Patient Derived Xenografts (PDX) Models as an Avatar to Assess Personalized Therapy Options in Uveal Melanoma: A Feasibility Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients and Tumor Samples
2.2. Establishment of Uveal Melanoma Xenografts
2.3. In Vivo Therapeutic Assessment
2.4. Histopathological Analyses
2.5. Establishment of the Tumor Molecular Profile
3. Results
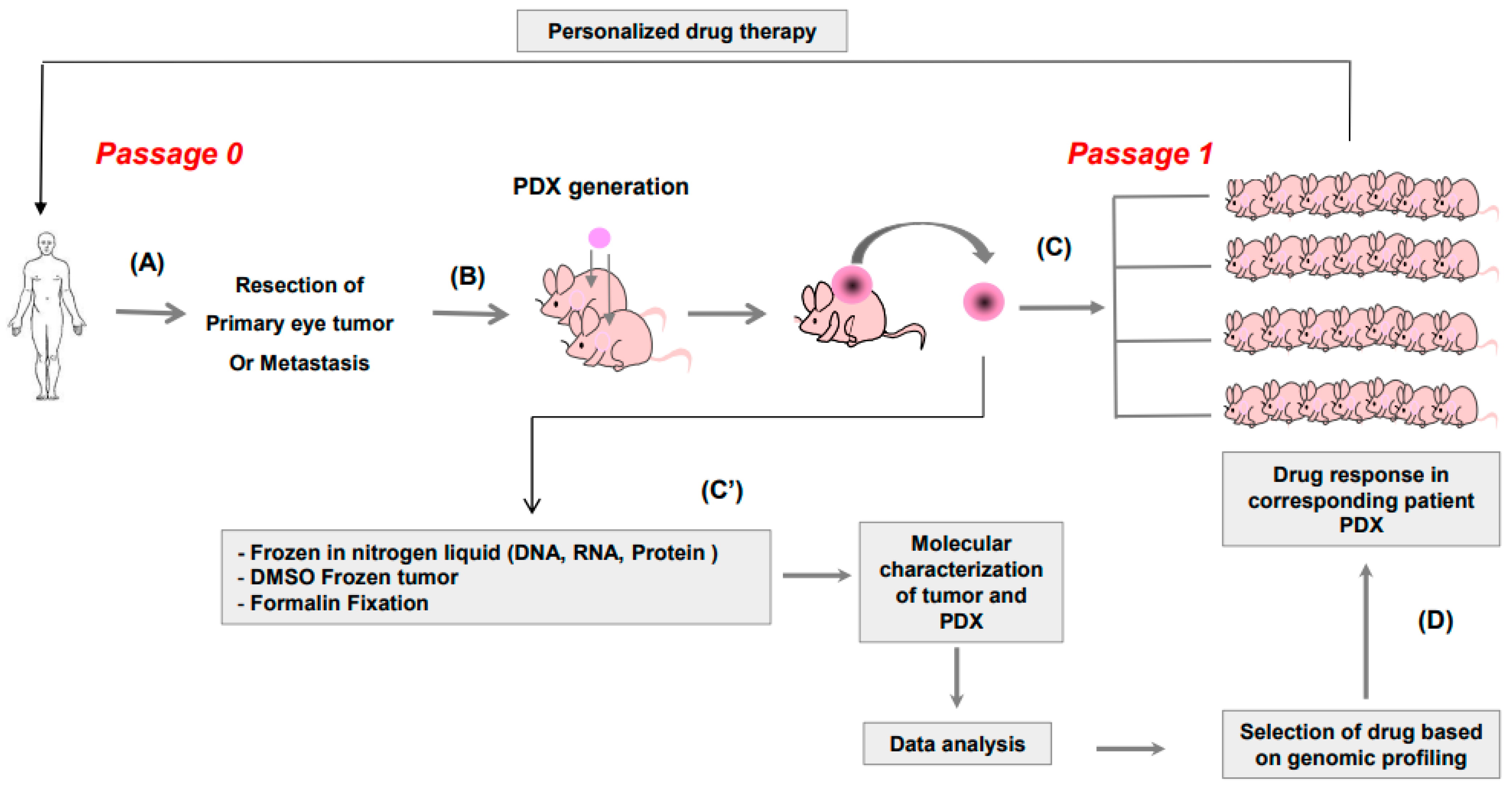
3.1. Study Design
3.2. Establishment of PDX
3.3. Genomic Risk of the Patient Correlates with Tumor Take Rate in Mice
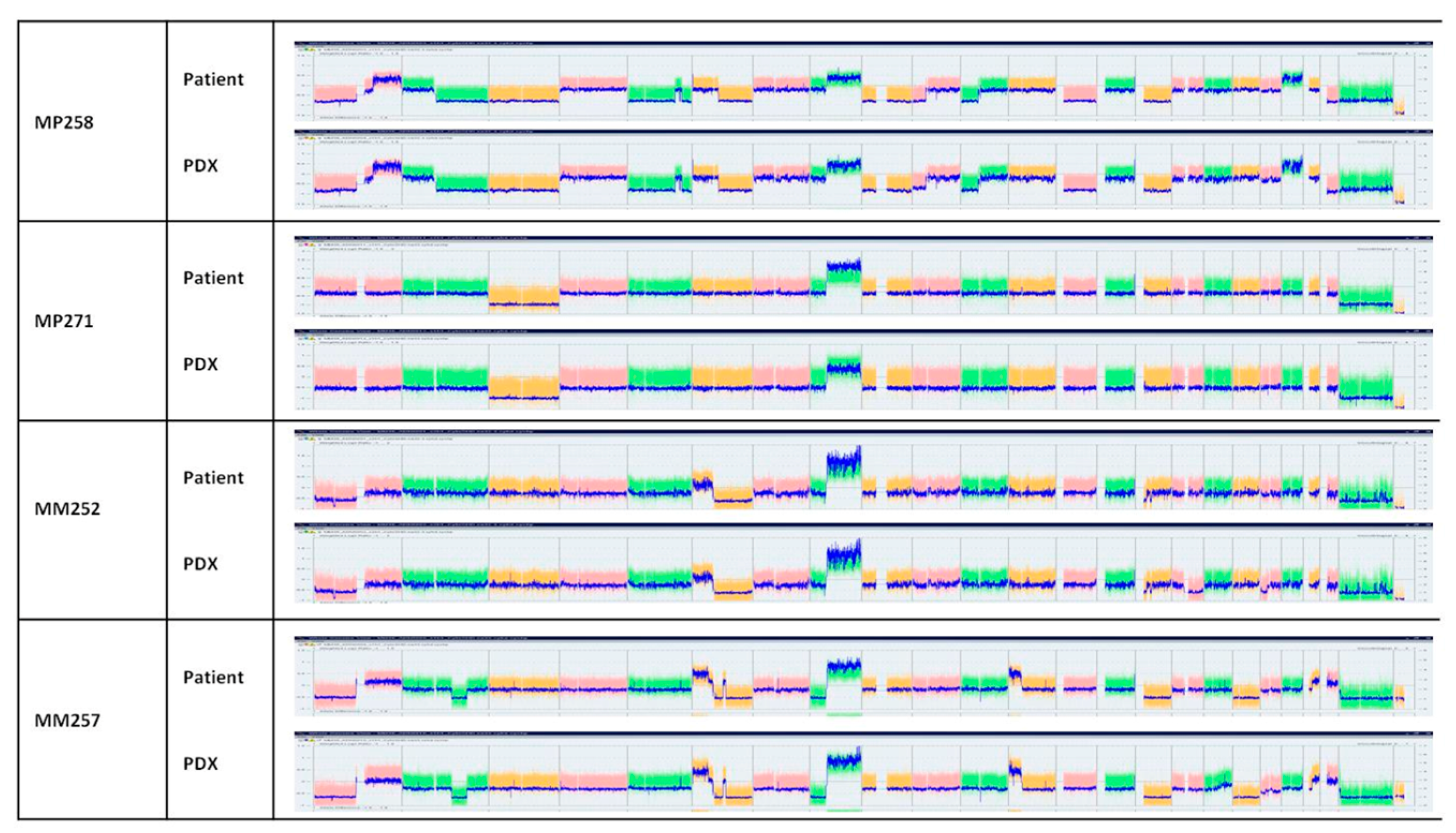
3.4. Conservation of Histological and Genomic Features between Patient’s Tumor and Their PDX
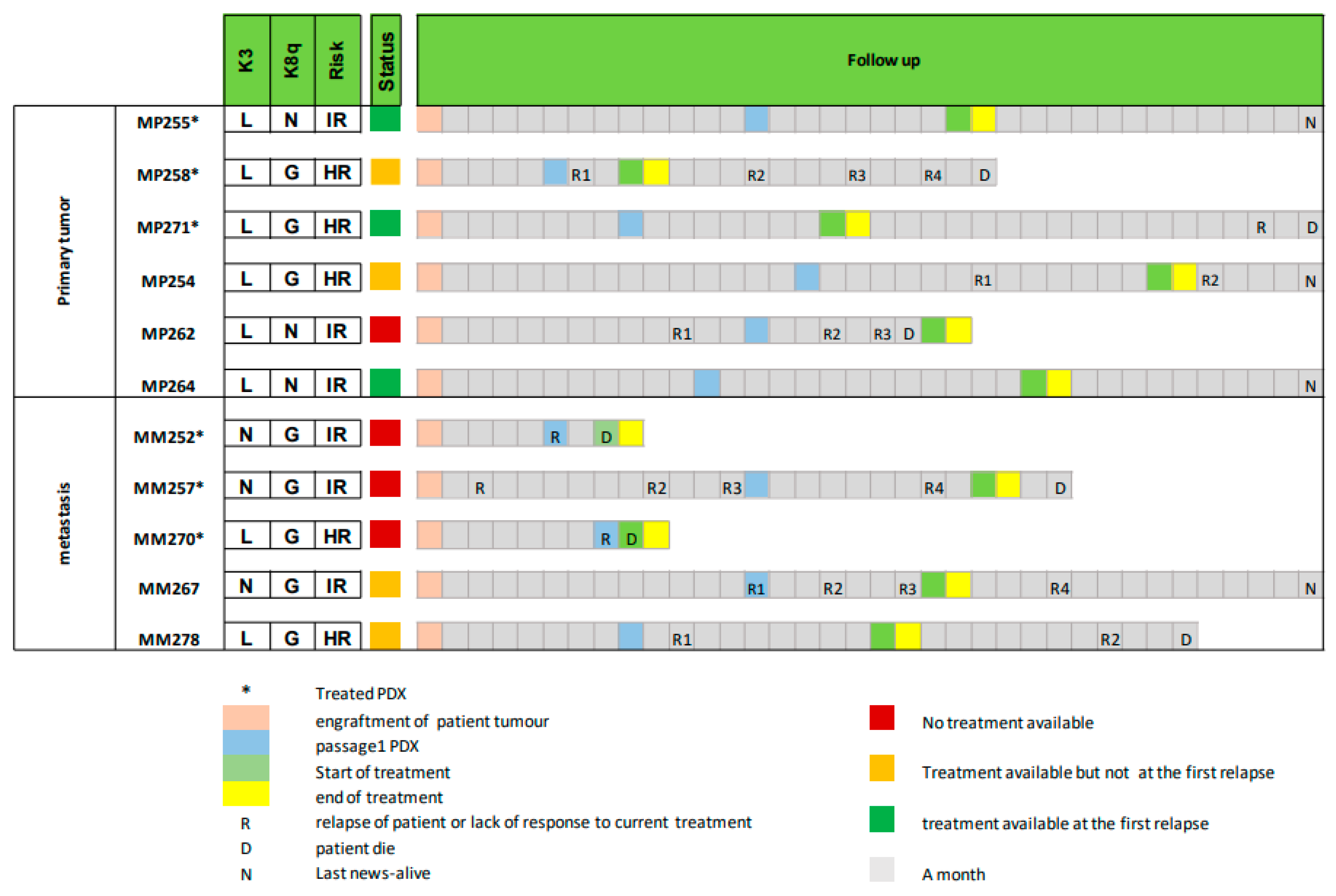
3.5. Using Avatar Models to Direct Patient Therapy
3.6. Follow-Up of Patient and Corresponding PDX Models
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahendraraj, K.; Lau, C.S.; Lee, I.; Chamberlain, R.S. Trends in incidence, survival, and management of uveal melanoma: A population-based study of 7,516 patients from the Surveillance, Epidemiology, and End Results database (1973–2012). Clin. Ophthalmol. 2016, 10, 2113–2119. [Google Scholar] [CrossRef] [PubMed]
- Kujala, E.; Mäkitie, T.; Kivelä, T. Very Long-Term Prognosis of Patients with Malignant Uveal Melanoma. Investig. Ophthalmol. Vis. Sci. 2003, 44, 4651–4659. [Google Scholar] [CrossRef] [PubMed]
- Carvajal, R.D.; Schwartz, G.K.; Tezel, T.; Marr, B.; Francis, J.H.; Nathan, P.D. Metastatic disease from uveal melanoma: Treatment options and future prospects. Br. J. Ophthalmol. 2017, 101, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Mariani, P.; Piperno-Neumann, S.; Servois, V.; Berry, M.G.; Dorval, T.; Plancher, C.; Couturier, J.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Desjardins, L.; et al. Surgical management of liver metastases from uveal melanoma: 16 years’ experience at the Institut Curie. Eur. J. Surg. Oncol. 