Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy?
Abstract
1. Introduction
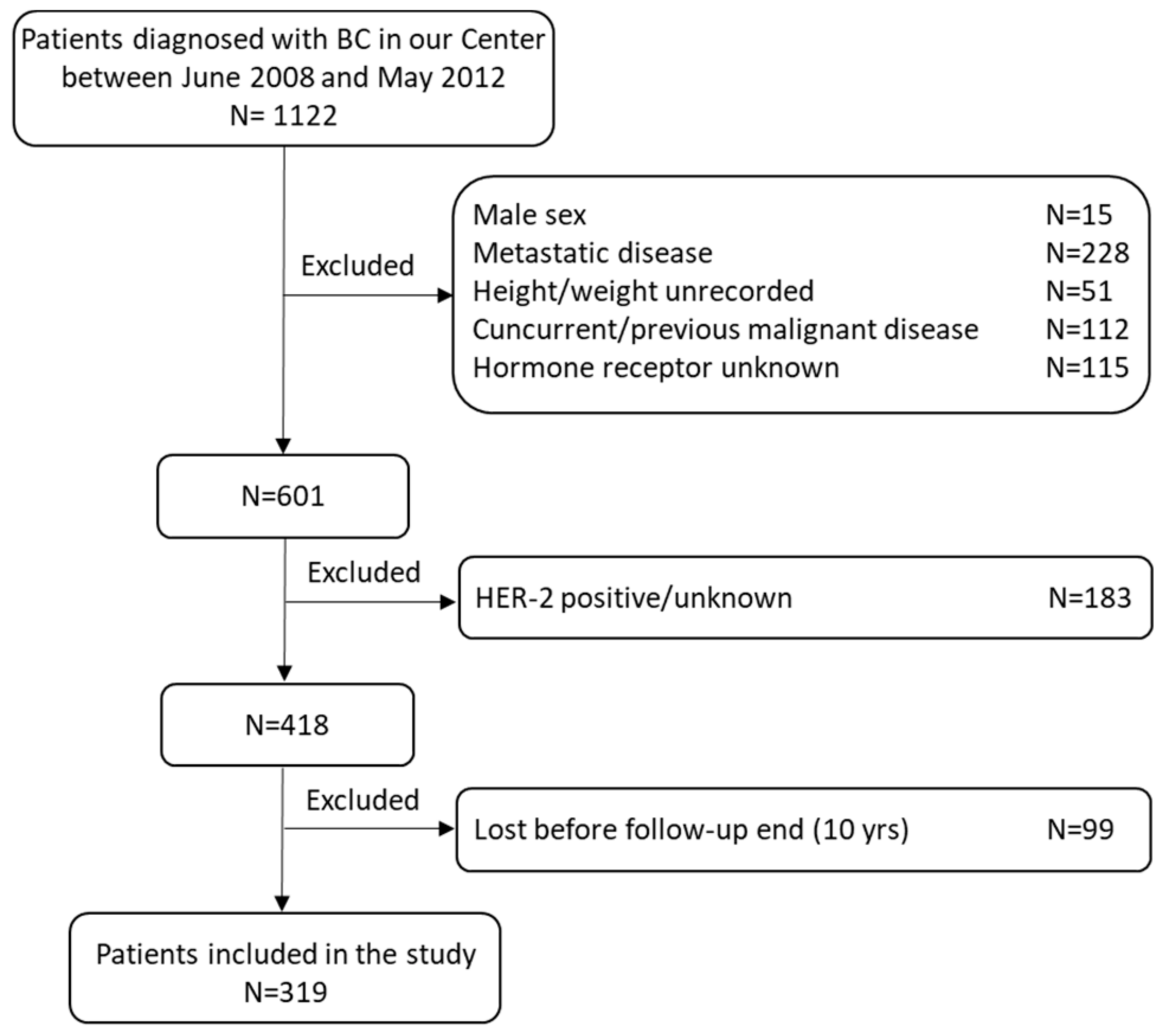
2. Materials and Methods
2.1. Patients
2.2. Statistical Analysis
3. Results
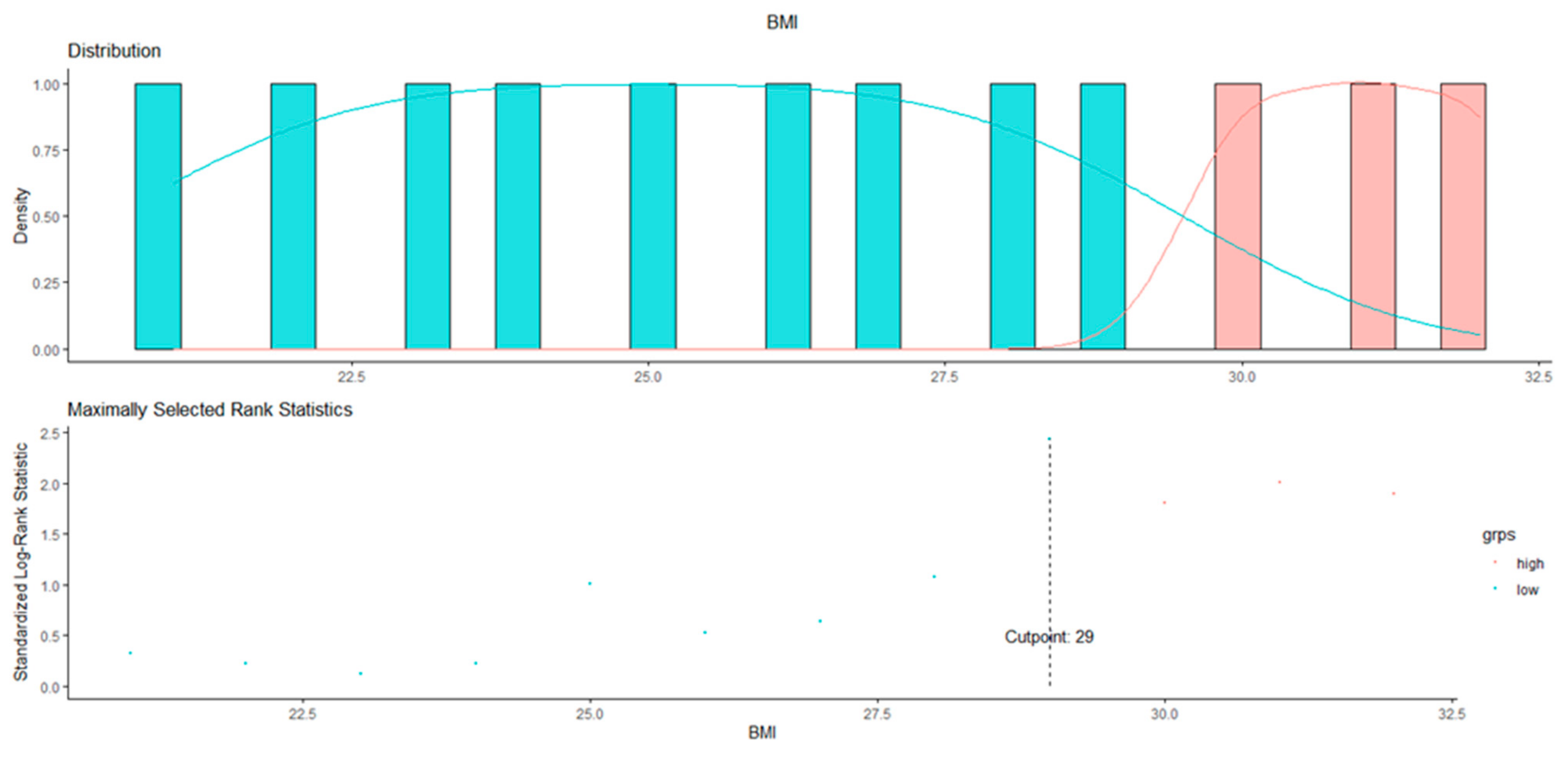
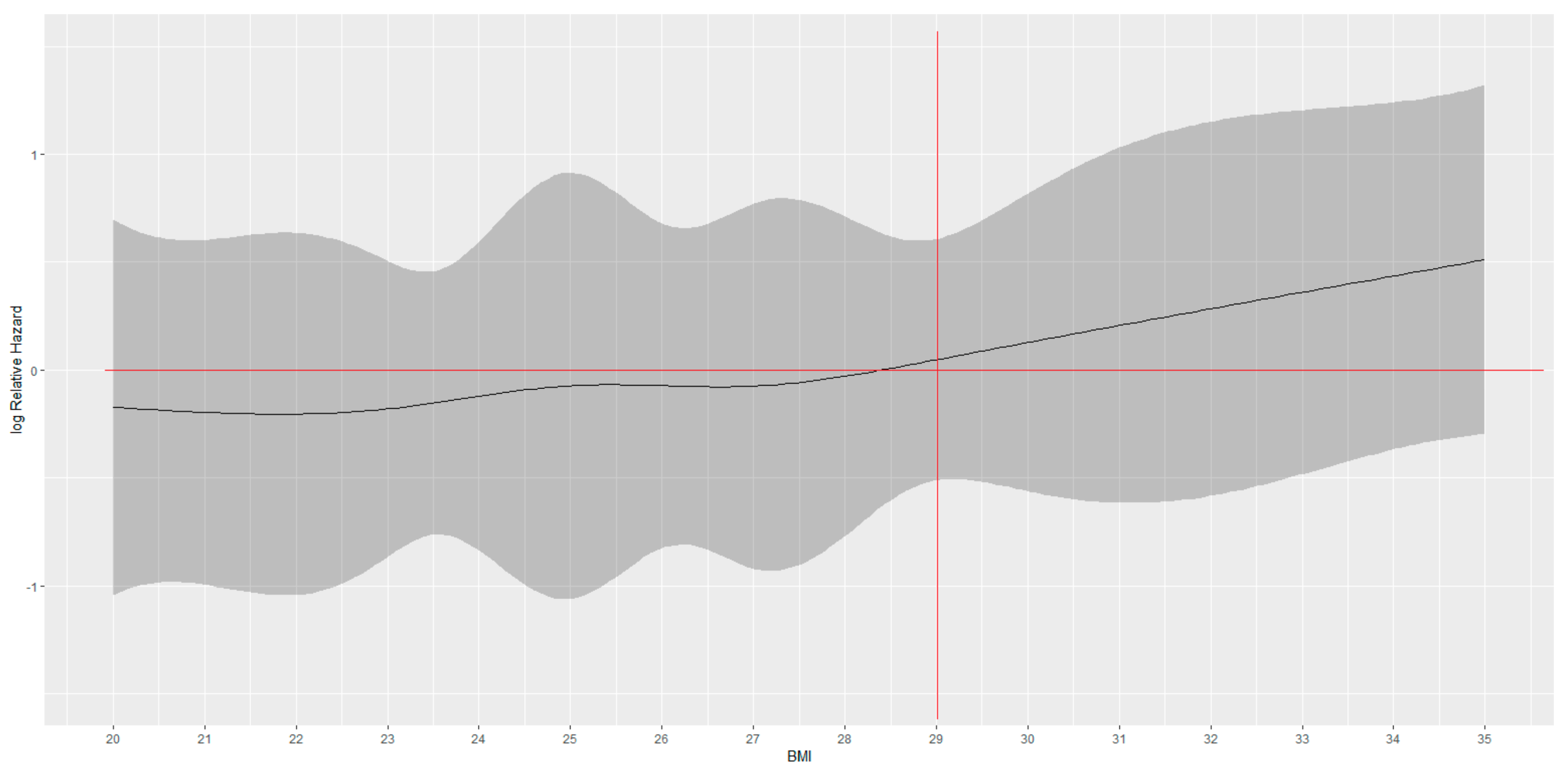
3.1. BMI Cut-Off Identification
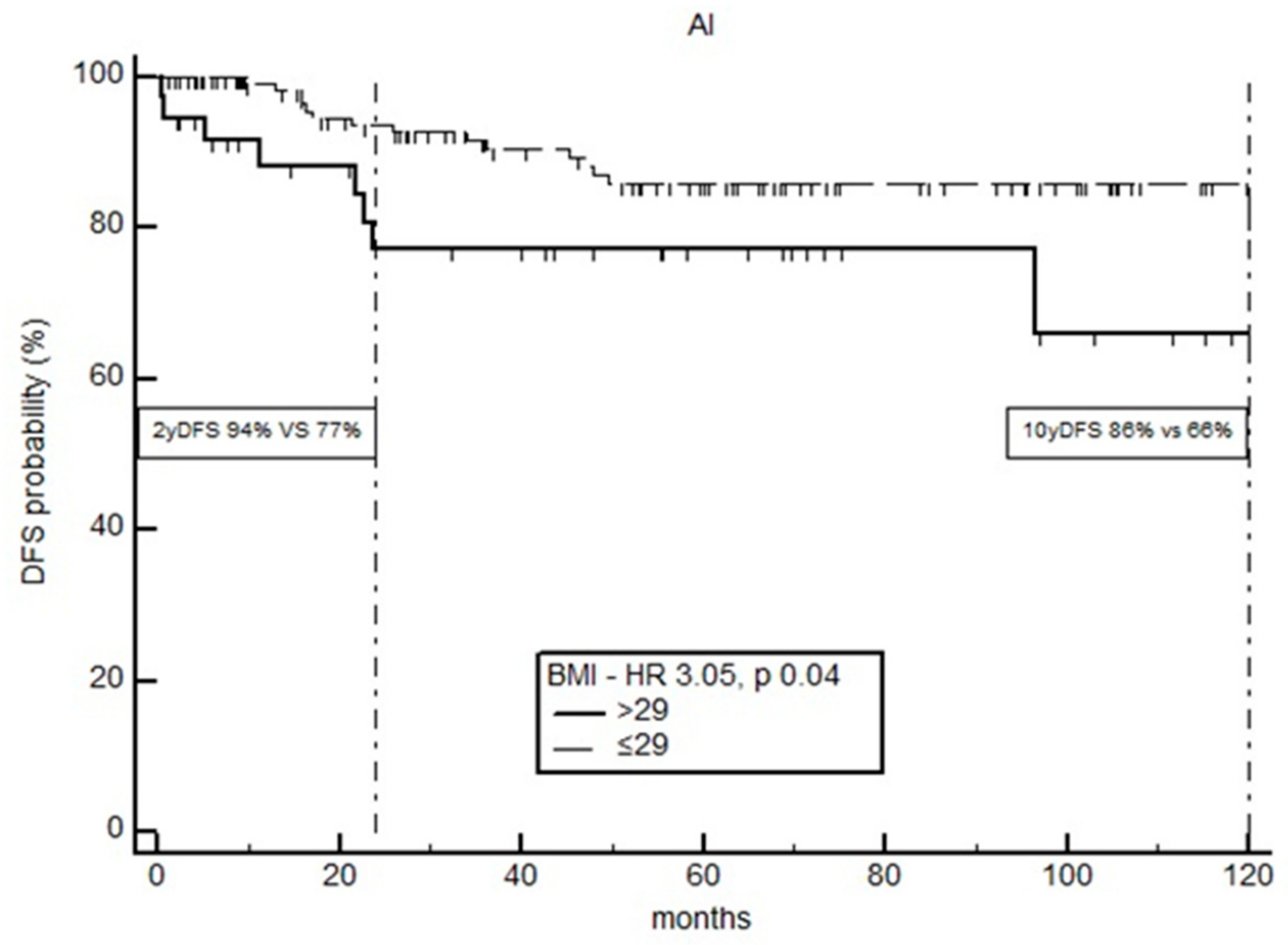
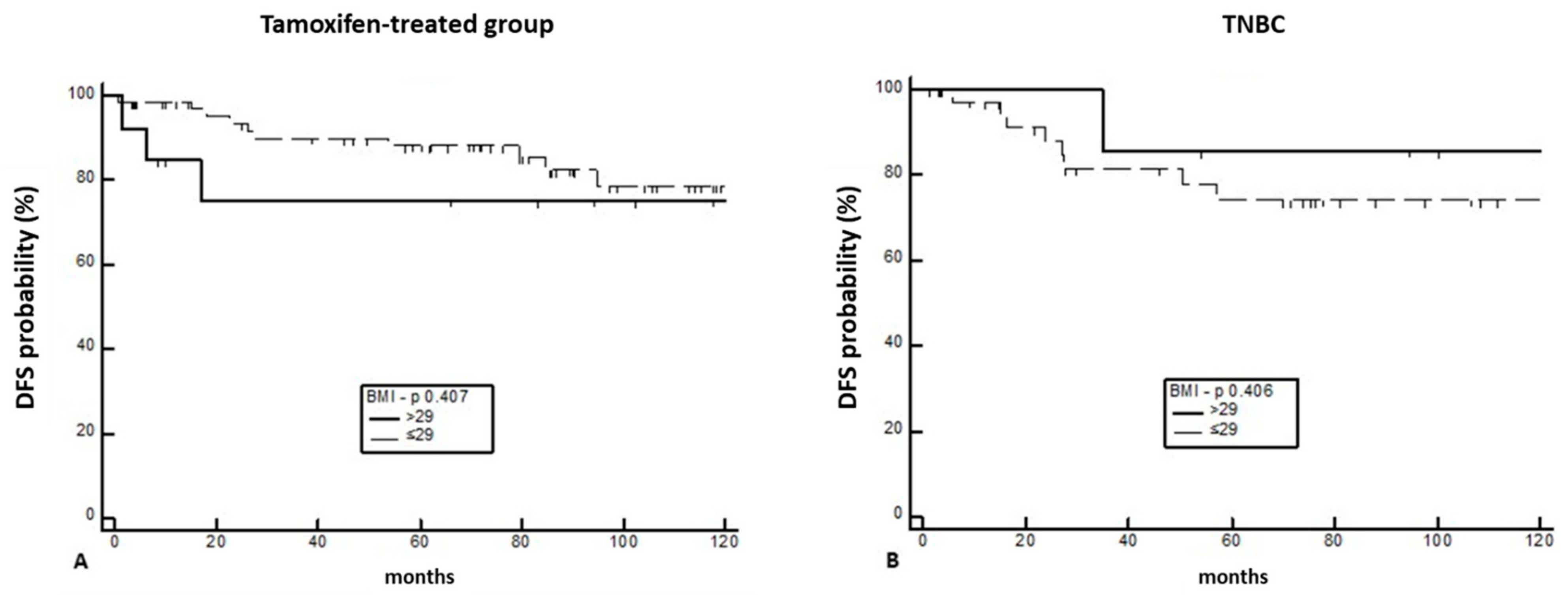
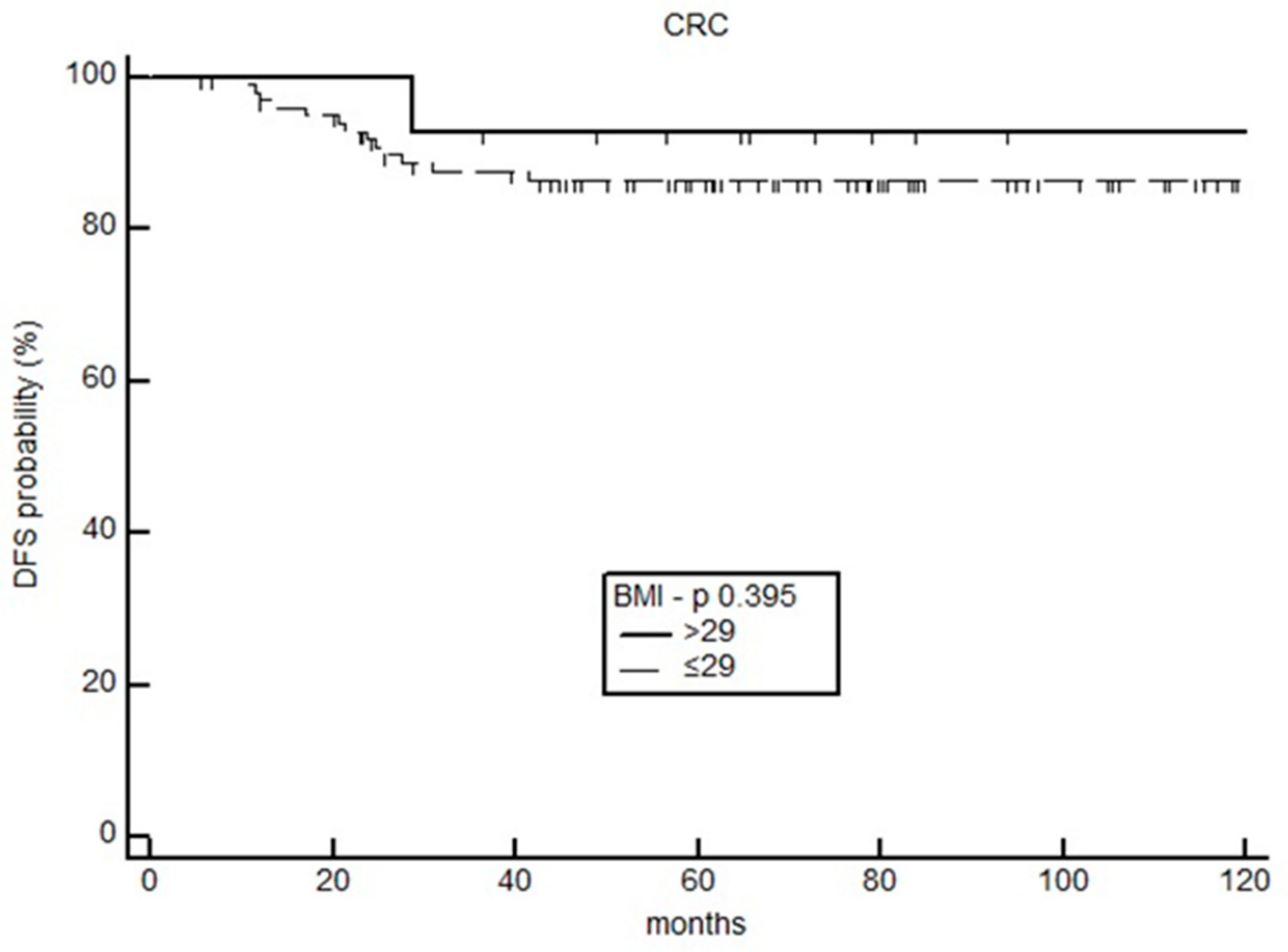
3.2. Impact of BMI on Survival
3.3. Biochemical and Histopathological Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perou, C.M.; Sørlie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; Rees, C.A.; Pollack, J.R.; Ross, D.T.; Johnsen, H.; Akslen, L.A.; et al. Molecular portraits of human breast tumours. Nature 2000, 406, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Yersal, O.; Barutca, S. Biological subtypes of breast cancer: Prognostic and therapeutic implications. World J. Clin. Oncol. 2014, 5, 412–424. [Google Scholar] [CrossRef] [PubMed]
- Ratosa, I.; Plavc, G.; Pislar, N.; Zagar, T.; Perhavec, A.; Franco, P. Improved Survival after Breast-Conserving Therapy Compared with Mastectomy in Stage I-IIA Breast Cancer. Cancers 2021, 13, 4044. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Thürlimann, B.; Weber, W.P.; Poortmans, P.; Regan, M.M.; Senn, H.J.; Winer, E.P.; Gnant, M.; Panelists of the St Gallen Consensus Conference. Customizing local and systemic therapies for women with early breast cancer: The St. Gallen International Consensus Guidelines for treatment of early breast cancer 2021. Ann Oncol. 2021, 32, 1216–1235. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.E.; Dowsett, M. Aromatase inhibitors in breast cancer. N. Engl. J. Med. 2003, 348, 2431–2442. [Google Scholar] [CrossRef]
- Conte, B.; Poggio, F.; Del Mastro, L. Luteininzing hormone releasing hormones analogs in combination with tamoxifen for the adjuvant treatment of premenopausal women with hormone receptor positive breast cancer. Expert Opin. Pharmacother. 2017, 18, 1357–1362. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in premenopausal women with oestrogen receptor-positive early-stage breast cancer treated with ovarian suppression: A patient-level meta-analysis of 7030 women from four randomised trials. Lancet 2022, 23, 382–392. [Google Scholar] [CrossRef]
