Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer
Abstract
1. Introduction
2. Methods
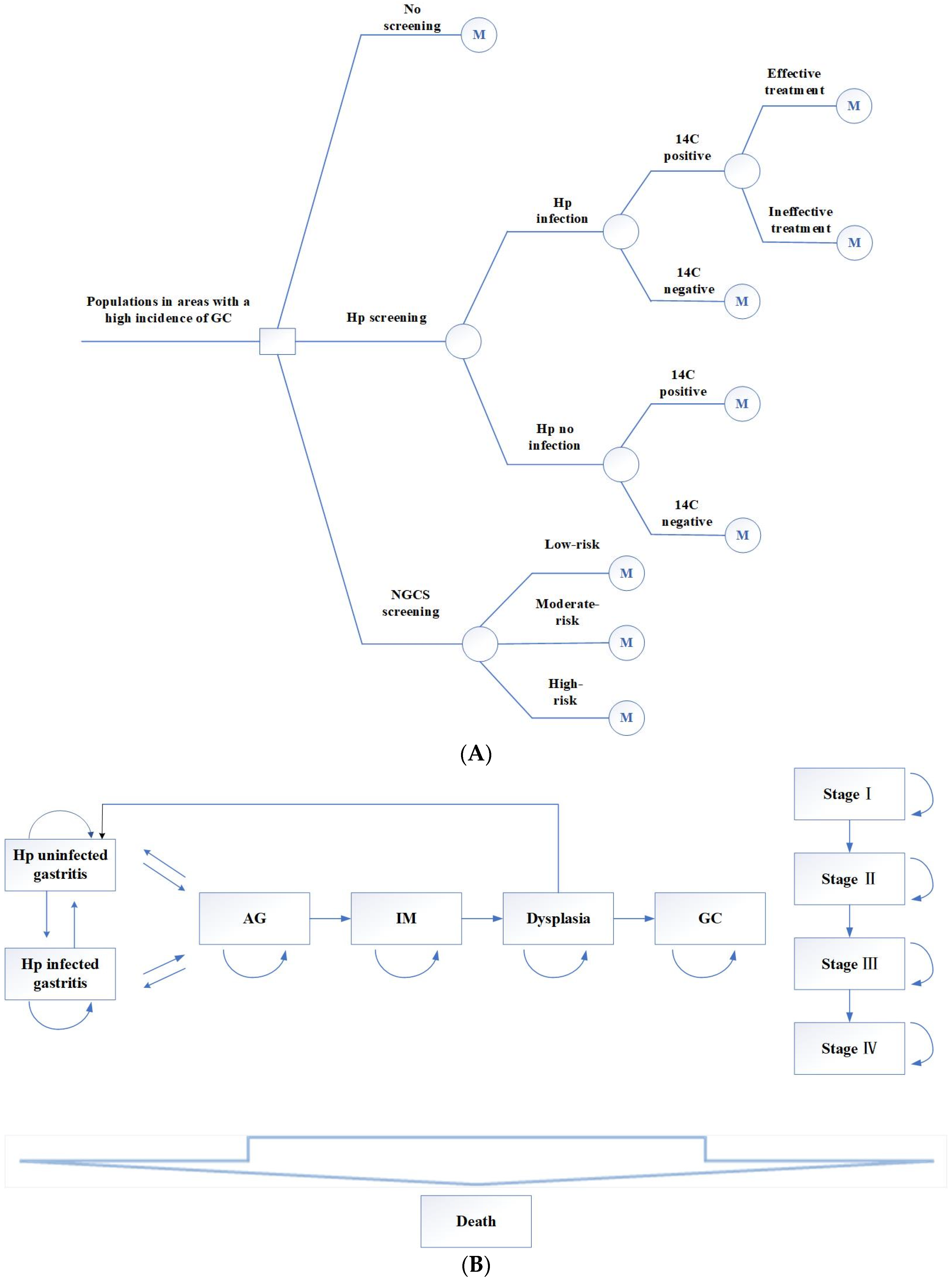
2.1. Establish the Markov Model
- (1)
- No screening.