2009, 35, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Aaberg, T.M.; Covington, K.R.; Tsai, T.; Shildkrot, Y.; Plasseraud, K.M.; Alsina, K.M.; Oelschlager, K.M.; Monzon, F.A. Gene Expression Profiling in Uveal Melanoma: Five-Year Prospective Outcomes and Meta-Analysis. Ocul. Oncol. Pathol. 2020, 6, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Cassoux, N.; Rodrigues, M.J.; Plancher, C.; Asselain, B.; Levy-Gabriel, C.; Lumbroso-Le Rouic, L.; Piperno-Neumann, S.; Dendale, R.; Sastre, X.; Desjardins, L.; et al. Genome-wide profiling is a clinically relevant and affordable prognostic test in posterior uveal melanoma. Br. J. Ophthalmol. 2014, 98, 769–774. [Google Scholar] [CrossRef] [PubMed]
- Khoja, L.; Atenafu, E.G.; Suciu, S.; Leyvraz, S.; Sato, T.; Marshall, E.; Keilholz, U.; Zimmer, L.; Patel, S.P.; Piperno-Neumann, S.; et al. Meta-Analysis in Metastatic Uveal Melanoma to Determine Progression-Free and Overall Survival Benchmarks: An International Rare Cancers Initiative (IRCI) Ocular Melanoma study. Ann. Oncol. 2019, 31, 1370–1380. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.D.; Turell, M.E.; Topham, A.K. Uveal melanoma: Trends in incidence, treatment, and survival. Ophthalmology 2011, 118, 1881–1885. [Google Scholar] [CrossRef]
- Arvelo, F.; Poupon, M.F.; Goguel, A.F.; Lizard, G.; Bourgeois, Y.; Arriagada, R.; Le Chevalier, T. Response of a multidrug-resistant human small-cell lung cancer xenograft to chemotherapy. J. Cancer Res. Clin. Oncol. 1993, 120, 17–23. [Google Scholar] [CrossRef]
- Malaney, P.; Nicosia, S.V.; Davé, V. One mouse, one patient paradigm: New avatars of personalized cancer therapy. Cancer Lett. 2014, 344, 1–12. [Google Scholar] [CrossRef]
- Xu, C.; Li, X.; Liu, P.; Li, M.; Luo, F. Patient-derived xenograft mouse models: A high fidelity tool for individualized medicine. Oncol. Lett. 2019, 17, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Koga, Y.; Ochiai, A. Systematic Review of Patient-Derived Xenograft Models for Preclinical Studies of Anti-Cancer Drugs in Solid Tumors. Cells 2019, 8, 418. [Google Scholar] [CrossRef]