- Sella, T.; Ruddy, K.J.; Carey, L.A.; Partridge, A.H. Optimal Endocrine Therapy in Premenopausal Women: A Pragmatic Approach to Unanswered Questions. JCO Oncol. Pract. 2021, 18, 211–216. [Google Scholar] [CrossRef]
- Del Mastro, L.; Mansutti, M.; Bisagni, G.; Ponzone, R.; Durando, A.; Amaducci, L.; Campadelli, E.; Cognetti, F.; Frassoldati, A.; Michelotti, A.; et al. Extended therapy with letrozole as adjuvant treatment of postmenopausal patients with early-stage breast cancer: A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2021, 22, 1458–1467. [Google Scholar] [CrossRef]
- Gnant, M.; Fitzal, F.; Rinnerthaler, G.; Steger, G.G.; Greil-Ressler, S.; Balic, M.; Heck, D.; Jakesz, R.; Thaler, J.; Egle, D.; et al. Duration of adjuvant aromatase-inhibitor therapy in postmenopausal breast cancer. N. Engl. J. Med. 2021, 385, 395–405. [Google Scholar] [CrossRef]
- Reeves, G.K.; Pirie, K.; Beral, V.; Green, J.; Spencer, E.; Bull, D.; Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: Cohort study. BMJ 2007, 335, 1134. [Google Scholar] [CrossRef]
- Smith, S.G.; Sestak, I.; Morris, M.A.; Harvie, M.; Howell, A.; Forbes, J.; Cuzick, J. The impact of body mass index on breast cancer incidence among women at increased risk: An observational study from the International Breast Intervention Studies. Breast Cancer Res. Treat. 2021, 188, 215–223. [Google Scholar] [CrossRef]
- Picon-Ruiz, M.; Morata-Tarifa, C.; Valle-Goffin, J.J.; Friedman, E.R.; Slingerland, J.M. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA A. Cancer J. Clin. 2017, 67, 378–397. [Google Scholar] [CrossRef]
- Sparano, J.A.; Wang, M.; Zhao, F.; Stearns, V.; Martino, S.; Ligibel, J.A.; Perez, E.A.; Saphner, T.; Wolff, A.C.; Sledge, G.W., Jr.; et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer 2012, 118, 5937–5946. [Google Scholar] [CrossRef]