- (2)
- Hp screening. The 14C urea breath test was used to screen this population. The positive screening results were treated with a regular 14-day treatment: esmprazole 20 mg bid, colloidal pectin bismuth 150 mg tid, furazolidone 100 mg bid, amoxicillin 1 g bid. Those allergic to amoxicillin were replaced with tetracycline 500 mg bid. If the Hp eradication treatment fails, the next cycle still enters the Hp-positive cycle.
- (3)
- NGCS screening. According to the NGCS, patients were divided into three groups: low-risk, moderate-risk, and high-risk (Table 1 and Table 2). The high-risk GC group was examined using a magnifying endoscopy and monitored by gastroscopy annually. The moderate-risk GC group was examined using magnifying endoscopy and monitored by gastroscopy every two years. Gastroscopy was performed every three years in the low-risk GC group [7]. If the result was dysplasia or early GC, individuals were treated with endoscopic submucosal dissection (ESD) or surgical treatment, followed by gastroscopy monitoring every half year for one year, every year for three years [16].
2.2. Model Parameters
2.3. Cost-Effectiveness Analysis (CEA)
2.4. Sensitivity Analysis
3. Results
3.1. Cost-Effectiveness Analysis
3.2. Sensitivity Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lage, J.; Uedo, N.; Dinis-Ribeiro, M.; Yao, K. Surveillance of patients with gastric precancerous conditions. Best Pract. Res. Clin. Gastroenterol. 2016, 30, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, W.Q.; Li, Z.S.; Li, N.; Ren, J.S.; Tian, J.H.; Tian, W.J.; Hu, F.L.; Peng, J. China Guideline for the Screening, Early Detection and Early Treatment of Gastric Cancer (2022, Beijing). Zhonghua Zhong Liu Za Zhi Chin. J. Oncol. 2022, 31, 488–527. [Google Scholar]
- Li, T.; Chai, P.P.; Zhang, Y.H.; Wan, Q.; Zhai, T.M.; Guo, F.; Li, Y.; Wang, R.R.; Chen, C.M.; Liu, G.X. Analysis of Cost Accounting and Financing Burden of Tumor Treatment in China. Health Econ. Res. 2021, 38, 17–20. [Google Scholar]
- Katai, H.; Ishikawa, T.; Akazawa, K.; Isobe, Y.; Miyashiro, I.; Oda, I.; Tsujitani, S.; Ono, H.; Tanabe, S.; Fukagawa, T.; et al. Registration Committee of the Japanese Gastric Cancer Association. Five-year survival analysis of surgically resected gastric cancer cases in Japan: A retrospective analysis of more than 100,000 patients from the nationwide registry of the Japanese Gastric Cancer Association (2001-2007). Gastric Cancer 2018, 21, 144–154. [Google Scholar]
- Sumiyama, K. Past and current trends in endoscopic diagnosis for early stage gastric cancer in Japan. Gastric Cancer 2017, 20 (Suppl. S1), 20–27. [Google Scholar] [CrossRef]
- Du, Y.Q.; Cai, Q.C.; Liao, Z. Expert consensus opinion on early gastric cancer screening process in China (Draft) (2017, Shanghai). Chin. J. Gastroenterol. 2018, 23, 92–97. [Google Scholar]
- Chinese consensus on eradication of Helicobacter pylori and prevention and control of gastric cancer (2019, Shanghai). Chin. J. Health Manag. 2019, 13, 285–291.