- Tentler, J.J.; Tan, A.C.; Weekes, C.D.; Jimeno, A.; Leong, S.; Pitts, T.M.; Arcaroli, J.J.; Messersmith, W.A.; Eckhardt, S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat. Rev. Clin. Oncol. 2012, 9, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Corso, S.; Isella, C.; Bellomo, S.E.; Apicella, M.; Durando, S.; Migliore, C.; Ughetto, S.; D’Errico, L.; Menegon, S.; Moya-Rull, D.; et al. A Comprehensive PDX Gastric Cancer Collection Captures Cancer Cell-Intrinsic Transcriptional MSI Traits. Cancer Res. 2019, 79, 5884–5896. [Google Scholar] [CrossRef]
- Izumchenko, E.; Paz, K.; Ciznadija, D.; Sloma, I.; Katz, A.; Vasquez-Dunddel, D.; Ben-Zvi, I.; Stebbing, J.; McGuire, W.; Harris, W.; et al. Patient-derived xenografts effectively capture responses to oncology therapy in a heterogeneous cohort of patients with solid tumors. Ann. Oncol. 2017, 28, 2595–2605. [Google Scholar] [CrossRef] [PubMed]
- Garralda, E.; Paz, K.; López-Casas, P.P.; Jones, S.; Katz, A.; Kann, L.M.; López-Rios, F.; Sarno, F.; Al-Shahrour, F.; Vasquez, D.; et al. Integrated next-generation sequencing and avatar mouse models for personalized cancer treatment. Clin. Cancer Res. 2014, 20, 2476–2484. [Google Scholar] [CrossRef] [PubMed]
- Hidalgo, M.; Amant, F.; Biankin, A.V.; Budinská, E.; Byrne, A.T.; Caldas, C.; Clarke, R.B.; de Jong, S.; Jonkers, J.; Mælandsmo, G.M.; et al. Patient-derived xenograft models: An emerging platform for translational cancer research. Cancer Discov. 2014, 4, 998–1013. [Google Scholar] [CrossRef]
- Bertotti, A.; Migliardi, G.; Galimi, F.; Sassi, F.; Torti, D.; Isella, C.; Corà, D.; Di Nicolantonio, F.; Buscarino, M.; Petti, C.; et al. A molecularly annotated platform of patient-derived xenografts (“xenopatients”) identifies HER2 as an effective therapeutic target in cetuximab-resistant colorectal cancer. Cancer Discov. 2011, 1, 508–523. [Google Scholar] [CrossRef]
