Mapping the Scientific Landscape of Bacterial Influence on Oral Cancer: A Bibliometric Analysis of the Last Decade’s Medical Progress
Abstract
:1. Introduction
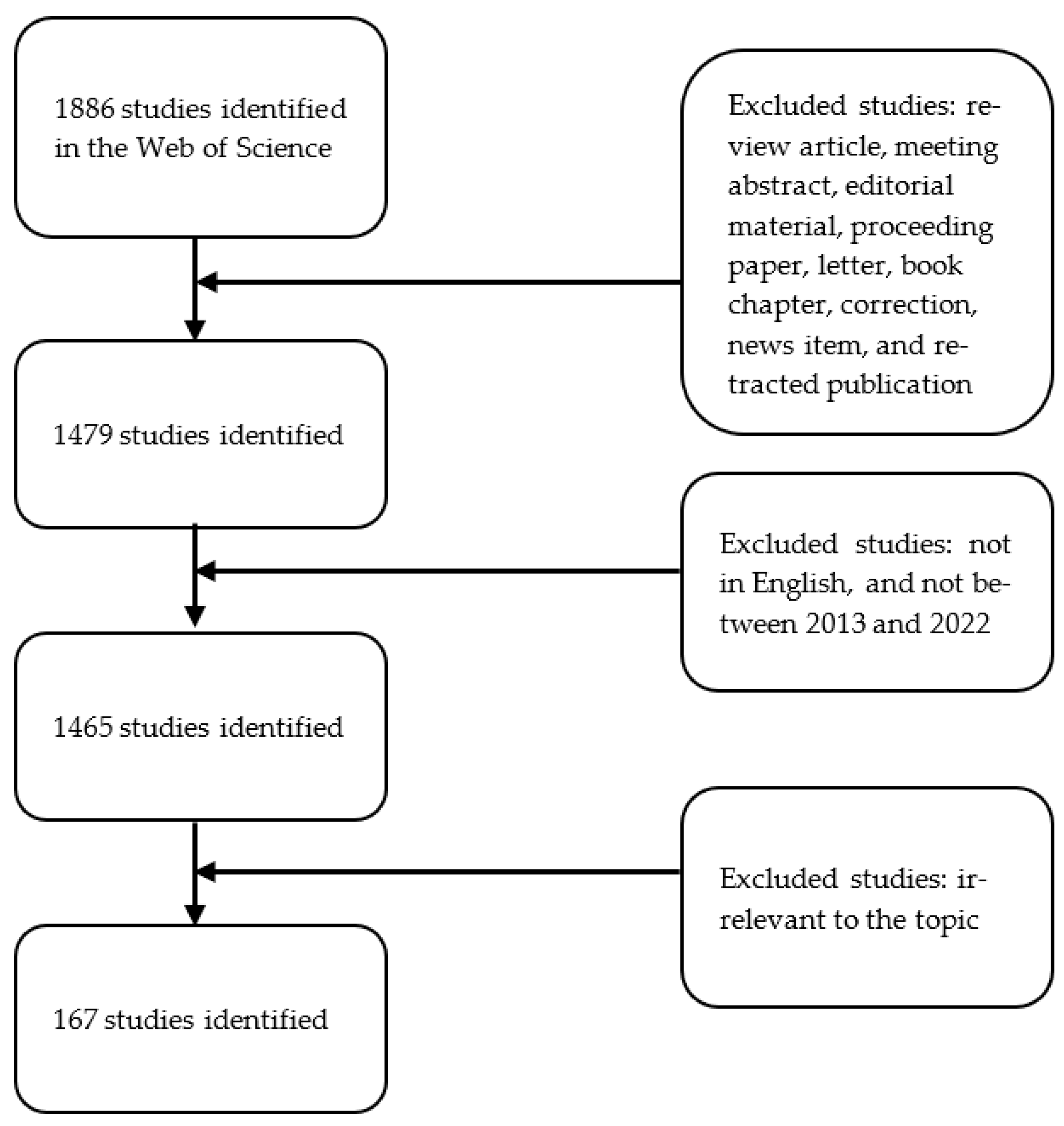
2. Materials and Methods
2.1. Data Sources and Search Strategies
2.2. Data Collection and Processing
2.3. Bibliometric Analysis
3. Results
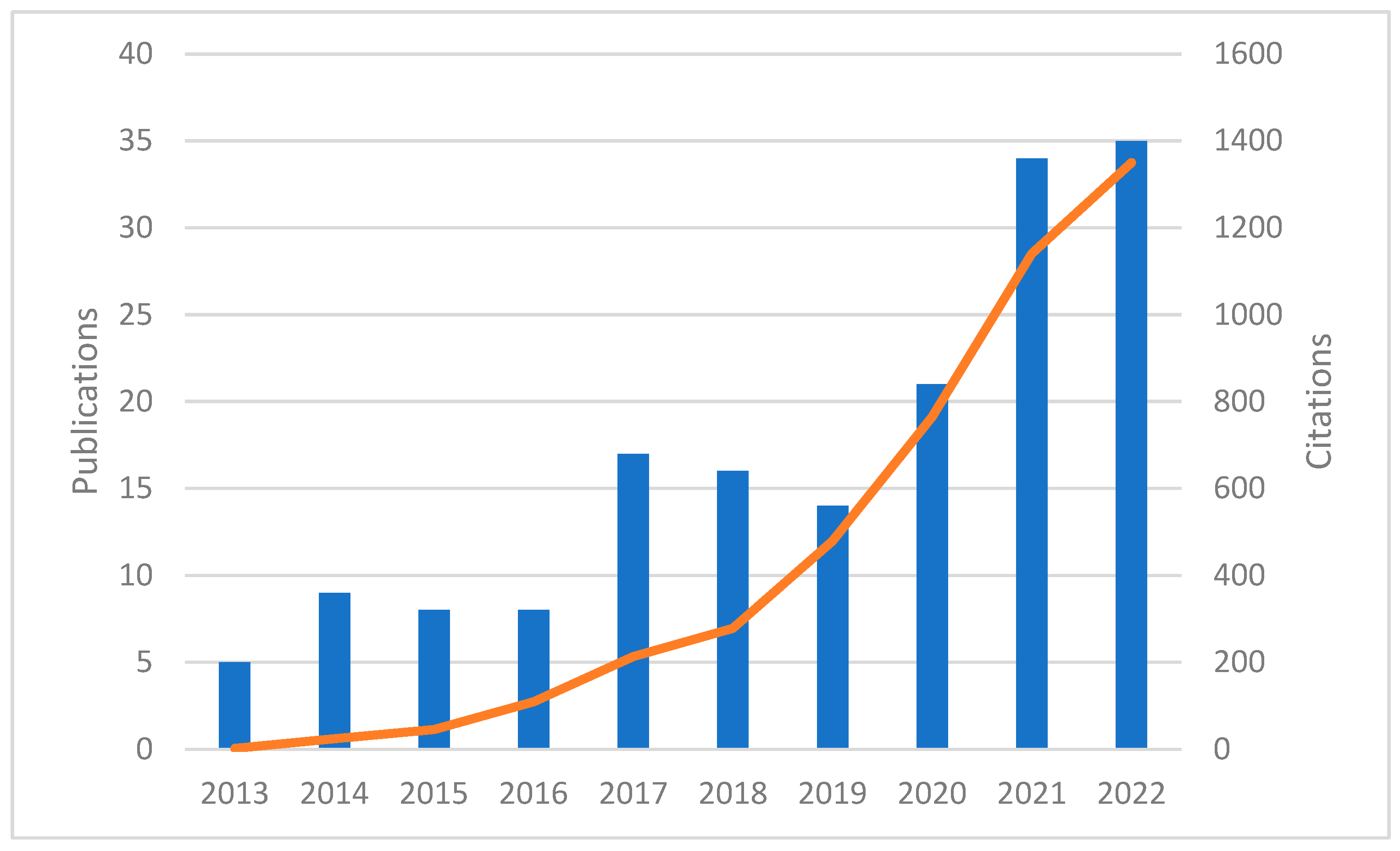
3.1. Publications and Their Citations
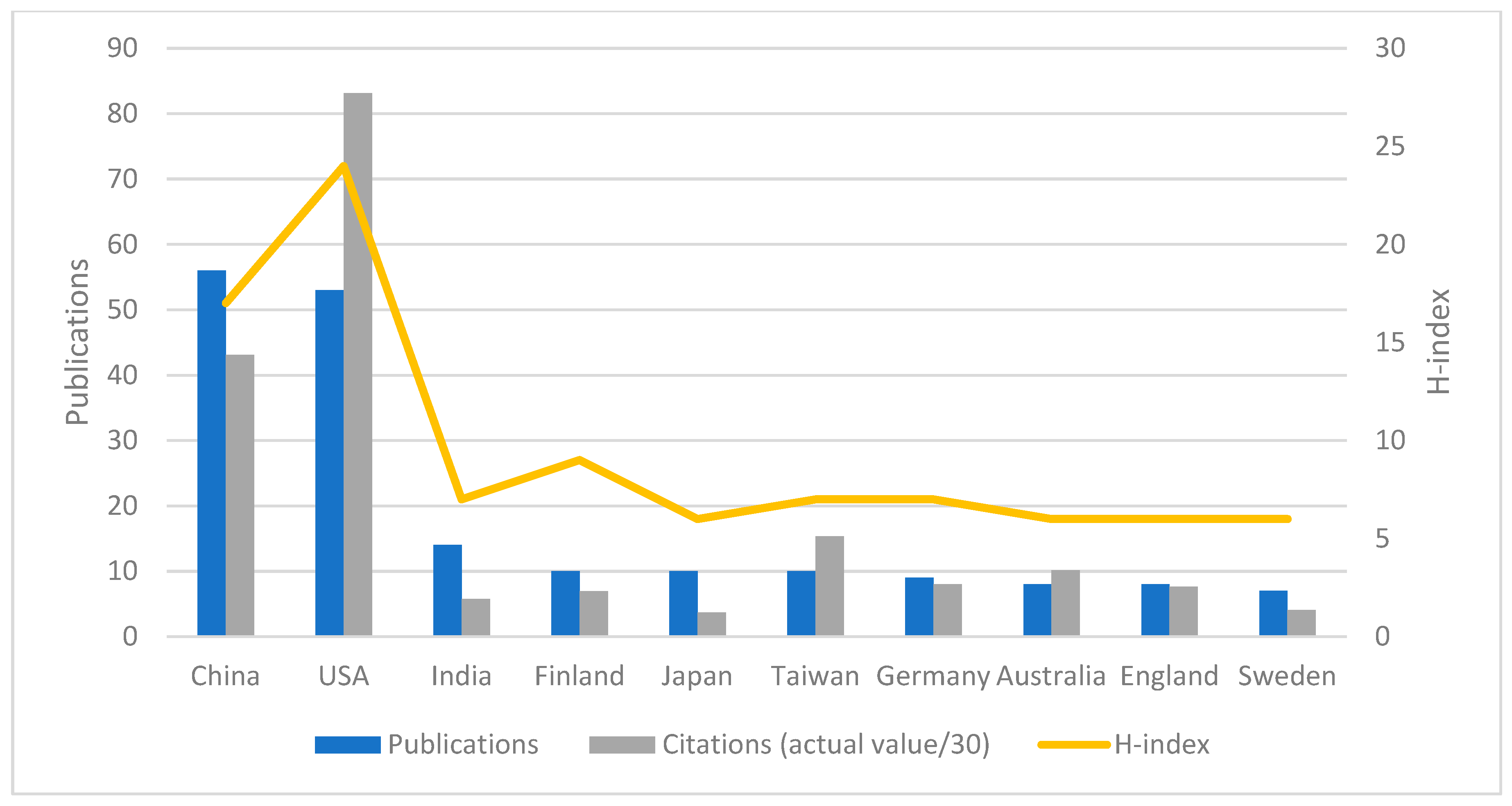
3.2. Authors, Affiliations, and Countries/Regions
3.3. Journals
3.4. Top 10 Most Cited Articles
3.5. Keyword Analysis of Research Themes in Oral Cancer and Bacteria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Zanoni, D.K.; Montero, P.H.; Migliacci, J.C.; Shah, J.P.; Wong, R.J.; Ganly, I.; Patel, S.G. Survival Outcomes after Treatment of Cancer of the Oral Cavity (1985–2015). Oral Oncol. 2019, 90, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Finney Rutten, L.J.; Blake, K.D.; Skolnick, V.G.; Davis, T.; Moser, R.P.; Hesse, B.W. Data Resource Profile: The National Cancer Institute’s Health Information National Trends Survey (HINTS). Int. J. Epidemiol. 2020, 49, 17-17j. [Google Scholar] [CrossRef] [PubMed]
- Irani, S. Distant Metastasis from Oral Cancer: A Review and Molecular Biologic Aspects. J. Int. Soc. Prev. Community Dent. 2016, 6, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Oral Cancer 5-Year Survival Rates by Race, Gender, and Stage of Diagnosis|National Institute of Dental and Craniofacial Research. Available online: https://www.nidcr.nih.gov/research/data-statistics/oral-cancer/survival-rates (accessed on 30 May 2023).