- Rugge, M.; Genta, R.M.; Di Mario, F.; El-Omar, E.M.; El-Serag, H.B.; Fassan, M.; Hunt, R.H.; Kuipers, E.J.; Malfertheiner, P.; Sugano, K.; et al. Gastric Cancer as Preventable Disease. Clin. Gastroenterol. Hepatol. 2017, 15, 1833–1843. [Google Scholar] [CrossRef]
- Miki, K.; Urita, Y. Using serum pepsinogens wisely in a clinical practice. J. Dig. Dis. 2007, 8, 8–14. [Google Scholar] [CrossRef]
- Chen, Y.; Huang, J.; Sun, Z.J. Evaluation on the application of ″Nanhai Model″ early gastric cancer screening strategy in grass-roots communities. Mod. Dig. Interv. 2021, 26, 939–943. [Google Scholar]
- Miao, R.L.; Li, Z.Y.; Ji, J.F. Current treatment status and trends of early gastric cancer in China: Analyzed based on the data of China Gastrointestinal Cancer Surgery Union. Chin. J. Pract. Surg. 2019, 39, 419–423. [Google Scholar]
- Hamashima, C. Current issues and future perspectives of gastric cancer screening. World J. Gastroenterol. 2014, 20, 13767–13774. [Google Scholar] [CrossRef] [PubMed]
- Kowada, A. Cost-effectiveness of Helicobacter pylori test and eradication versus upper gastrointestinal series versus endoscopy for gastric cancer mortality and outcomes in high prevalence countries. Scand. J. Gastroenterol. 2019, 54, 685–689. [Google Scholar] [CrossRef]
- Saito, S.; Azumi, M.; Muneoka, Y.; Nishino, K.; Ishikawa, T.; Sato, Y.; Terai, S.; Akazawa, K. Cost-effectiveness of combined serum anti-Helicobacter pylori IgG antibody and serum pepsinogen concentrations for screening for gastric cancer risk in Japan. Eur. J. Health Econ. 2018, 19, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Standard for diagnosis and treatment of gastric cancer (2018 Edition). Chin. J. Digest. Med. Imageol. (Electron. Ed.) 2019, 9, 118–144.
- National Clinical Research Center for Digestive Diseases (Shanghai); National Early Gastrointestinal-Cancer Prevention & Treatment Center Alliance (GECA); Helicobacter Pylori Group; Chinese Society of Gastroenterology; Chinese Society of Health Management; Digestive Endoscopy Professional Committee of Chinese Endoscopist Association; Cancer Endoscopy Professional Committee of China Anti-Cancer Association. Chinese consensus on management of gastric mucosal precancerous conditions and lesions (2020). Chin. J. Dig. Endosc. 2020, 37, 769–780. [Google Scholar]
- Rokkas, T.; Rokka, A.; Portincasa, P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann. Gastroenterol. 2017, 30, 414–423. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.J.; Kim, N.; Lee, H.S.; Lee, J.B.; Choi, Y.J.; Yoon, H.; Shin, C.M.; Park, Y.S.; Lee, D.H. Reversibility of atrophic gastritis and intestinal metaplasia after Helicobacter pylori eradication: A prospective study for up to 10 years. Aliment. Pharmacol. Ther. 2018, 47, 380–390. [Google Scholar] [CrossRef]
- Gray, A.; Clarke, P.M.; Wolstenholme, J.L.; Wordsworth, S. Applied Methods of Cost-Effectiveness Analysis in Healthcare; Oxford University Press: Oxford, UK, 2010; pp. 3–30. [Google Scholar]
- Kowada, A. Cost-effectiveness of Helicobacter pylori screening followed by eradication treatment for employees in Japan. Epidemiol. Infect. 2018, 146, 1834–1840. [Google Scholar] [CrossRef]