- Calle, E.E.; Rodriguez, C.; Walker-Thurmond, K.; Thun, M.J. Overweight, Obesity, and Mortality from Cancer in a Prospectively Studied Cohort of U.S. Adults. N. Engl. J. Med. 2003, 348, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.R.; Clyne, C.; Rubin, G.; Boon, W.C.; Robertson, K.; Britt, K.; Speed, C.; Jones, M. Aromatase—A Brief Overview. Annu. Rev. Physiol. 2002, 64, 93–127. [Google Scholar] [CrossRef]
- Yager, J.D.; Davidson, N.E. Estrogen Carcinogenesis in Breast Cancer. N. Engl. J. Med. 2006, 354, 270–282. [Google Scholar] [CrossRef]
- Simpson, E.R.; Brown, K.A. Minireview: Obesity and breast cancer: A tale of inflammation and dysregulated metabolism. Mol. Endocrinol. 2013, 27, 715–725. [Google Scholar] [CrossRef]
- De Placido, S.; Gallo, C.; De Laurentiis, M.; Bisagni, G.; Arpino, G.; Sarobba, M.G.; Riccardi, F.; Russo, A.; Del Mastro, L.; Cogoni, A.A.; et al. Adjuvant anastrozole versus exemestane versus letrozole, upfront or after 2 years of tamoxifen, in endocrine-sensitive breast cancer (FATA-GIM3): A randomised, phase 3 trial. Lancet Oncol. 2018, 19, 474–485. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of Incidence and mortality worldwide for 36 cancers in 185 countries. CA A. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Protani, M.; Coory, M.; Martin, J.H. Effect of obesity on survival of women with breast cancer: Systematic review and meta-analysis. Breast Cancer Res. Treat. 2010, 123, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Gnant, M.; Pfeiler, G.; Stoger, H.; Mlineritsch, B.; Fitzal, F.; Balic, M.; Kwasny, W.; Seifert, M.; Stierer, M.; Dubsky, P.; et al. The predictive impact of body mass index on the efficacy of extended adjuvant endocrine treatment with anastrozole in postmenopausal patients with breast cancer: An analysis of the randomised ABCSG-6a trial. Br. J. Cancer 2013, 109, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Sestak, I.; Distler, W.; Forbes, J.F.; Dowsett, M.; Howell, A.; Cuzick, J. Effect of body mass index on recurrences in tamoxifen and anastrozole treated women: An exploratory analysis from the ATAC trial. J. Clin. Oncol. 2010, 28, 3411–3415. [Google Scholar] [CrossRef] [PubMed]