- Abati, S.; Bramati, C.; Bondi, S.; Lissoni, A.; Trimarchi, M. Oral Cancer and Precancer: A Narrative Review on the Relevance of Early Diagnosis. Int. J. Environ. Res. Public Health 2020, 17, 9160. [Google Scholar] [CrossRef]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2021, 88, 116–126. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, S.; Ray, J.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota During Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Pandey, D.; Szczesniak, M.; Maclean, J.; Yim, H.; Zhang, F.; Graham, P.; El-Omar, E.; Wu, P. Dysbiosis in Head and Neck Cancer: Determining Optimal Sampling Site for Oral Microbiome Collection. Pathogens 2022, 11, 1550. [Google Scholar] [CrossRef]
- Zeng, B.; Tan, J.; Guo, G.; Li, Z.; Yang, L.; Lao, X.; Wang, D.; Ma, J.; Zhang, S.; Liao, G.; et al. The Oral Cancer Microbiome Contains Tumor Space-Specific and Clinicopathology-Specific Bacteria. Front. Cell. Infect. Microbiol. 2022, 12, 942328. [Google Scholar] [CrossRef]
- Frank, D.; Qiu, Y.; Cao, Y.; Zhang, S.; Lu, L.; Kofonow, J.; Robertson, C.; Liu, Y.; Wang, H.; Levens, C.; et al. A Dysbiotic Microbiome Promotes Head and Neck Squamous Cell Carcinoma. Oncogene 2022, 41, 1269–1280. [Google Scholar] [CrossRef]
- Geleijnse, G.; Chiang, R.C.-J.; Sieswerda, M.; Schuurman, M.; Lee, K.C.; van Soest, J.; Dekker, A.; Lee, W.-C.; Verbeek, X.A.A.M. Prognostic Factors Analysis for Oral Cavity Cancer Survival in the Netherlands and Taiwan Using a Privacy-Preserving Federated Infrastructure. Sci. Rep. 2020, 10, 20526. [Google Scholar] [CrossRef] [PubMed]
- Minarovits, J. Anaerobic Bacterial Communities Associated with Oral Carcinoma: Intratumoral, Surface-Biofilm and Salivary Microbiota. Anaerobe 2021, 68, 102300. [Google Scholar] [CrossRef] [PubMed]
- Teles, F.; Alawi, F.; Castilho, R.; Wang, Y. Association or Causation? Exploring the Oral Microbiome and Cancer Links. J. Dent. Res. 2020, 99, 1411–1424. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.; Queiroz, E.; Nightingale, K.; Kerr, A.; DeLacure, M.; Veeramachaneni, R.; et al. Changes in Abundance of Oral Microbiota Associated with Oral Cancer. PLoS ONE 2014, 9, e98741. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, S.; Nagao, Y.; Ma, J.; Asakawa, M.; Yoshida, R.; Takeshita, T.; Hirosue, A.; Yamashita, Y.; Nakayama, H. Compositional Shift of Oral Microbiota Following Surgical Resection of Tongue Cancer. Front. Cell. Infect. Microbiol. 2020, 10, 600884. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Wu, L.; Zhang, Y.; Shi, L.; Yang, X. Bibliometric Analysis of Research Trends and Characteristics of Oral Potentially Malignant Disorders. Clin. Oral Investig. 2020, 24, 447–454. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Software Survey: VOSviewer, a Computer Program for Bibliometric Mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef]
- Chen, X.; Xian, B.; Wei, J.; Chen, Y.; Yang, D.; Lai, X.; Liu, L.; Wu, Y.; Lin, X.; Deng, Y.; et al. Predictive Value of the Presence of Prevotella and the Ratio of Porphyromonas gingivalis to Prevotella in Saliva for Esophageal Squamous Cell Carcinoma. Front. Cell. Infect. Microbiol. 2022, 12, 1463. [Google Scholar] [CrossRef]
- Arthur, R.; Bezerra, R.; Ximenez, J.; Merlin, B.; Morraye, R.; Neto, J.; Fava, N.; Figueiredo, D.; de Biagi, C.; Montibeller, M.; et al. Microbiome and Oral Squamous Cell Carcinoma: A Possible Interplay on Iron Metabolism and Its Impact on Tumor Microenvironment. Braz. J. Microbiol. 2021, 52, 1287–1302. [Google Scholar] [CrossRef]
- Tsai, M.; Chen, Y.; Chen, W.; Chen, M. Streptococcus mutans Promotes Tumor Progression in Oral Squamous Cell Carcinoma. J. Cancer 2022, 13, 3358–3367. [Google Scholar] [CrossRef]
- Chadegani, A.A.; Salehi, H.; Yunus, M.M.; Farhadi, H.; Fooladi, M.; Farhadi, M.; Ebrahim, N.A. A Comparison between Two Main Academic Literature Collections: Web of Science and Scopus Databases. Asian Soc. Sci. 2013, 9, p18. [Google Scholar] [CrossRef]
