disease at the time of initial diagnosis: A total of 15 patients presented with metastatic disease at the time of diagnosis (Table 1). These patients demonstrated significantly lower OS post-embolization compared to patients without evidence of metastatic disease (12.9 vs. 31.6; p = 0.03).
- Indication for embolization: Patients that were treated as the first line of treatment (n = 10) had higher OS compared to chemorefractory patients (28.5 vs. 17 months). However, this difference was not statistically significant.
- Venous invasion: A total of 7 (20.5%) patients presented with venous involvement at the time of first embolization. The inferior vena cava was involved in 2, the hepatic vein in 1, and the portal vein was invaded in 4 patients. Patients with venous invasion had significantly lower OS compared to patients with no venous involvement (7 vs. 28.6 months; p = 0.046).
- Tumor pattern:
- Solitary vs. multifocal: The majority of patients (88.2%) presented with multifocal disease at the time of embolization. No significant difference was detected between the two groups (29.8 months in solitary tumors vs. 20.7 in multifocal; p = 0.45).
- Tumor diameter: Tumors larger than 10 cm (n = 8) were associated with worse OS compared to the other two groups (p = 0.0038). Larger than 10 cm tumors had an OS of 7.3 months (Figure 2B).
- Percent of liver involvement by the tumor: Patients with less than 25% (n = 19) tumor involvement in the liver showed significantly longer OS compared to patients with liver involvement of more than 25% (n = 15) (34 vs. 13 months; p = 0.0076).
- Degree of enhancement on pre-embolization cross-sectional imaging: Tumors were hypervascular in 12 (35%) patients and hypo- or iso-vascular in 22 (65%). Hypervascular tumors were associated with better OS compared to hypo- and iso-vascular tumors (32.9 months vs. 14.4; p = 0.04).
3.3. Follow Up and Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaib, Y.; El-Serag, H.B. The epidemiology of cholangiocarcinoma. Semin. Liver Dis. 2004, 24, 115–125. [Google Scholar] [CrossRef]
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [Google Scholar] [CrossRef] [PubMed]