- Niraula, S.; Ocana, A.; Ennis, M.; Goodwin, P.J. Body size and breast cancer prognosis in relation to hormone receptor and menopausal status: A meta-analysis. Breast Cancer Res. Treat. 2012, 134, 769–781. [Google Scholar] [CrossRef]
- Geisler, J.; Haynes, B.; Anker, G.; Dowsett, M.; Lønning, P.E. Influence of letrozole and anastrozole on total body aromatization and plasma estrogen levels in postmenopausal breast cancer patients evaluated in a randomized.; cross-over study. J. Clin. Oncol. 2002, 20, 751–757. [Google Scholar] [CrossRef]
- Iyengar, N.M.; Gucalp, A.; Dannenberg, A.J.; Hudis, C.A. Obesity and Cancer Mechanisms: Tumor Microenvironment and Inflammation. J. Clin. Oncol. 2016, 34, 4270–4276. [Google Scholar] [CrossRef]
- Muti, P.; Quattrin, T.; Grant, B.J.; Krogh, V.; Micheli, A.; Schünemann, H.J.; Ram, M.; Freudenheim, J.L.; Sieri, S.; Trevisan, M.; et al. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 1361–1368. [Google Scholar]
- Takeda, Y.; Fujita, Y.; Bessho, R.; Sato, M.; Abe, T.; Yanagimachi, T.; Sakagami, H.; Abiko, A.; Takiyama, Y.; Ota, T.; et al. Increment of plasma glucose by exogenous glucagon is associated with present and future renal function in type 2 diabetes: A retrospective study from glucagon stimulation test. BMC Endocr. Disord. 2019, 19, 99. [Google Scholar] [CrossRef]
- Jepson, C.; Hsu, J.Y.; Fischer, M.J.; Kusek, J.W.; Lash, J.P.; Ricardo, A.C.; Schelling, J.R.; Feldman, H.I. Chronic Renal Insufficiency Cohort (CRIC) Study Investigators. Incident Type 2 Diabetes Among Individuals With CKD: Findings From the Chronic Renal Insufficiency Cohort (CRIC) Study. Am. J. Kidney Dis. 2019, 73, 72–81. [Google Scholar] [CrossRef]
- Desai, P.; Lehman, A.; Chlebowski, R.T.; Kwan, M.L.; Arun, M.; Manson, J.E.; Lavasani, S.; Wasswertheil-Smoller, S.; Sarto, G.E.; LeBoff, M.; et al. Statins and breast cancer stage and mortality in the Women’s Health Initiative. Cancer Causes Control. 2015, 26, 529–539. [Google Scholar] [CrossRef]
- Kabat, G.C.; Kim, M.Y.; Lee, J.S.; Ho, G.Y.; Going, S.B.; Beebe-Dimmer, J.; Manson, J.E.; Chlebowski, R.T.; Rohan, T.E. Metabolic obesity phenotypes and risk of breast cancer in postmenopausal women. Cancer Epidemiol. Biomark. Prev. 2017, 12, 1730–1735. [Google Scholar] [CrossRef]