- Hirsch, J.E. An Index to Quantify an Individual’s Scientific Research Output. Proc. Natl. Acad. Sci. USA 2005, 102, 16569–16572. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. Citation-Based Clustering of Publications Using CitNetExplorer and VOSviewer. Scientometrics 2017, 111, 1053–1070. [Google Scholar] [CrossRef] [PubMed]
- Baraniya, D.; Chitrala, K.; Al-Hebshi, N. Global Transcriptional Response of Oral Squamous Cell Carcinoma Cell Lines to Health-Associated Oral Bacteria-an in Vitro Study. J. Oral Microbiol. 2022, 14, 2073866. [Google Scholar] [CrossRef]
- Baraniya, D.; Jain, V.; Lucarelli, R.; Tam, V.; Vanderveer, L.; Puri, S.; Yang, M.; Al-hebshi, N. Screening of Health-Associated Oral Bacteria for Anticancer Properties in Vitro. Front. Cell. Infect. Microbiol. 2020, 10, 575656. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.; Perera, I.; Ipe, D.; Ulett, G.; Speicher, D.; Chen, T.; Johnson, N. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732. [Google Scholar] [CrossRef]
- Al-Hebshi, N.; Nasher, A.; Maryoud, M.; Homeida, H.; Chen, T.; Idris, A.; Johnson, N. Inflammatory Bacteriome Featuring Fusobacterium nucleatum and Pseudomonas aeruginosa Identified in Association with Oral Squamous Cell Carcinoma. Sci. Rep. 2017, 7, 1834. [Google Scholar] [CrossRef]
- Al-Hebshi, N.; Alharbi, F.; Mahri, M.; Chen, T. Differences in the Bacteriome of Smokeless Tobacco Products with Different Oral Carcinogenicity: Compositional and Predicted Functional Analysis. Genes 2017, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Al-Hebshi, N.; Nasher, A.; Idris, A.; Chen, T. Robust Species Taxonomy Assignment Algorithm for 16S RRNA NGS Reads: Application to Oral Carcinoma Samples. J. Oral Microbiol. 2015, 7, 28934. [Google Scholar] [CrossRef] [PubMed]
- Stashenko, P.; Yost, S.; Choi, Y.; Danciu, T.; Chen, T.; Yoganathan, S.; Kressirer, C.; Ruiz-Tourrella, M.; Das, B.; Kokaras, A.; et al. The Oral Mouse Microbiome Promotes Tumorigenesis in Oral Squamous Cell Carcinoma. MSYSTEMS 2019, 4, 10–1128. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Zhang, Z.; Li, Y.; Li, Q.; Geng, F.; Liu, J.; Pan, Y. Analysis of Differentially Expressed Genes in Oral Epithelial Cells Infected with Fusobacterium Nucleatum for Revealing Genes Associated with Oral Cancer. J. Cell. Mol. Med. 2021, 25, 892–904. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhao, X.; Xu, Z.; Dai, W.; Duan, W.; Huang, S.; Zhang, E.; Liu, J.; Zhang, S.; et al. Composition and Function of Oral Microbiota between Gingival Squamous Cell Carcinoma and Periodontitis. Oral Oncol. 2020, 107, 104710. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium Nucleatum Promotes Epithelial-Mesenchymal Transiton through Regulation of the lncRNA MIR4435-2HG/miR-296-5p/Akt2/SNAI1 Signaling Pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Geng, F.; Zhang, Y.; Lu, Z.; Zhang, S.; Pan, Y. Fusobacterium Nucleatum Caused DNA Damage and Promoted Cell Proliferation by the Ku70/P53 Pathway in Oral Cancer Cells. DNA Cell Biol. 2020, 39, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Geng, F.; Shi, X.; Li, Y.; Zhang, X.; Zhao, X.; Pan, Y. The Prevalence Rate of Periodontal Pathogens and Its Association with Oral Squamous Cell Carcinoma. Appl. Microbiol. Biotechnol. 2019, 103, 1393–1404. [Google Scholar] [CrossRef] [PubMed]
- Kylmae, A.; Sorsa, T.; Jouhi, L.; Mustonen, H.; Mohamed, H.; Randen-Brady, R.; Maekitie, A.; Atula, T.; Hagstrom, J.; Haglund, C. Prognostic Role of Porphyromonas gingivalis Gingipain Rgp and Matrix Metalloproteinase 9 in Oropharyngeal Squamous Cell Carcinoma. Anticancer Res. 2022, 42, 5415–5430. [Google Scholar] [CrossRef]
