Impact of COVID-19 Pandemic on Non-Small Cell Lung Cancer Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data Source
2.2. Patient Population
2.3. Statistical Analyses
2.3.1. Measurement of Diagnosis and Treatment Encounters
2.3.2. Measurement of Time to Treatment Initiation (TTI)
2.3.3. Measurement of Delay in Administration of Immunotherapy
2.3.4. Measurement of Dosing Schedule Switching Rate
3. Results
3.1. Study Attrition
3.2. Baseline Characteristics
3.3. Changes in Cancer Care Utilization before vs. during COVID-19 Timeframe
3.4. Time to Treatment Initiation before vs. during COVID-19 Timeframe
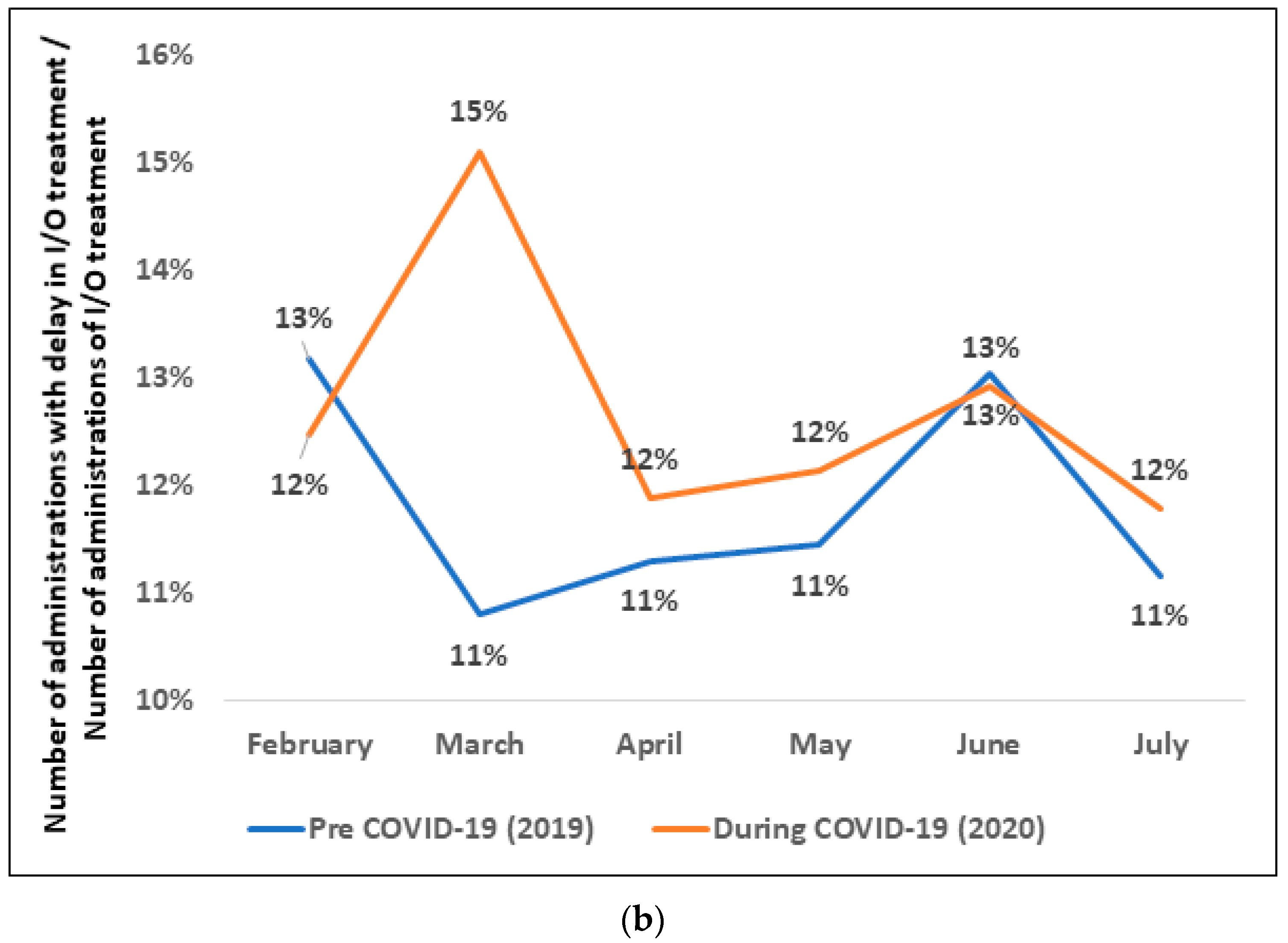
3.5. Delay in Immunotherapy Treatment before vs. during COVID-19 Timeframe
3.6. Switching Rate from Shorter to Longer Immunotherapy Dosing Schedules before vs. during COVID-19 Timeframe
4. Discussion
Strengths and Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burki, T.K. Cancer guidelines during the COVID-19 pandemic. Lancet Oncol. 2020, 21, 629–630. [Google Scholar] [CrossRef] [PubMed]
- National Cancer Institute Surveillance, Epidemiology and End Results Program. SEER*Explorer. Available online: https://seer.cancer.gov/explorer/ (accessed on 3 June 2022).