- Nathan, H.; Pawlik, T.M.; Wolfgang, C.L.; Choti, M.A.; Cameron, J.L.; Schulick, R.D. Trends in survival after surgery for cholangiocarcinoma: A 30-year population-based SEER database analysis. J. Gastrointest. Surg. 2007, 11, 1488–1496; discussion 1496–1487. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, S.; Khan, S.A.; Hallemeier, C.L.; Kelley, R.K.; Gores, G.J. Cholangiocarcinoma—Evolving concepts and therapeutic strategies. Nat. Rev. Clin. Oncol. 2018, 15, 95–111. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.C.; Choti, M.A.; Bellavance, E.C.; Pawlik, T.M. Palliation of hepatic tumors. Surg. Oncol. 2007, 16, 277–291. [Google Scholar] [CrossRef]
- Burger, I.; Hong, K.; Schulick, R.; Georgiades, C.; Thuluvath, P.; Choti, M.; Kamel, I.; Geschwind, J.F.H. Transcatheter arterial chemoembolization in unresectable cholangiocarcinoma: Initial experience in a single institution. J. Vasc. Interv. Radiol. JVIR 2005, 16, 353–361. [Google Scholar] [CrossRef]
- Hyder, O.; Marsh, J.W.; Salem, R.; Petre, E.N.; Kalva, S.; Liapi, E.; Cosgrove, D.; Neal, D.; Kamel, I.; Zhu, A.X.; et al. Intra-arterial therapy for advanced intrahepatic cholangiocarcinoma: A multi-institutional analysis. Ann. Surg. Oncol. 2013, 20, 3779–3786. [Google Scholar] [CrossRef]
- Jordan, O.; Denys, A.; De Baere, T.; Boulens, N.; Doelker, E. Comparative study of chemoembolization loadable beads: In vitro drug release and physical properties of DC bead and hepasphere loaded with doxorubicin and irinotecan. J. Vasc. Interv. Radiol. JVIR 2010, 21, 1084–1090. [Google Scholar] [CrossRef]
- Maluccio, M.; Covey, A.M.; Gandhi, R.; Gonen, M.; Getrajdman, G.I.; Brody, L.A.; Fong, Y.; Jarnagin, W.; D’Angelica, M.; Blumgart, L.; et al. Comparison of survival rates after bland arterial embolization and ablation versus surgical resection for treating solitary hepatocellular carcinoma up to 7 cm. J. Vasc. Interv. Radiol. JVIR 2005, 16, 955–961. [Google Scholar] [CrossRef]
- Llovet, J.M.; Di Bisceglie, A.M.; Bruix, J.; Kramer, B.S.; Lencioni, R.; Zhu, A.X.; Sherman, M.; Schwartz, M.; Lotze, M.; Talwalkar, J.; et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J. Natl. Cancer Inst. 2008, 100, 698–711. [Google Scholar] [CrossRef]
- Valle, J.; Wasan, H.; Palmer, D.H.; Cunningham, D.; Anthoney, A.; Maraveyas, A.; Madhusudan, S.; Iveson, T.; Hughes, S.; Pereira, S.P.; et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N. Engl. J. Med. 2010, 362, 1273–1281. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; McClenny, T.E.; Cardella, J.F.; Lewis, C.A. Society of Interventional Radiology clinical practice guidelines. J. Vasc. Interv. Radiol. JVIR 2003, 14, S199–S202. [Google Scholar] [CrossRef] [PubMed]