- Yeh, J.M.; Hur, C.; Ward, Z.; Schrag, D.; Goldie, S.J. Gastric adenocarcinoma screening and prevention in the era of new biomarker and endoscopic technologies: A cost-effectiveness analysis. Gut 2016, 65, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Saumoy, M.; Schneider, Y.; Shen, N.; Kahaleh, M.; Sharaiha, R.Z.; Shah, S.C. Cost Effectiveness of Gastric Cancer Screening According to Race and Ethnicity. Gastroenterology 2018, 155, 648–660. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.H.; Qin, X.X.; Zhang, Y. Screening for gastric cancer in China: Advances, challenges, and visions. Chin. J. Cancer Res. 2021, 33, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.Z.; Xie, Y.; Lu, H.; Cheng, H.; Zeng, Z.R.; Zhou, L.Y. The fifth national consensus report on the treatment of Helicobacter pylori infection. Chin. J. Gastroenterol. 2017, 37, 364–378. [Google Scholar]
- Zheng, Y.S.; Chen, Z.L.; Sai, X.Y. A cross sectional survey of infection rate of helicobacter pylori in the health physical examination population. Chin. J. Clin. (Electron. Ed.) 2013, 7, 10044–10047. [Google Scholar]
- Zhou, Q.; Li, L.; Ai, Y.; Pan, Z.; Guo, M.; Han, J. Diagnostic accuracy of the 14C-urea breath test in Helicobacter pylori infections: A meta-analysis. Wien. Klin. Wochenschr. 2017, 129, 38–45. [Google Scholar] [CrossRef]
- Alboraie, M.; Saad, M.; Al-Ali, J.; Malik, M.; Asem, N.; Schmidt, I.; Alfadhli, A.A. Quadruple therapy versus standard triple therapy for eradication of Helicobacter pylori in Kuwait. Arab. J. Gastroenterol. 2015, 16, 131–135. [Google Scholar] [CrossRef]
- Pan, K.F.; Zhang, L.; Gerhard, M.; Ma, J.L.; Liu, W.D.; Ulm, K.; Wang, J.X.; Zhang, L.; Zhang, Y.; Bajbouj, M.; et al. A large randomised controlled intervention trial to prevent gastric cancer by eradication of Helicobacter pylori in Linqu County, China: Baseline results and factors affecting the eradication. Gut 2016, 65, 9–18. [Google Scholar] [CrossRef]
- Han, Y.; Yan, T.; Ma, H.; Yao, X.; Lu, C.; Li, Y.; Li, L. Cost-Effectiveness Analysis of Helicobacter pylori Eradication Therapy for Prevention of Gastric Cancer: A Markov Model. Dig. Dis. Sci. 2020, 65, 1679–1688. [Google Scholar] [CrossRef]
- Bae, S.E.; Choi, K.D.; Choe, J.; Kim, S.O.; Na, H.K.; Choi, J.Y.; Ahn, J.Y.; Jung, K.W.; Lee, J.; Kim, D.H.; et al. The effect of eradication of Helicobacter pylori on gastric cancer prevention in healthy asymptomatic populations. Helicobacter 2018, 23, e12464. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, T.H.; Chou, C.K.; Tu, Y.K.; Liao, W.C.; Wu, M.S.; Graham, D.Y. Association Between Helicobacter pylori Eradication and Gastric Cancer Incidence: A Systematic Review and Meta-analysis. Gastroenterology 2016, 150, 1113–1124.e5. [Google Scholar] [CrossRef] [PubMed]
- Sugano, K. Effect of Helicobacter pylori eradication on the incidence of gastric cancer: A systematic review and meta-analysis. Gastric Cancer 2019, 22, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Ford, A.C.; Yuan, Y.; Moayyedi, P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut 2020, 69, 2113–2121. [Google Scholar] [CrossRef]
- Matsuo, T.; Ito, M.; Takata, S.; Tanaka, S.; Yoshihara, M.; Chayama, K. Low prevalence of Helicobacter pylori-negative gastric cancer among Japanese. Helicobacter 2011, 16, 415–419. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.H.; Ma, C.T.; Feng, H. Application of New Gastric Cancer Screening Scoring System in Early Gastric Cancer Screening: A Preliminary Community-based Study. Chin. J. Gastroenterol. 2020, 25, 395–399. [Google Scholar]