- American Cancer Society. Cancer Facts and Figures 2021. Special Section: COVID-19 and Cancer. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2021/special-section-covid19-and-cancer-2021.pdf (accessed on 3 June 2022).
- Elkrief, A.; Kazandjian, S.; Bouganim, N. Changes in Lung Cancer Treatment as a Result of the Coronavirus Disease 2019 Pandemic. JAMA Oncol. 2020, 6, 1805–1806. [Google Scholar] [CrossRef] [PubMed]
- Onder, G.; Rezza, G.; Brusaferro, S. Case-Fatality Rate and Characteristics of Patients Dying in Relation to COVID-19 in Italy. JAMA 2020, 323, 1775–1776. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Guan, W.; Chen, R.; Wang, W.; Li, J.; Xu, K.; Li, C.; Ai, Q.; Lu, W.; Liang, H.; et al. Cancer patients in SARS-CoV-2 infection: A nationwide analysis in China. Lancet Oncol. 2020, 21, 335–337. [Google Scholar] [CrossRef] [PubMed]
- Yekedüz, E.; Utkan, G.; Ürün, Y. A systematic review and meta-analysis: The effect of active cancer treatment on severity of COVID-19. Eur. J. Cancer 2020, 141, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Kalathil, S.G.; Thanavala, Y. High immunosuppressive burden in cancer patients: A major hurdle for cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 813–819. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Cancer Screening, Diagnosis, Staging, and Surveillance. Available online: https://www.asco.org/asco-coronavirus-resources/care-individuals-cancer-during-covid-19/cancer-screening-diagnosis-staging (accessed on 3 June 2022).
- Centers for Medicare & Medicaid Services. Non-Emergent, Elective Medical Services, and Treatment Recommendations. Available online: https://www.cms.gov/files/document/cms-non-emergent-elective-medical-recommendations.pdf (accessed on 23 June 2022).
- Bhalla, S.; Bakouny, Z.; Schmidt, A.L.; Labaki, C.; Steinharter, J.A.; Tremblay, D.A.; Awad, M.M.; Kessler, A.J.; Haddad, R.I.; Evans, M.; et al. Care disruptions among patients with lung cancer: A COVID-19 and cancer outcomes study. Lung Cancer 2021, 160, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Steel, J.L.; Amin, A.; Peyser, T.; Olejniczak, D.; Antoni, M.; Carney, M.; Tillman, E.; Hecht, C.L.; Pandya, N.; Miceli, J.; et al. The benefits and consequences of the COVID-19 pandemic for patients diagnosed with cancer and their family caregivers. Psychooncology 2022, 31, 1003–1012. [Google Scholar] [CrossRef] [PubMed]
- London, J.W.; Fazio-Eynullayeva, E.; Palchuk, M.B.; Sankey, P.; McNair, C. Effects of the COVID-19 Pandemic on Cancer-Related Patient Encounters. JCO Clin. Cancer Inform. 2020, 4, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Patt, D.; Gordan, L.; Diaz, M.; Okon, T.; Grady, L.; Harmison, M.; Markward, N.; Sullivan, M.; Peng, J.; Zhou, A. Impact of COVID-19 on Cancer Care: How the Pandemic Is Delaying Cancer Diagnosis and Treatment for American Seniors. JCO Clin. Cancer Inform. 2020, 4, 1059–1071. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Berman, A.T.; Marmarelis, M.E.; Haas, A.R.; Feigenberg, S.J.; Braun, J.; Ciunci, C.A.; Bauml, J.M.; Cohen, R.B.; Kucharczuk, J.C.; et al. Management of Lung Cancer During the COVID-19 Pandemic. JCO Oncol. Pract. 2020, 16, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Papautsky, E.L.; Hamlish, T. Patient-reported treatment delays in breast cancer care during the COVID-19 pandemic. Breast Cancer Res. Treat. 2020, 184, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Tecentriq Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/761041lbl.pdf (accessed on 8 June 2022).
- US Food and Drug Administration. Modification of the Dosage Regimen for Nivolumab. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/modification-dosage-regimen-nivolumab (accessed on 8 June 2022).