- Inoue, A.; Deem, A.K.; Kopetz, S.; Heffernan, T.P.; Draetta, G.F.; Carugo, A. Current and Future Horizons of Patient-Derived Xenograft Models in Colorectal Cancer Translational Research. Cancers 2019, 11, 1321. [Google Scholar] [CrossRef]
- Calles, A.; Rubio-Viqueira, B.; Hidalgo, M. Primary human non-small cell lung and pancreatic tumorgraft models--utility and applications in drug discovery and tumor biology. Curr. Protoc. Pharmacol. 2013, 61, 14–26. [Google Scholar] [CrossRef]
- Russo, M.V.; Faversani, A.; Gatti, S.; Ricca, D.; Del Gobbo, A.; Ferrero, S.; Palleschi, A.; Vaira, V.; Bosari, S. A new mouse avatar model of non-small cell lung cancer. Front. Oncol. 2015, 5, 52. [Google Scholar] [CrossRef]
- Bihani, T.; Patel, H.K.; Arlt, H.; Tao, N.; Jiang, H.; Brown, J.L.; Purandare, D.M.; Hattersley, G.; Garner, F. Elacestrant (RAD1901), a Selective Estrogen Receptor Degrader (SERD), Has Antitumor Activity in Multiple ER(+) Breast Cancer Patient-derived Xenograft Models. Clin. Cancer Res. 2017, 23, 4793–4804. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Rath, O.; Schueler, J.; Sung, H.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Lee, H.M.; Choi, H.Y.; et al. Development of Novel Patient-Derived Preclinical Models from Malignant Effusions in Patients with Tyrosine Kinase Inhibitor-Resistant Clear Cell Renal Cell Carcinoma. Transl. Oncol. 2017, 10, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.; Mobuchon, L.; Houy, A.; Alsafadi, S.; Baulande, S.; Mariani, O.; Marande, B.; Ait Rais, K.; Van der Kooij, M.K.; Kapiteijn, E.; et al. Evolutionary Routes in Metastatic Uveal Melanomas Depend on MBD4 Alterations. Clin. Cancer Res. 2019, 25, 5513–5524. [Google Scholar] [CrossRef] [PubMed]
- Le Tourneau, C.; Delord, J.P.; Goncalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; Campone, M.; Tredan, O.; Massiani, M.A.; Mauborgne, C.; et al. Molecularly targeted therapy based on tumour molecular profiling versus conventional therapy for advanced cancer (SHIVA): A multicentre, open-label, proof-of-concept, randomised, controlled phase 2 trial. Lancet Oncol. 2015, 16, 1324–1334. [Google Scholar] [CrossRef] [PubMed]