- Ferroni, P.; Riondino, S.; Buonomo, O.; Palmirotta, R.; Guadagni, F.; Roselli, M. Type 2 Diabetes and Breast Cancer: The Interplay between Impaired Glucose Metabolism and Oxidant Stress. Oxid. Med. Cell. Longev. 2015, 2015, 183928. [Google Scholar] [CrossRef]
- Dibaba, D.T.; Ogunsina, K.; Braithwaite, D.; Akinyemiju, T. Metabolic syndrome and risk of breast cancer mortality by menopause, obesity, and subtype. Breast Cancer Res. Treat. 2019, 174, 209–218. [Google Scholar] [CrossRef]
- Zheng, W.; Cao, L.; Ouyang, L.; Zhang, Q.; Duan, B.; Zhou, W.; Chen, S.; Peng, W.; Xie, Y.; Fan, Q.; et al. Anticancer activity of 1,25-(OH)2D3 against human breast cancer cell lines by targeting Ras/MEK/ERK pathway. Onco. Targets Ther. 2019, 12, 721–732. [Google Scholar] [CrossRef]
- Swami, S.; Krishnan, A.V.; Feldman, D. 1α,25-Dihydroxyvitamin D3 Down-Regulates Estrogen Receptor Abundance and Suppresses Estrogen Actions in MCF-7 Human Breast Cancer Cells. Clin. Cancer Res. 2000, 6, 3371–3379. [Google Scholar]
- Swami, S.; Krishnan, A.V.; Wang, J.Y.; Jensen, K.; Peng, L.; Albertelli, M.A.; Feldman, D. Inhibitory Effects of Calcitriol on the Growth of MCF-7 Breast Cancer Xenografts in Nude Mice: Selective Modulation of Aromatase Expression in vivo. Horm. Canc. 2011, 2, 190–202. [Google Scholar] [CrossRef]
- Ma, Y.; Trump, D.L.; Johnson, C.S. Vitamin D in combination cancer treatment. J. Cancer 2010, 1, 101–107. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Swami, S.; Feldman, D. The potential therapeutic benefits of vitamin D in the treatment of estrogen receptor positive breast cancer. Steroids 2012, 77, 1107–1112. [Google Scholar] [CrossRef]
- Morton, M.L.; Thompson, C.L. Decreasing 25-hydroxy-vitamin D levels account for portion of the effect of increasing body mass index on breast cancer mortality. Mol. Nutr. Food Res. 2013, 57, 260–266. [Google Scholar] [CrossRef]
- Vrieling, A.; Hein, R.; Abbas, S.; Schneeweiss, A.; Flesch-Janys, D.; Chang-Claude, J. Serum 25- hydroxyvitamin D and postmenopausal breast cancer survival: A prospective patient cohort study. Breast Cancer Res. 2011, 13, R74. [Google Scholar] [CrossRef] [PubMed]