- Zhong, L.; Huang, X.M.; Yang, G.Z. Effect of new gastric cancer screening scoring system combined with gastroscopy in the diagnosis of early gastric cancer in Bao′an District, Shenzhen. J. Imaging Res. Med. Appl. 2021, 5, 223–224. [Google Scholar]
- Wang, G.Q.; Zhang, Q.H.; Naren, M. Clinical study on application of new gastric cancer screening and scoring system in primary physical examination center. J. Hulunbeir Univ. 2020, 28, 90–93. [Google Scholar]
- Probst, A.; Schneider, A.; Schaller, T.; Anthuber, M.; Ebigbo, A.; Messmann, H. Endoscopic submucosal dissection for early gastric cancer: Are expanded resection criteria safe for Western patients. Endoscopy 2017, 49, 855–865. [Google Scholar] [CrossRef]
- Bang, C.S.; Baik, G.H.; Shin, I.S.; Suk, K.T.; Yoon, J.H.; Kim, D.J. Endoscopic submucosal dissection of gastric subepithelial tumors: A systematic review and meta-analysis. Korean J. Intern. Med. 2016, 31, 860–871. [Google Scholar] [CrossRef]
- Weck, M.N.; Brenner, H. Prevalence of chronic atrophic gastritis in different parts of the world. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1083–1094. [Google Scholar] [CrossRef]
- Shah, S.C.; Canakis, A.; Peek, R.M., Jr.; Saumoy, M. Endoscopy for Gastric Cancer Screening Is Cost Effective for Asian Americans in the United States. Clin. Gastroenterol. Hepatol. 2020, 18, 3026–3039. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.X.; Liu, Q.; Zhao, B.; Zhang, H.H.; Sang, H.M.; Djaleel, S.M.; Zhang, G.X.; Xu, S.F. Risk factors for intestinal metaplasia in a southeastern Chinese population: An analysis of 28,745 cases. J. Cancer Res. Clin. Oncol. 2017, 143, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Ang, T.L.; Fock, K.M. Prevalence and Distribution of Intestinal Metaplasia and Correlation With Demographics in the Singaporean Chinese Population. Clin. Gastroenterol. Hepatol. 2015, 13, e92. [Google Scholar] [CrossRef]
- Choi, C.E.; Sonnenberg, A.; Turner, K.; Genta, R.M. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig. Dis. Sci. 2015, 60, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.K. Research on the Incidence Trends and Risk Factors of Digestive System Cancer in China in Recent Rorty Years; Zunyi Medical University: Zunyi, China, 2021. [Google Scholar]
- Chen, Z.J.; Tang, Y.H.; Diao, Z. Guiding role of new gastric cancer screening scoring system in opportunistic screening of early gastric cancer. Guizhou Med. J. 2020, 44, 1876–1877. [Google Scholar]
- Yang, W.S.; Wu, F.; Zhou, C. The value of a new gastric cancer screening scoring system in screening gastric cancer and precancerous lesions. Jiangxi Med. J. 2020, 55, 641–644. [Google Scholar]
- Zhou, H.J.; Dan, Y.Y.; Naidoo, N.; Li, S.C.; Yeoh, K.G. A cost-effectiveness analysis evaluating endoscopic surveillance for gastric cancer for populations with low to intermediate risk. PLoS ONE 2013, 8, e83959. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Zhou, J.; Naidoo, N.; Yang, W.Y.; Lin, X.C.; Wang, P.; Ding, J.Q.; Wu, C.B.; Zhou, H.J. Determining the cost-effectiveness of endoscopic surveillance for gastric cancer in patients with precancerous lesions. Asia Pac. J. Clin. Oncol. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- Yeh, J.M.; Hur, C.; Schrag, D.; Kuntz, K.M.; Ezzati, M.; Stout, N.; Ward, Z.; Goldie, S.J. Contribution of H. pylori and smoking trends to US incidence of intestinal-type noncardia gastric adenocarcinoma: A microsimulation model. PLoS Med. 2013, 10, e1001451. [Google Scholar] [CrossRef]