- US Food and Drug Administration. Pembrolizumab (Keytruda) 5-10-2017. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/pembrolizumab-keytruda-5-10-2017#:~:text=On%20May%2010%2C%202017%2C%20the,cell%20lung%20cancer%20(NSCLC) (accessed on 8 June 2022).
- US Food and Drug Administration. FDA Approves Atezolizumab for First-Line Treatment of Metastatic NSCLC with High PD-L1 Expression. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-atezolizumab-first-line-treatment-metastatic-nsclc-high-pd-l1-expression (accessed on 8 June 2022).
- US Food and Drug Administration. FDA Approves New Dosing Regimen for Pembrolizumab. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-new-dosing-regimen-pembrolizumab (accessed on 8 June 2022).
- OPDIVO (Nivolumab) Injection. Highlights of Prescribing Information. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/125554s058lbl.pdf (accessed on 8 June 2022).
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- American Society of Clinical Oncology. Cancer Treatment and Supportive Care. Available online: https://www.asco.org/covid-resources/patient-care-info/cancer-treatment-supportive-care (accessed on 3 June 2022).
- Shankar, A.; Saini, D.; Bhandari, R.; Bharati, S.J.; Kumar, S.; Yadav, G.; Durga, T.; Goyal, N. Lung cancer management challenges amidst COVID-19 pandemic: Hope lives here. Lung Cancer Manag. 2020, 9, Lmt33. [Google Scholar] [CrossRef] [PubMed]
- Ontada. Available online: https://www.ontada.com/ (accessed on 8 June 2022).
- The US Oncology Network. Available online: https://www.usoncology.com/our-company (accessed on 11 September 2020).
- Centers for Disease Control and Prevention. CDC Museum COVID-19 Timeline. Available online: https://www.cdc.gov/museum/timeline/covid19.html (accessed on 3 June 2022).
- NPR. Coronavirus Updates. All 50 U.S. States Have Now Started to Reopen, Easing COVID-19 Shutdown. Available online: https://www.npr.org/sections/coronavirus-live-updates/2020/05/20/859723846/all-50-u-s-states-have-now-started-to-reopen-easing-covid-19-shutdown (accessed on 14 July 2022).
- Lou, J.; Kooragayala, K.; Williams, J.P.; Sandilos, G.; Butchy, M.V.; Yoon-Flannery, K.; Kwiatt, M.; Hong, Y.K.; Shersher, D.D.; Burg, J.M. The Early Impact of the COVID-19 Pandemic on Lung, Colorectal, and Breast Cancer Screening and Treatment at a Tertiary Cancer Center. Am. J. Clin. Oncol. 2022, 45, 381–390. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lam, A.Y.; Jensen, C.D.; Marks, A.R.; Badalov, J.; Layefsky, E.; Kao, K.; Ho, N.J.; Schottinger, J.E.; Ghai, N.R.; et al. Impact of the COVID-19 Pandemic on Fecal Immunochemical Testing, Colonoscopy Services, and Colorectal Neoplasia Detection in a Large United States Community-based Population. Gastroenterology 2022, 163, 723–731.e726. [Google Scholar] [CrossRef] [PubMed]
- Parikh, R.B.; Takvorian, S.U.; Vader, D.; Wileyto, E.P.; Clark, A.S.; Lee, D.J.; Goyal, G.; Rocque, G.B.; Dotan, E.; Geynisman, D.M.; et al. Impact of the COVID-19 Pandemic on Treatment Patterns for Patients with Metastatic Solid Cancer in the United States. J. Natl. Cancer Inst. 2022, 114, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Neilson, L.; Kohli, M.; Munshi, K.D.; Peasah, S.K.; Henderson, R.; Passero, V.; Good, C.B. Impact of the COVID-19 pandemic on new starts to oral oncology medications in the US. J. Oncol. Pharm. Pract. Off. Publ. Int. Soc. Oncol. Pharm. Pract. 2022, 10781552211073778. [Google Scholar] [CrossRef] [PubMed]