- Boehm, L.M.; Jayakrishnan, T.T.; Miura, J.T.; Zacharias, A.J.; Johnston, F.M.; Turaga, K.K.; Gamblin, T.C. Comparative effectiveness of hepatic artery based therapies for unresectable intrahepatic cholangiocarcinoma. J. Surg. Oncol. 2015, 111, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Bragazzi, M.C.; Venere, R.; Ribichini, E.; Covotta, F.; Cardinale, V.; Alvaro, D. Intrahepatic cholangiocarcinoma: Evolving strategies in management and treatment. Dig. Liver Dis. 2023; Online ahead of print. [Google Scholar] [CrossRef]
- Brown, K.T.; Do, R.K.; Gonen, M.; Covey, A.M.; Getrajdman, G.I.; Sofocleous, C.T.; Jarnagin, W.R.; D’Angelica, M.I.; Allen, P.J.; Erinjeri, J.P.; et al. Randomized Trial of Hepatic Artery Embolization for Hepatocellular Carcinoma Using Doxorubicin-Eluting Microspheres Compared With Embolization With Microspheres Alone. J. Clin. Oncol. 2016, 34, 2046–2053. [Google Scholar] [CrossRef]
- Gusani, N.J.; Balaa, F.K.; Steel, J.L.; Geller, D.A.; Marsh, J.W.; Zajko, A.B.; Carr, B.I.; Gamblin, T.C. Treatment of unresectable cholangiocarcinoma with gemcitabine-based transcatheter arterial chemoembolization (TACE): A single-institution experience. J. Gastrointest. Surg. 2008, 12, 129–137. [Google Scholar] [CrossRef]
- Kuhlmann, J.B.; Euringer, W.; Spangenberg, H.C.; Breidert, M.; Blum, H.E.; Harder, J.; Fischer, R. Treatment of unresectable cholangiocarcinoma: Conventional transarterial chemoembolization compared with drug eluting bead-transarterial chemoembolization and systemic chemotherapy. Eur. J. Gastroenterol. Hepatol. 2012, 24, 437–443. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.H.; Yoon, H.J.; Lee, I.S.; Yoon, H.K.; Kim, K.P. Transarterial chemoembolization versus supportive therapy in the palliative treatment of unresectable intrahepatic cholangiocarcinoma. Clin. Radiol. 2011, 66, 322–328. [Google Scholar] [CrossRef]
- Rafi, S.; Piduru, S.M.; El-Rayes, B.; Kauh, J.S.; Kooby, D.A.; Sarmiento, J.M.; Kim, H.S. Yttrium-90 radioembolization for unresectable standard-chemorefractory intrahepatic cholangiocarcinoma: Survival, efficacy, and safety study. Cardiovasc. Interv. Radiol. 2013, 36, 440–448. [Google Scholar] [CrossRef]