- Belin, L.; Kamal, M.; Mauborgne, C.; Plancher, C.; Mulot, F.; Delord, J.P.; Gonçalves, A.; Gavoille, C.; Dubot, C.; Isambert, N.; et al. Randomized phase II trial comparing molecularly targeted therapy based on tumor molecular profiling versus conventional therapy in patients with refractory cancer: Cross-over analysis from the SHIVA trial. Ann. Oncol. 2017, 28, 590–596. [Google Scholar] [CrossRef] [PubMed]
- Damato, B.; Dopierala, J.A.; Coupland, S.E. Genotypic profiling of 452 choroidal melanomas with multiplex ligation-dependent probe amplification. Clin. Cancer Res. 2010, 16, 6083–6092. [Google Scholar] [CrossRef] [PubMed]
- Amirouchene-Angelozzi, N.; Frisch-Dit-Leitz, E.; Carita, G.; Dahmani, A.; Raymondie, C.; Liot, G.; Gentien, D.; Nemati, F.; Decaudin, D.; Roman-Roman, S.; et al. The mTOR inhibitor Everolimus synergizes with the PI3K inhibitor GDC0941 to enhance anti-tumor efficacy in uveal melanoma. Oncotarget 2016, 7, 23633–23646. [Google Scholar] [CrossRef][Green Version]
- Némati, F.; Sastre-Garau, X.; Laurent, C.; Couturier, J.; Mariani, P.; Desjardins, L.; Piperno-Neumann, S.; Lantz, O.; Asselain, B.; Plancher, C.; et al. Establishment and characterization of a panel of human uveal melanoma xenografts derived from primary and/or metastatic tumors. Clin. Cancer Res. 2010, 16, 2352–2362. [Google Scholar] [CrossRef]
- Wang, J.; Xing, B.; Liu, W.; Li, J.; Wang, X.; Li, J.; Yang, J.; Ji, C.; Li, Z.; Dong, B.; et al. Molecularly annotation of mouse avatar models derived from patients with colorectal cancer liver metastasis. Theranostics 2019, 9, 3485–3500. [Google Scholar] [CrossRef]
- Marangoni, E.; Vincent-Salomon, A.; Auger, N.; Degeorges, A.; Assayag, F.; de Cremoux, P.; de Plater, L.; Guyader, C.; De Pinieux, G.; Judde, J.G.; et al. A new model of patient tumor-derived breast cancer xenografts for preclinical assays. Clin. Cancer Res. 2007, 13, 3989–3998. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lin, W.; Huang, Y.; Chen, X.; Wang, H.; Teng, L. The Essential Factors of Establishing Patient-derived Tumor Model. J. Cancer 2021, 12, 28–37. [Google Scholar] [CrossRef] [PubMed]