- De Pergola, G.; Martino, T.; Zupo, R.; Caccavo, D.; Pecorella, C.; Paradiso, S.; Silvestris, F.; Triggiani, V. 25 Hydroxyvitamin D Levels are Negatively and Independently Associated with Fat Mass in a Cohort of Healthy Overweight and Obese Subjects. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 838–844. [Google Scholar] [CrossRef] [PubMed]
- Pereira-Santos, M.; Costa, P.R.F.; Assis, A.M.O.; Santos, C.A.S.T.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Vashi, P.G.; Lammersfeld, C.A.; Braun, D.P.; Gupta, D. Serum 25-hydroxyvitamin D is inversely associated with body mass index in cancer. Nutr. J. 2011, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- Lagunova, Z.; Porojnicu, A.C.; Grant, W.B.; Bruland, O.; Moan, J.E. Obesity and increased risk of cancer: Does decrease of serum 25-hydroxyvitamin D level with increasing body mass index explain some of the association? Mol. Nutr. Food Res. 2010, 54, 1127–1133. [Google Scholar] [CrossRef]
- McGill, A.T.; Stewart, J.M.; Lithander, F.E.; Strik, C.M.; Poppitt, S.D. Relationships of low serum vitamin D3 with anthropometry and markers of the metabolic syndrome and diabetes in overweight and obesity. Nutr. J. 2008, 7, 4. [Google Scholar] [CrossRef]
- Rodriguez-Rodriguez, E.; Navia, B.; Lopez-Sobaler, A.M.; Ortega, R.M. Vitamin D in overweight/obese women and its relationship with dietetic and anthropometric variables. Obesity 2009, 17, 778–782. [Google Scholar] [CrossRef]
- Stein, E.M.; Strain, G.; Sinha, N.; Ortiz, D.; Pomp, A.; Dakin, G.; McMahon, D.J.; Bockman, R.; Silverberg, S.J. Vitamin D insufficiency prior to bariatric surgery: Risk factors and a pilot treatment study. Clin. Endocrinol. 2009, 71, 176–183. [Google Scholar] [CrossRef]
- Earthman, C.P.; Beckman, L.M.; Masodkar, K.; Sibley, S.D. The link between obesity and low circulating 25-hydroxyvitamin D concentrations: Considerations and implications. Int. J. Obes. 2012, 36, 387–396. [Google Scholar] [CrossRef]
- Pantelimon, I.; Gales, L.N.; Anghel, R.M.; Gruia, M.I.; Nita, I.; Matei, C.V.; Bodea, D.; Stancu, A.M.; Pirvu, E.; Radu, M.C.; et al. Aspects Regarding the Influence of Obesity on the Molecular Characteristics of Breast Tumors. Cureus 2022, 14, e26952. [Google Scholar] [CrossRef]