- Edeline, J.; Lamarca, A.; McNamara, M.G.; Jacobs, T.; Hubner, R.A.; Palmer, D.; Groot Koerkamp, B.; Johnson, P.; Guiu, B.; Valle, J.W. Locoregional therapies in patients with intrahepatic cholangiocarcinoma: A systematic review and pooled analysis. Cancer Treat. Rev. 2021, 99, 102258. [Google Scholar] [CrossRef]
- Fiore, F.; Del Prete, M.; Franco, R.; Marotta, V.; Ramundo, V.; Marciello, F.; Di Sarno, A.; Carratù, A.C.; de Luca di Roseto, C.; Colao, A.; et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine 2014, 47, 177–182. [Google Scholar] [CrossRef]
- Strosberg, J.R.; Choi, J.; Cantor, A.B.; Kvols, L.K. Selective hepatic artery embolization for treatment of patients with metastatic carcinoid and pancreatic endocrine tumors. Cancer Control 2006, 13, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.T.; Omary, R.A.; Takehana, C.; Ibrahim, S.; Lewandowski, R.J.; Ryu, R.K.; Salem, R. The role of tumor vascularity in predicting survival after yttrium-90 radioembolization for liver metastases. J. Vasc. Interv. Radiol. JVIR 2009, 20, 1564–1569. [Google Scholar] [CrossRef] [PubMed]
- Shimohira, M.; Sato, Y.; Yasumoto, T.; Kodama, Y.; Masada, T.; Inaba, Y.; Yamakado, K. Arterial Embolization Using Microspheres for Hypervascular Liver Metastases Refractory to Standard Treatments: A Multicenter Prospective Clinical Trial. Cardiovasc. Interv. Radiol. 2021, 44, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Ridouani, F.; Soliman, M.M.; England, R.W.; Hsu, M.; Moskowitz, C.S.; Doustaly, R.; Sofocleous, C.T.; Boas, F.E.; Yarmohammadi, H.; Deipolyi, A.R. Relationship of radiation dose to efficacy of radioembolization of liver metastasis from breast cancer. Eur. J. Radiol. 2021, 136, 109539. [Google Scholar] [CrossRef]
- Öcal, O.; Schinner, R.; Schütte, K.; de Toni, E.N.; Loewe, C.; van Delden, O.; Vandecaveye, V.; Gebauer, B.; Zech, C.J.; Sengel, C.; et al. Early tumor shrinkage and response assessment according to mRECIST predict overall survival in hepatocellular carcinoma patients under sorafenib. Cancer Imaging 2022, 22, 1. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Age (mean +/− SD, range) | 62 ± 14.03; 31–92 |
| Sex | |
| Male | 11 (32%) |
| Female | 23 (68%) |
| Metastasis at presentation | |
| Yes | 15 (44.1%) |
| No | 19 (55.9%) |
| Location of Metastasis | |
| Lymph node | 6 (17.6%) |
| Lung | 1 (3%) |
| Lung + Lymph node | 4 (11.7%) |
| IVC | 1 (3%) |
| Bone | 1 (3%) |
| Multifocal | 2 (5.8%) |
| Pathology type | |
| Moderately differentiated | 21 (61.8%) |