- Listyarifah, D.; Nieminen, M.; Makinen, L.; Haglund, C.; Grenier, D.; Hayry, V.; Nordstrom, D.; Hernandez, M.; Yucel-Lindberg, T.; Tervahartiala, T.; et al. Treponema denticola Chymotrypsin-like Proteinase Is Present in Early-Stage Mobile Tongue Squamous Cell Carcinoma and Related to the Clinicopathological Features. J. Oral Pathol. Med. 2018, 47, 764–772. [Google Scholar] [CrossRef]
- Kylma, A.; Jouhi, L.; Listyarifah, D.; Mohamed, H.; Makitie, A.; Remes, S.; Haglund, C.; Atula, T.; Nieminen, M.; Sorsa, T.; et al. Treponema denticola Chymotrypsin-like Protease as Associated with HPV-Negative Oropharyngeal Squamous Cell Carcinoma. Br. J. Cancer 2018, 119, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Nieminen, M.; Listyarifah, D.; Hagstrom, J.; Haglund, C.; Grenier, D.; Nordstrom, D.; Uitto, V.; Hernandez, M.; Yucel-Lindberg, T.; Tervahartiala, T.; et al. Treponema denticola Chymotrypsin-like Proteinase May Contribute to Orodigestive Carcinogenesis through Immunomodulation. Br. J. Cancer 2018, 118, 428–434. [Google Scholar] [CrossRef]
- Kauppila, J.; Korvala, J.; Siirila, K.; Manni, M.; Makinen, L.; Hagstrom, J.; Atula, T.; Haglund, C.; Selander, K.; Saarnio, J.; et al. Toll-like Receptor 9 Mediates Invasion and Predicts Prognosis in Squamous Cell Carcinoma of the Mobile Tongue. J. Oral Pathol. Med. 2015, 44, 571–577. [Google Scholar] [CrossRef]
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 Protein of Fusobacterium nucleatum to Human Inhibitory Receptor TIGIT Protects Tumors from Immune Cell Attack. Immunity 2015, 42, 344–355. [Google Scholar] [CrossRef]
- Gallimidi, A.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.; Nussbaum, G.; Elkin, M. Periodontal Pathogens Porphyromonas gingivalis and Fusobacterium nucleatum Promote Tumor Progression in an Oral-Specific Chemical Carcinogenesis Model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Guerrero-Preston, R.; Godoy-Vitorino, F.; Jedlicka, A.; Rodriguez-Hilario, A.; Gonzalez, H.; Bondy, J.; Lawson, F.; Folawiyo, O.; Michailidi, C.; Dziedzic, A.; et al. 16S rRNA Amplicon Sequencing Identifies Microbiota Associated with Oral Cancer, Human Papilloma Virus Infection and Surgical Treatment. Oncotarget 2016, 7, 51320–51334. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in Oral Microbiota Associated with Oral Cancer. Sci. Rep. 2017, 7, 11773. [Google Scholar] [CrossRef]
- Yang, C.; Yeh, Y.; Yu, H.; Chin, C.; Hsu, C.; Liu, H.; Huang, P.; Hu, S.; Liao, C.; Chang, K.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862. [Google Scholar] [CrossRef]
- Hayes, R.; Ahn, J.; Fan, X.; Peters, B.; Ma, Y.; Yang, L.; Agalliu, I.; Burk, R.; Ganly, I.; Purdue, M.; et al. Association of Oral Microbiome with Risk for Incident Head and Neck Squamous Cell Cancer. JAMA Oncol. 2018, 4, 358–365. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, Y.; Zheng, H.; Zhang, C. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell. Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Gupta, R.; Acharya, A.K.; Patthi, B.; Goud, V.; Reddy, S.; Garg, A.; Singla, A. Changing Trends in Oral Cancer—A Global Scenario. Nepal J. Epidemiol. 2016, 6, 613–619. [Google Scholar] [CrossRef]
- Srivastava, A.; Mishra, S.; Garg, P.; Dubey, A.; Deo, S.; Verma, D. Comparative and Analytical Characterization of the Oral Bacteriome of Smokeless Tobacco Users with Oral Squamous Cell Carcinoma. Appl. Microbiol. Biotechnol. 2022, 106, 4115–4128. [Google Scholar] [CrossRef]
- Gopinath, D.; Menon, R.; Wie, C.; Banerjee, M.; Panda, S.; Mandal, D.; Behera, P.; Roychoudhury, S.; Kheur, S.; Botelho, M.; et al. Salivary Bacterial Shifts in Oral Leukoplakia Resemble the Dysbiotic Oral Cancer Bacteriome. J. Oral Microbiol. 2021, 13, 1857998. [Google Scholar] [CrossRef]
- Ha, N.; Woo, B.; Kim, D.; Ha, E.; Choi, J.; Kim, S.; Park, B.; Lee, J.; Park, H. Prolonged and Repetitive Exposure to Porphyromonas gingivalis Increases Aggressiveness of Oral Cancer Cells by Promoting Acquisition of Cancer Stem Cell Properties. Tumor Biol. 2015, 36, 9947–9960. [Google Scholar] [CrossRef]