- Song, H.; Ekheden, I.G.; Zheng, Z.; Ericsson, J.; Nyrén, O.; Ye, W. Incidence of gastric cancer among patients with gastric precancerous lesions: Observational cohort study in a low risk Western population. BMJ 2015, 351, h3867. [Google Scholar] [CrossRef]
- Kosaka, T.; Endo, M.; Toya, Y.; Abiko, Y.; Kudara, N.; Inomata, M.; Chiba, T.; Takikawa, Y.; Suzuki, K.; Sugai, T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: A single-center retrospective study. Dig. Endosc. 2014, 26, 183–191. [Google Scholar] [CrossRef]
- Kowada, A. Endoscopy Is Cost-Effective for Gastric Cancer Screening After Successful Helicobacter pylori Eradication. Dig. Dis. Sci. 2021, 66, 4220–4226. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Lin, G.W.; Xu, S.R. Determination of health utility value of chronic gastritis, peptic ulcer and gastric cancer. Chin. J. Dig. Dis. 2000, 4, 53–54. [Google Scholar]
- Zhou, H.J.; So, J.B.; Yong, W.P.; Luo, N.; Zhu, F.; Naidoo, N.; Li, S.C.; Yeoh, K.G. Validation of the functional assessment of cancer therapy-gastric module for the Chinese population. Health Qual. Life Outcomes 2012, 10, 145. [Google Scholar] [CrossRef] [PubMed]






| Variables Name | Class | Score |
|---|---|---|
| Age (y) | 40~49 | 0 |
| 50~59 | 5 | |
| 60~69 | 6 | |
| >69 | 10 | |
| Sex | woman | 0 |
| man | 4 | |
| Hp infection | absent | 0 |
| present | 1 | |
| PGR | ≥3.89 | 0 |
| <3.89 | 3 | |
| G-17 (pmol/L) | <1.50 | 0 |
| 1.50~5.70 | 3 | |
| >5.70 | 5 |
| Class | Score |
|---|---|
| Low-risk | 0–11 |
| Moderate-risk | 12–16 |
| High-risk | 17–23 |
| Scheme | Cost (yuan) | Incremental Cost | Effect (QALY) | Incremental Effect | ICER (yuan/QALY) |
|---|---|---|---|---|---|
| No screening | 91,636.78 | - | 19.98036 | - | - |
| Hp screening | 92,227.87 | 591.09 | 21.08228 | 1.10192 | 536.42 |
| Scheme | Cost (yuan) | Incremental Cost | Effect (QALY) | Incremental Effect | ICER (yuan/QALY) |
|---|---|---|---|---|---|
| NGCS screening | 50,710.36 | - | 20.00893 | - | - |
| no screening | 91,636.78 | 40,926.42 | 19.98036 | −0.02857 | Dominated |
| Hp screening | 92,227.87 | 41,517.51 | 21.08228 | 1.07335 | 38,680.31 |
| Gastritis | AG | IM | Dysplasia | Stage I | Stage II | Stage III | Stage IV | Death | |
|---|---|---|---|---|---|---|---|---|---|
| no screening | 10.95 | 26.27 | 27.79 | 10.93 | 0.03 | 0.04 | 0.03 | 0.06 | 23.90 |
| Low-risk | 33.11 | 32.04 | 11.329 | 0.08 | 0.03 | 0.009 | 0.001 | 0.001 | 23.40 |
| moderate-risk | 30.17 | 31.9 | 13.85 | 0.10 | 0.14 | 0.04 | 0.005 | 0.005 | 23.79 |
| High-risk | 22.49 | 29.99 | 21.33 | 0.16 | 0.56 | 0.16 | 0.02 | 0.02 | 25.27 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zheng, P.; Liu, J. Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer. Curr. Oncol. 2023, 30, 1132-1145. https://doi.org/10.3390/curroncol30010086
Zheng P, Liu J. Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer. Current Oncology. 2023; 30(1):1132-1145. https://doi.org/10.3390/curroncol30010086
Chicago/Turabian StyleZheng, Peiyu, and Jinchun Liu. 2023. "Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer" Current Oncology 30, no. 1: 1132-1145. https://doi.org/10.3390/curroncol30010086
APA StyleZheng, P., & Liu, J. (2023). Cost-Effectiveness Analysis of Hp and New Gastric Cancer Screening Scoring System for Screening and Prevention of Gastric Cancer. Current Oncology, 30(1), 1132-1145. https://doi.org/10.3390/curroncol30010086