- Saleh, R.R.; Nadler, M.B.; Desnoyers, A.; Meti, N.; Fazelzad, R.; Amir, E. Platinum-based chemotherapy in early-stage triple negative breast cancer: A meta-analysis. Cancer Treat. Rev. 2021, 100, 102283. [Google Scholar] [CrossRef]
- Gong, S.; Wang, K.; Li, Y.; Zhou, Z.; Alamian, A. Ethnic group differences in obesity in Asian Americans in California, 2013–2014. BMC Public Health 2021, 21, 1589. [Google Scholar] [CrossRef]
- Vicks, W.S.; Lo, J.C.; Guo, L.; Rana, J.S.; Zhang, S.; Ramalingam, N.D.; Gordon, N.P. Prevalence of prediabetes and diabetes vary by ethnicity among U.S. Asian adults at healthy weight, overweight, and obesity ranges: An electronic health record study. BMC Public Health 2022, 22, 1954. [Google Scholar] [CrossRef]
- Bandera, E.V.; Maskarinec, G.; Romieu, I.; John, E.M. Racial and ethnic disparities in the impact of obesity on breast cancer risk and survival: A global perspective. Adv. Nutr. 2015, 6, 803–819. [Google Scholar] [CrossRef]
| Variable | N (%) |
|---|---|
| Number of patients | 319 |
| Age at diagnosis (mean and range) | 53.7 (29.2–85.1) |
| Menopausal status | |
| Pre-menopause | 96 (30.1) |
| Post-menopause | 223 (69.9) |
| BMI | |
| Underweight (<18.5) | 8 (2.5) |
| Normal weight (18.5–24.99) | 151 (47.3) |
| Overweight (25–29.99) | 101 (31.7) |
| Obese (≥30) | 59 (18.5) |
| Histology | |
| Ductal | 273 (85.6) |
| Lobular | 40 (12.5) |
| Other | 6 (1.9) |
| Grading | |
| G1 | 44 (13.8) |
| G2 | 134 (42) |
| G3 | 141 (44.2) |
| TNM | |
| T1N0 | 77 (24.1) |
| T2-T3N0 | 68 (21.3) |
| N+ | 174 (54.6) |
| Molecular subtype | |
| Luminal A-like | 103 (32.3) |
| Luminal B-like | 151 (47.3) |
| Triple-negative | 65 (20.4) |
| ECOG-PS | |
| 0 | 315 (98.7) |
| 1 | 4 (1.3) |
| Chemotherapy | |
| Neoadjuvant | 101 (31.6) |
| Adjuvant | 218 (68.4) |
| Chemotherapy Scheme | |
| Antracycline | 88 (27.6) |
| Antracycline + Taxanes | 198 (62) |
| Taxanes | 33 (10.4) |
| Endocrine therapy | |
| Aromatase inhibitors | 172 (67.7) |
| Tamoxifene | 82 (32.3) |
| Type of AI | |
| Anastrozole | 122 (71) |
| Letrozole | 32 (18.6) |