- Nomoto, D.; Baba, Y.; Liu, Y.; Tsutsuki, H.; Okadome, K.; Harada, K.; Ishimoto, T.; Iwatsuki, M.; Iwagami, S.; Miyamoto, Y.; et al. Fusobacterium nucleatum Promotes Esophageal Squamous Cell Carcinoma Progression via the NOD1/RIPK2/NF-Kappa B Pathway. Cancer Lett. 2022, 530, 59–67. [Google Scholar] [CrossRef]
- McIlvanna, E.; Linden, G.J.; Craig, S.G.; Lundy, F.T.; James, J.A. Fusobacterium nucleatum and Oral Cancer: A Critical Review. BMC Cancer 2021, 21, 1212. [Google Scholar] [CrossRef]
- Abed, J.; Maalouf, N.; Manson, A.; Earl, A.; Parhi, L.; Emgard, J.; Klutstein, M.; Tayeb, S.; Almogy, G.; Atlan, K.; et al. Colon Cancer-Associated Fusobacterium nucleatum May Originate From the Oral Cavity and Reach Colon Tumors via the Circulatory System. Front. Cell. Infect. Microbiol. 2020, 10, 400. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.; Sarkar, S.; Umar, S.; Lee, S.; Thomas, S. The Contribution of the Human Oral Microbiome to Oral Disease: A Review. Microorganisms 2023, 11, 318. [Google Scholar] [CrossRef]
- Lamont, R.; Fitzsimonds, Z.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in Oral and Orodigestive Squamous Cell Carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Michaud, D.S.; Kelsey, K.T.; Papathanasiou, E.; Genco, C.A.; Giovannucci, E. Periodontal Disease and Risk of All Cancers among Male Never Smokers: An Updated Analysis of the Health Professionals Follow-up Study. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2016, 27, 941–947. [Google Scholar] [CrossRef]
- Michaud, D.S.; Lu, J.; Peacock-Villada, A.Y.; Barber, J.R.; Joshu, C.E.; Prizment, A.E.; Beck, J.D.; Offenbacher, S.; Platz, E.A. Periodontal Disease Assessed Using Clinical Dental Measurements and Cancer Risk in the ARIC Study. J. Natl. Cancer Inst. 2018, 110, 843–854. [Google Scholar] [CrossRef]
- Hoare, A.; Soto, C.; Rojas-Celis, V.; Bravo, D. Chronic Inflammation as a Link between Periodontitis and Carcinogenesis. Mediators Inflamm. 2019, 2019, e1029857. [Google Scholar] [CrossRef] [PubMed]
- Tezal, M.; Sullivan, M.A.; Hyland, A.; Marshall, J.R.; Stoler, D.; Reid, M.E.; Loree, T.R.; Rigual, N.R.; Merzianu, M.; Hauck, L.; et al. Chronic Periodontitis and the Incidence of Head and Neck Squamous Cell Carcinoma. Cancer Epidemiol. Biomark. Prev. Publ. Am. Assoc. Cancer Res. Cosponsored Am. Soc. Prev. Oncol. 2009, 18, 2406–2412. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, Y.; Zhang, L. Role of the Microbiome in Oral Cancer Occurrence, Progression and Therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef] [PubMed]
- Pignatelli, P.; Romei, F.; Bondi, D.; Giuliani, M.; Piattelli, A.; Curia, M. Microbiota and Oral Cancer as A Complex and Dynamic Microenvironment: A Narrative Review from Etiology to Prognosis. Int. J. Mol. Sci. 2022, 23, 8323. [Google Scholar] [CrossRef] [PubMed]



| Authors | Publications | Affiliation | Citations | H-Index |
|---|---|---|---|---|
| Al-Hebshi NN [25,26,27,28,29,30] | 6 | Temple Univ, USA; Jazan Univ, Saudi Arabia | 342 | 5 |
| Chen T [27,28,29,30,31] | 5 | Harvard Univ, USA; Forsyth Institute, USA | 368 | 5 |
| Pan YP [32,33,34,35,36] | 5 | China Medical Univ, China | 177 | 5 |
| Haglund C [37,38,39,40,41] | 5 | University of Helsinki, Finland | 98 | 4 |
| Hagstrom J [37,38,39,40,41] | 5 | University of Helsinki, Finland | 98 | 4 |
| Affiliations | Publications | Citations | H-Index |
|---|---|---|---|
| Harvard University, USA | 12 | 1207 | 11 |
| University of Helsinki, Finland | 10 | 212 | 9 |
| Sichuan University, China | 10 | 91 | 5 |
| Pennsylvania Commonwealth System of Higher Education PCSHE, USA | 7 | 299 | 6 |
| State University System of Florida, USA | 7 | 218 | 7 |
| Forsyth Institute, USA | 6 | 368 | 5 |
| China Medical University, China | 6 | 193 | 6 |
| Helsinki University Central Hospital, Finland | 6 | 109 | 5 |
| Karolinska Institutet, Finland | 6 | 113 | 5 |
| Journal | IF | Publisher | Publications | Average Cites | Scope |