- Laurent, C.; Gentien, D.; Piperno-Neumann, S.; Nemati, F.; Nicolas, A.; Tesson, B.; Desjardins, L.; Mariani, P.; Rapinat, A.; Sastre-Garau, X.; et al. Patient-derived xenografts recapitulate molecular features of human uveal melanomas. Mol. Oncol. 2013, 7, 625–636. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, G.V.; Powell, E.; Seth, S.; Ge, Z.; Carugo, A.; Bristow, C.; Peoples, M.; Robinson, F.; Qiu, H.; Shao, J.; et al. High-resolution clonal mapping of multi-organ metastasis in triple negative breast cancer. Nat. Commun. 2018, 9, 5079. [Google Scholar] [CrossRef] [PubMed]
- Eirew, P.; Steif, A.; Khattra, J.; Ha, G.; Yap, D.; Farahani, H.; Gelmon, K.; Chia, S.; Mar, C.; Wan, A.; et al. Dynamics of genomic clones in breast cancer patient xenografts at single-cell resolution. Nature 2015, 518, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Dobrolecki, L.E.; Airhart, S.D.; Alferez, D.G.; Aparicio, S.; Behbod, F.; Bentires-Alj, M.; Brisken, C.; Bult, C.J.; Cai, S.; Clarke, R.B.; et al. Patient-derived xenograft (PDX) models in basic and translational breast cancer research. Cancer Metastasis Rev. 2016, 35, 547–573. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Ellis, M.J.; Li, S.; Larson, D.E.; Chen, K.; Wallis, J.W.; Harris, C.C.; McLellan, M.D.; Fulton, R.S.; Fulton, L.L.; et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature 2010, 464, 999–1005. [Google Scholar] [CrossRef]
- Decaudin, D.; Frisch Dit Leitz, E.; Nemati, F.; Tarin, M.; Naguez, A.; Zerara, M.; Marande, B.; Vivet-Noguer, R.; Halilovic, E.; Fabre, C.; et al. Preclinical evaluation of drug combinations identifies co-inhibition of Bcl-2/XL/W and MDM2 as a potential therapy in uveal melanoma. Eur. J. Cancer 2020, 126, 93–103. [Google Scholar] [CrossRef]