| Poorly differentiated | 10 (29.4%) |
| Unknown | 3 (8.8%) |
| Treatment prior to embolization | |
| Chemotherapy (systemic or arterial infusion) | 24 (70.6%) |
| Hepatic arterial infusion pump | 4 (11.7%) |
| Surgery only | 4 (11.7%) |
| Combination of surgery and chemotherapy | 7 (20.5%) |
| Combination of chemotherapy and external beam radiation | 4 (11.7%) |
| Alcohol ablation and chemotherapy | 1 (2.9%) |
| RFA plus chemotherapy and surgery | 2 (5.8%) |
| None | 10 (29.4%) |
| Tumor characteristics | |
| Tumor pattern | |
| Solitary | 4 (11.8%) |
| Multifocal | 30 (88.2%) |
| Largest tumor size (cm) | |
| Mean, SD | 7.6 +/− 3.9 |
| Range | 2–17.8 |
| <5 cm | 11 (32.3%) |
| ≥5 to ≤10 | 15 (44.2%) |
| >10 | 8 (23.5%) |
| % of liver involvement | |
| <25% | 19 (55.8%) |
| 25–50% | 15 (44.2%) |
| 50–75% | 0 (0%) |
| Drug/Regimens | Number of Patients (%) |
|---|---|
| Commonly used systemic chemotherapy | |
| Gemcitabine and cisplatin | 8 (23.5) |
| Gemcitabine single therapy | 6 (17.6) |
| Other used systemic therapy | |
| Gemcitabine + capecitabine | 3 (8.8) |
| Gemcitabine + carboplatin | 1 (2.9) |
| Gemcitabine + carboplatin + paclitaxel | 1 (2.9) |
| Gemcitabine + carboplatin + capecitabine | 1 (2.9) |
| Gemcitabine + docetaxel + capecitabine (GTX) | 1 (2.9) |
| Gemcitabine + 5FU | 1 (2.9) |
| Gemcitabine + oxaliplatin | 2 (5.8) |
| Gemcitabine + bevacizumab | 1 (2.9) |
| Gemcitabine + carboplatin + bevacizumab | 1 (2.9) |
| Oxaliplatin + 5FU + Leucovorin | 1 (2.9) |
| Capecitabine + oxaliplatin (XELOX) | 1 (2.9) |
| Capecitabine single therapy | 1 (2.9) |
| Irinotecan single therapy | 2 (5.8) |
| Flurouracil (5FU) single therapy | 1 (2.9) |
| Irinotecan + capecitabine | 1 (2.9) |
| FOLFIRI | 2 (5.8) |
| FOLFOX-6 | 1 (2.9) |
| Targeted therapy as a monotherapy after progression on multiple regimens | |
| Sorafenib | 1 (2.9) |
| Immunotherapy | |
| Nivolumab | 1 (2.9) |
| Chemotherapy with hepatic arterial infusion pump | |
| Floxuridine (FUDR) + Mitomycin to side port of the pump | 2 (5.8) |
| Floxuridine (FUDR) | 2 (5.8) |
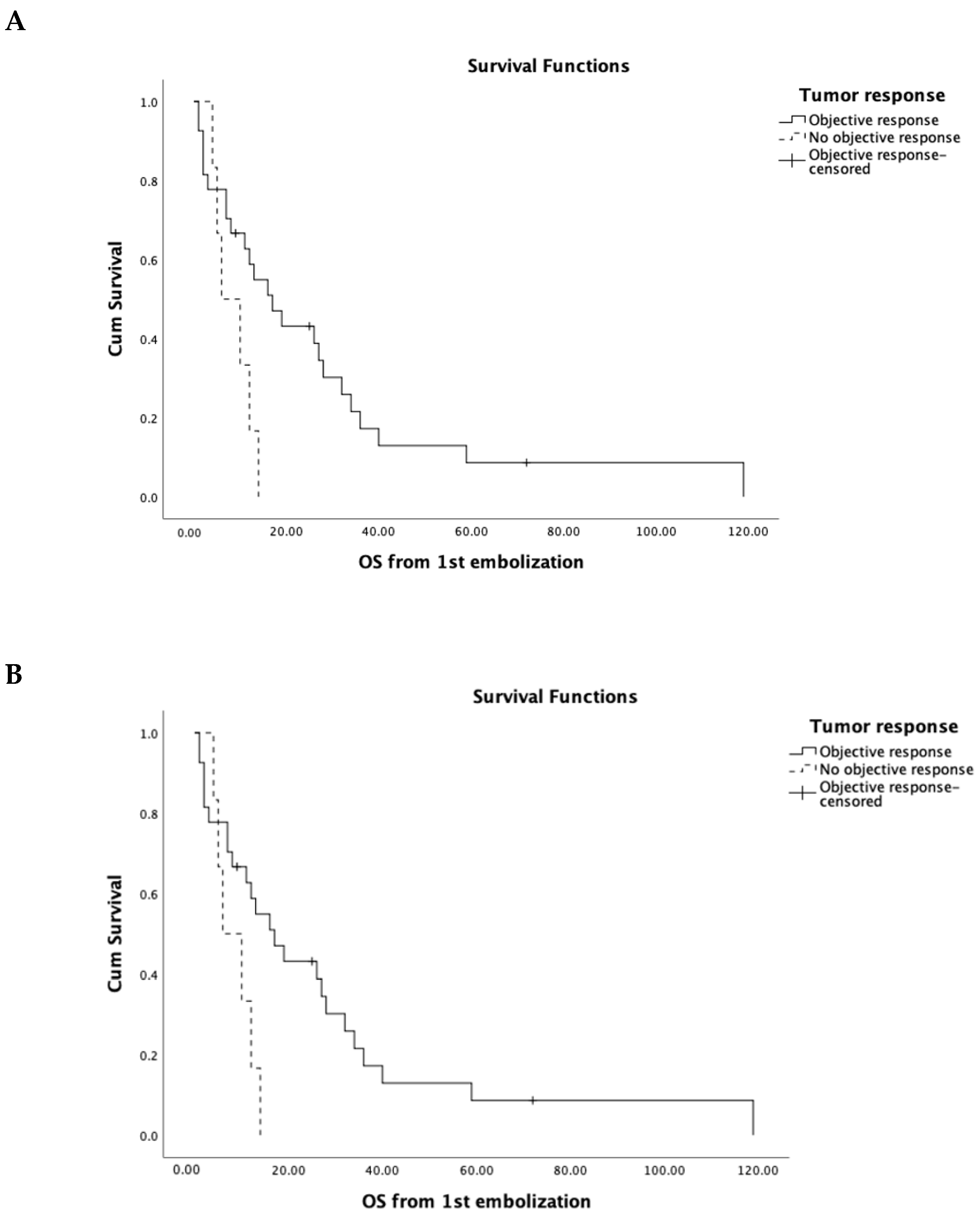
| Treatment Response Category | Value | Objective Response (OR) |
|---|---|---|
| Complete Response (CR) | 14 (41.1%) | 27 (79.4%) |
| Partial Response (PR) | 13 (38.2%) | |
| Stable Disease (SD) | 2 (5.8%) | |
| Progressive Disease (PD) | 4 (11.7%) | |
| Not evaluated (NE) | 1 (2.9%) |