|---|---|---|---|---|---|
| Frontiers in Cellular and Infection Microbiology | 6.073 | Frontiers Media SA | 10 | 18.1 | Microbiology |
| Scientific Reports | 4.997 | Nature Portfolio | 9 | 58.0 | Multidisciplinary Sciences |
| Frontiers in Microbiology | 6.064 | Frontiers Media SA | 7 | 47.1 | Microbiology |
| Journal of Oral Microbiology | 5.833 | Taylor & Francis Ltd | 6 | 23.7 | Microbiology |
| Oncotarget | 5.168 | Impact Journals LLC | 5 | 101.0 | Cancer |
| Journal of Dental Research | 8.924 | Sage Publications Inc | 4 | 46.3 | Dentistry |
| Oral Oncology | 5.972 | Elsevier | 4 | 26.3 | Dentistry, Oncology |
| Frontiers in Oncology | 5.738 | Frontiers Media SA | 4 | 10.8 | Cancer |
| International Journal of Molecular Sciences | 6.208 | MDPI | 4 | 5.5 | Biochemistry and Molecular Biology |
| Journal of Cancer | 4.478 | Ivyspring Int Publ | 4 | 2.3 | Cancer |
| Rank | Article Title | Corresponding Author | Year of publication | Country/ Region | Journal | Citations | Reference |
|---|---|---|---|---|---|---|---|
| 1 | Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack | Bachrach, Gilad and Mandelboim, Ofer | 2015 | Israel | Immunity (IF =32.4) | 646 | [42] |
| 2 | Changes in abundance of oral microbiota associated with oral cancer | Albertson, Donna G. | 2014 | USA | PLOS one (IF = 3.7) | 212 | [15] |
| 3 | Periodontal pathogens Porphyromonas gingivalis and Fusobacterium nucleatum promote tumor progression in an oral-specific chemical carcinogenesis model | Elkin, Michael | 2015 | Israel | Oncotarget (IF = 5.168) | 208 | [43] |
| 4 | 16S rRNA amplicon sequencing identifies microbiota associated with oral cancer, human papilloma virus infection, and surgical treatment | Guerrero-Preston, Rafael; Sidransky, David | 2016 | USA | Oncotarget (IF = 5.168) | 178 | [44] |
| 5 | Variations in oral microbiota associated with oral cancer | Liang, Jingping | 2017 | China | Scientific Reports (IF = 4.6) | 164 | [45] |
| 6 | Oral microbiota community dynamics associated with oral squamous cell carcinoma staging | Chang, Kai-Ping | 2018 | Taiwan | Frontiers in Microbiology (IF = 5.2) | 155 | [46] |
| 7 | Association of oral microbiome with risk for incident head and neck squamous cell cancer | Hayes, Richard B. | 2018 | USA | JAMA Oncology (IF = 28.4) | 150 | [47] |
| 8 | The oral microbiota may have influence on oral cancer | Zhang, Chen Ping | 2020 | China | Frontiers in Cellular and Infection Microbiology (IF = 5.7) | 132 | [48] |
| 9 | Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma | Al-hebshi, Nezar Noor | 2017 | USA, Saudi Arabia | Scientific Reports (IF = 4.6) | 126 | [28] |
| 10 | Inflammatory bacteriome and oral squamous cell carcinoma | Al-hebshi, Nezar Noor | 2018 | USA | Journal of Dental Research (IF = 7.6) | 96 | [27] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, S.-W.; Yang, J.-J.; Lin, Y.-Y. Mapping the Scientific Landscape of Bacterial Influence on Oral Cancer: A Bibliometric Analysis of the Last Decade’s Medical Progress. Curr. Oncol. 2023, 30, 9004-9018. https://doi.org/10.3390/curroncol30100650
Hu S-W, Yang J-J, Lin Y-Y. Mapping the Scientific Landscape of Bacterial Influence on Oral Cancer: A Bibliometric Analysis of the Last Decade’s Medical Progress. Current Oncology. 2023; 30(10):9004-9018. https://doi.org/10.3390/curroncol30100650
Chicago/Turabian StyleHu, Suh-Woan, Jaw-Ji Yang, and Yuh-Yih Lin. 2023. "Mapping the Scientific Landscape of Bacterial Influence on Oral Cancer: A Bibliometric Analysis of the Last Decade’s Medical Progress" Current Oncology 30, no. 10: 9004-9018. https://doi.org/10.3390/curroncol30100650
APA StyleHu, S.-W., Yang, J.-J., & Lin, Y.-Y. (2023). Mapping the Scientific Landscape of Bacterial Influence on Oral Cancer: A Bibliometric Analysis of the Last Decade’s Medical Progress. Current Oncology, 30(10), 9004-9018. https://doi.org/10.3390/curroncol30100650