- Ben-David, U.; Ha, G.; Tseng, Y.Y.; Greenwald, N.F.; Oh, C.; Shih, J.; McFarland, J.M.; Wong, B.; Boehm, J.S.; Beroukhim, R.; et al. Patient-derived xenografts undergo mouse-specific tumor evolution. Nat. Genet. 2017, 49, 1567–1575. [Google Scholar] [CrossRef]
- Shain, A.H.; Bagger, M.M.; Yu, R.; Chang, D.; Liu, S.; Vemula, S.; Weier, J.F.; Wadt, K.; Heegaard, S.; Bastian, B.C.; et al. The genetic evolution of metastatic uveal melanoma. Nat. Genet. 2019, 51, 1123–1130. [Google Scholar] [CrossRef]
- Nathan, P.; Hassel, J.C.; Rutkowski, P.; Baurain, J.F.; Butler, M.O.; Schlaak, M.; Sullivan, R.J.; Ochsenreither, S.; Dummer, R.; Kirkwood, J.M.; et al. Overall Survival Benefit with Tebentafusp in Metastatic Uveal Melanoma. New Engl. J. Med. 2021, 385, 1196–1206. [Google Scholar] [CrossRef]
- Chen, A.; Neuwirth, I.; Herndler-Brandstetter, D. Modeling the Tumor Microenvironment and Cancer Immunotherapy in Next-Generation Humanized Mice. Cancers 2023, 15, 2989. [Google Scholar] [CrossRef]

| Characteristics | Nb of Patients |
|---|---|
| Patients | 29 * |
| Sex | |
| Male | 12 |
| Female | 17 |
| Age (year range) | |
| Male | 31–81 |
| Female | 31–83 |
| Tumors | |
| Primary | 20 |
| Metastasis | 9 |
| Grafted Patient Samples | Tumor Take Rate (TTR) | Origin | Tumor Take (TTR) | Genomic Classification | Tumor Take Rate (TTR) |
|---|---|---|---|---|---|
| 29 | 13/29 45% | Primary tumor (20 grafted tumors) | 7/20 (35%) | High Risk | 3/6 (50%) |
| Intermediate | 3/10 (30%) | ||||
| Low Risk | ¼ * (25%) | ||||
| Liver metastasis (9 grafted tumors) | 6/9 (67%) | High Risk | 3/3 * (100%) | ||
| Intermediate | 3/5 (60%) | ||||
| Low Risk | 0/1 (0%) |
| PDX | PDX Passage 0 $ | Chr 3 | Chr 8q | Risk of Metastases * | Time to Reach Volume > 60 mm3 (Months) £ | |
|---|---|---|---|---|---|---|
| PDX from Patients’ metastasis | MM252 | 24/06/13 | N | G | IR | 3 |
| MM257 | 23/09/13 | N | G | IR | 10 | |
| MM270 | 17/02/14 | L | G | HR | 7 | |
| MM267 | 03/02/14 | N | G | IR | 13 | |
| MM278 | 10/04/14 | L | G | HR | 4 | |
| MM287 | 25/08/14 | L | G | HR | 12 @ | |