| OS from 1st embolization | |
| mean | 23.7 ± 23.9 (CI: 13.4–34.1) |
| median | 13 (CI: 7.4–18.5) |
| range | 1–119 |
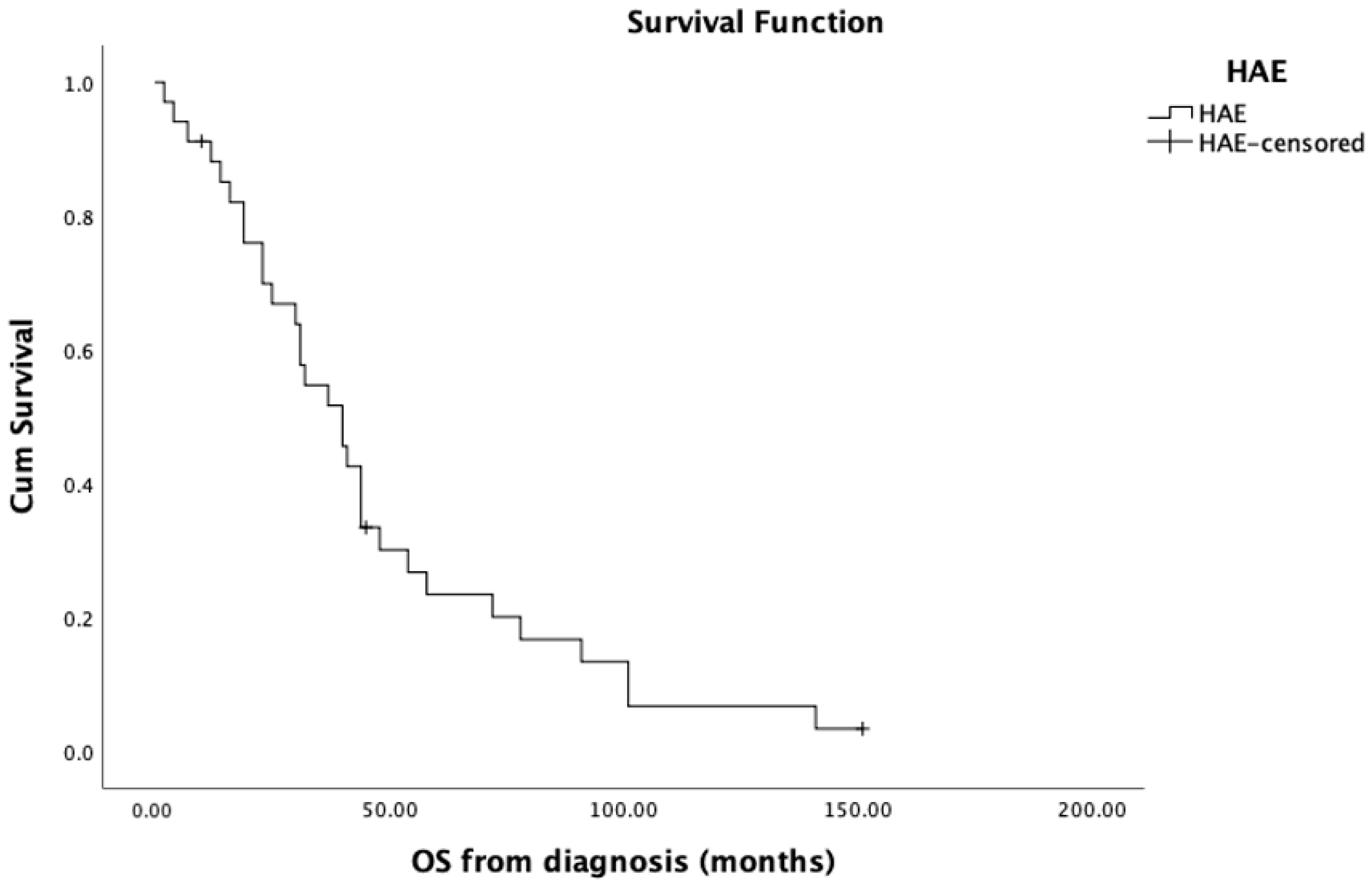
| OS from diagnosis | |
| mean, SD | 47.4 ± 36.3 (CI: 34.6–60.2) |
| median | 40 (CI: 28.8–51.1) |
| range | 2–151 |
| LT-PFS | |
| mean, SD | 7.7 ± 8.7 (CI: 4.5–10.9) |
| median | 4 (CI: 2.09–5.9) |
| range | 1–37 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Velayati, S.; Elsakka, A.; Zhao, K.; Erinjeri, J.P.; Marinelli, B.; Soliman, M.; Chevallier, O.; Ziv, E.; Brody, L.A.; Sofocleous, C.T.; et al. Safety and Efficacy of Hepatic Artery Embolization in Heavily Treated Patients with Intrahepatic Cholangiocarcinoma: Analysis of Clinicopathological and Radiographic Parameters Associated with Better Overall Survival. Curr. Oncol. 2023, 30, 9181-9191. https://doi.org/10.3390/curroncol30100663
Velayati S, Elsakka A, Zhao K, Erinjeri JP, Marinelli B, Soliman M, Chevallier O, Ziv E, Brody LA, Sofocleous CT, et al. Safety and Efficacy of Hepatic Artery Embolization in Heavily Treated Patients with Intrahepatic Cholangiocarcinoma: Analysis of Clinicopathological and Radiographic Parameters Associated with Better Overall Survival. Current Oncology. 2023; 30(10):9181-9191. https://doi.org/10.3390/curroncol30100663
Chicago/Turabian StyleVelayati, Sara, Ahmed Elsakka, Ken Zhao, Joseph P. Erinjeri, Brett Marinelli, Mohamed Soliman, Olivier Chevallier, Etay Ziv, Lynn A. Brody, Constantinos T. Sofocleous, and et al. 2023. "Safety and Efficacy of Hepatic Artery Embolization in Heavily Treated Patients with Intrahepatic Cholangiocarcinoma: Analysis of Clinicopathological and Radiographic Parameters Associated with Better Overall Survival" Current Oncology 30, no. 10: 9181-9191. https://doi.org/10.3390/curroncol30100663
APA StyleVelayati, S., Elsakka, A., Zhao, K., Erinjeri, J. P., Marinelli, B., Soliman, M., Chevallier, O., Ziv, E., Brody, L. A., Sofocleous, C. T., Solomon, S. B., Harding, J. J., Abou-Alfa, G. K., D’Angelica, M. I., Wei, A. C., Kingham, P. T., Jarnagin, W. R., & Yarmohammadi, H. (2023). Safety and Efficacy of Hepatic Artery Embolization in Heavily Treated Patients with Intrahepatic Cholangiocarcinoma: Analysis of Clinicopathological and Radiographic Parameters Associated with Better Overall Survival. Current Oncology, 30(10), 9181-9191. https://doi.org/10.3390/curroncol30100663