| Exemestane | 18 (10.4) |
| Variable | N (%) |
|---|---|
| Number of patients | 116 |
| Age at diagnosis (mean and range) | 61 (34.7–80.6) |
| Menopausal status | |
| Pre-menopause | 31 (26.7) |
| Post-menopause | 85 (73.3) |
| BMI | |
| Underweight (<18.5) | 6 (5) |
| Normal weight (18.5–24.99) | 59 (51.0) |
| Overweight (25–29.99) | 37 (32.0) |
| Obese (>30) | 14 (12.0) |
| Chemotherapy Scheme | |
| 5-Fluorouracil | 21 (18.0) |
| 5-Fluorouracil + Oxaliplatin | 95 (82) |
| BMI < 29 | BMI > 29 | ||
|---|---|---|---|
| Variable | Mean Value | Mean Value | p |
| Fibrinogen (mg/dL) | 292 | 335 | <0.0001 |
| Glucose (mg/dL) | 95 | 119 | 0.004 |
| ALT (UI/L) | 18 | 17 | 0.67 |
| AST (UI/L) | 18 | 18 | 0.89 |
| γGT (UI/L) | 21 | 24 | 0.92 |
| Bilirubin (mg/dL) | 0.44 | 0.40 | 0.47 |
| Urea (mg/dL) | 30 | 34 | 0.04 |
| Creatinine (mg/dL) | 0.7 | 0.8 | 0.01 |
| Total Cholesterol (mg/dL) | 194 | 209 | 0.03 |
| Triglycerides (mg/dL) | 125 | 117 | 0.68 |
| Vitamin D (UI/L) | 32 | 26 | 0.0001 |
| Ki-67 expression (%) | 20% | 29% | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riondino, S.; Formica, V.; Valenzi, E.; Morelli, C.; Flaminio, V.; Portarena, I.; Torino, F.; Roselli, M. Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy? Curr. Oncol. 2023, 30, 1220-1231. https://doi.org/10.3390/curroncol30010094
Riondino S, Formica V, Valenzi E, Morelli C, Flaminio V, Portarena I, Torino F, Roselli M. Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy? Current Oncology. 2023; 30(1):1220-1231. https://doi.org/10.3390/curroncol30010094
Chicago/Turabian StyleRiondino, Silvia, Vincenzo Formica, Elena Valenzi, Cristina Morelli, Valeria Flaminio, Ilaria Portarena, Francesco Torino, and Mario Roselli. 2023. "Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy?" Current Oncology 30, no. 1: 1220-1231. https://doi.org/10.3390/curroncol30010094
APA StyleRiondino, S., Formica, V., Valenzi, E., Morelli, C., Flaminio, V., Portarena, I., Torino, F., & Roselli, M. (2023). Obesity and Breast Cancer: Interaction or Interference with the Response to Therapy? Current Oncology, 30(1), 1220-1231. https://doi.org/10.3390/curroncol30010094