| PDX from Patients’primary tumor | MP255 | 03/09/13 | L | N | IR | 7 |
| MP258 | 25/09/13 | L | N | IR | 6 | |
| MP271 | 19/02/14 | L | G | HR | 6 | |
| MP254 | 30/08/13 | L | G | HR | 9 | |
| MP262 | 17/12/13 | L | N | IR | 8 | |
| MP264 | 27/01/14 | L | N | IR | 11 | |
| MP266 | 29/01/14 | N | N | LR | 14 @ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemati, F.; de Koning, L.; Gentien, D.; Assayag, F.; Henry, E.; Ait Rais, K.; Pierron, G.; Mariani, O.; Nijnikoff, M.; Champenois, G.; et al. Patient Derived Xenografts (PDX) Models as an Avatar to Assess Personalized Therapy Options in Uveal Melanoma: A Feasibility Study. Curr. Oncol. 2023, 30, 9090-9103. https://doi.org/10.3390/curroncol30100657
Nemati F, de Koning L, Gentien D, Assayag F, Henry E, Ait Rais K, Pierron G, Mariani O, Nijnikoff M, Champenois G, et al. Patient Derived Xenografts (PDX) Models as an Avatar to Assess Personalized Therapy Options in Uveal Melanoma: A Feasibility Study. Current Oncology. 2023; 30(10):9090-9103. https://doi.org/10.3390/curroncol30100657
Chicago/Turabian StyleNemati, Fariba, Leanne de Koning, David Gentien, Franck Assayag, Emilie Henry, Khadija Ait Rais, Gaelle Pierron, Odette Mariani, Michèle Nijnikoff, Gabriel Champenois, and et al. 2023. "Patient Derived Xenografts (PDX) Models as an Avatar to Assess Personalized Therapy Options in Uveal Melanoma: A Feasibility Study" Current Oncology 30, no. 10: 9090-9103. https://doi.org/10.3390/curroncol30100657
APA StyleNemati, F., de Koning, L., Gentien, D., Assayag, F., Henry, E., Ait Rais, K., Pierron, G., Mariani, O., Nijnikoff, M., Champenois, G., Nicolas, A., Meseure, D., Gardrat, S., Servant, N., Hupé, P., Kamal, M., Le Tourneau, C., Piperno-Neumann, S., Rodrigues, M., ... Cassoux, N. (2023). Patient Derived Xenografts (PDX) Models as an Avatar to Assess Personalized Therapy Options in Uveal Melanoma: A Feasibility Study. Current Oncology, 30(10), 9090-9103. https://doi.org/10.3390/curroncol30100657