- Strohbehn, G.W.; Holleman, R.; Burns, J.; Klamerus, M.L.; Kelley, M.J.; Kerr, E.A.; Ramnath, N.; Hofer, T.P. Adoption of Extended-Interval Dosing of Single-Agent Pembrolizumab and Comparative Effectiveness vs. Standard Dosing in Time-to-Treatment Discontinuation. JAMA Oncol. 2022, 8, 1663–1667. [Google Scholar] [CrossRef] [PubMed]
- Blumenthal, G.M.; Gong, Y.; Kehl, K.; Mishra-Kalyani, P.; Goldberg, K.B.; Khozin, S.; Kluetz, P.G.; Oxnard, G.R.; Pazdur, R. Analysis of time-to-treatment discontinuation of targeted therapy, immunotherapy, and chemotherapy in clinical trials of patients with non-small-cell lung cancer. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2019, 30, 830–838. [Google Scholar] [CrossRef]






| Baseline Characteristics | Stage IV NSCLC Patients (N = 7018) | Newly Diagnosed NSCLC Patients (N = 2579) | New and Continuing I/O Treated Patients (N = 3519) | Switchers (N = 8) |
|---|---|---|---|---|
| Gender—no. (%) | ||||
| Male | 3512 (50) | 1355 (53) | 1890 (54) | 6 (75) |
| Female | 3501 (50) | 1223 (47) | 1628 (46) | 2 (25) |
| Not documented | 5 (0) | 1 (0) | 1 (0) | 0 (0) |
| Age at Stage IV NSCLC diagnosis—years | ||||
| Mean (SD) | 69 (10) | 70 (10) | 69 (10) | 67 (7) |
| Race—no. (%) | ||||
| White | 4887 (70) | 1697 (66) | 2531 (72) | 7 (88) |
| Black or African American | 652 (9) | 237 (9) | 338 (10) | 0 (0) |
| Asian | 222 (3) | 67 (3) | 73 (2) | 1 (13) |
| Other | 118 (2) | 36 (1) | 46 (1) | 0 (0) |
| Not documented | 1139 (16) | 542 (21) | 531 (15) | 0 (0) |
| Region—no. (%) | ||||
| South | 2744 (39) | 1041 (40) | 1441 (41) | 4 (50) |
| Midwest | 1919 (27) | 715 (28) | 977 (28) | 1 (13) |
| West | 1944 (28) | 681 (26) | 899 (25) | 3 (38) |
| Northeast | 410 (6) | 141 (6) | 202 (6) | 0 (0) |
| Not documented | 1 (0) | 1 (0) | 0 (0) | 0 (0) |
| Histology Type—no. (%) | ||||
| Non-Squamous | 4877 (69) | 1761 (68) | 2443 (69) | 3 (38) |
| Squamous | 1447 (21) | 575 (22) | 834 (24) | 4 (50) |
| Not documented | 694 (10) | 243 (10) | 242 (7) | 1 (13) |
| ECOG PS score—no. (%) | ||||
| 0 | 876 (13) | 314 (12) | 489 (14) | 0 (0) |
| 1 | 2761 (39) | 1061 (41) | 1516 (43) | 4 (50) |
| 2+ | 936 (13) | 457 (18) | 438 (12) | 1 (13) |
| Not documented | 2445 (35) | 747 (29) | 1076 (31) | 3 (38) |
| Smoking history—no. (%) | ||||
| Former tobacco use | 3937 (56) | 1433 (56) | 2091 (59) | 5 (63) |
| Current tobacco use | 1441 (20) | 550 (21) | 800 (23) | 0 (0) |
| No history of tobacco use | 1237 (18) | 403 (16) | 442 (13) | 1 (13) |
| Not documented | 403 (6) | 193 (7) | 186 (5) | 2 (25) |
| Variable | Timing of Diagnosis | DF | t Value | p Value a | ||
|---|---|---|---|---|---|---|
| Total | Pre-COVID-19 (N = 986) | During-COVID-19 (N = 869) | ||||
| Gender, n | 2 | 0.89 | 0.644 | |||
| Female | 890 | 472 | 418 | |||
| Male | 964 | 513 | 451 | |||
| Not documented | 1 | 1 | 0 | |||
| Race, n | 4 | 7 | 0.136 | |||
| White | 1212 | 652 | 560 | |||
| Black | 178 | 97 | 81 | |||
| Asian | 54 | 31 | 23 | |||
| Other | 27 | 19 | 8 | |||
| Not documented | 384 | 187 | 197 | |||
| Histology, n | 2 | 3.65 | 0.161 | |||
| Nonsquamous | 1339 | 728 | 611 | |||
| Squamous | 416 | 204 | 212 | |||
| Not documented | 100 | 54 | 46 | |||
| ECOG PS, n | 4 | 11.45 | 0.022 | |||
| 0 | 266 | 150 | 116 | |||
| 1 | 849 | 467 | 382 | |||
| 2 | 240 | 133 | 107 | |||
| 3 | 49 | 19 | 30 | |||
| Not documented | 451 | 217 | 234 | |||
| Type of treatment, n | 3 | 1.95 | 0.582 | |||
| Chemo + I/O | 937 | 513 | 424 | |||
| Chemo | 258 | 133 | 125 | |||
| I/O | 415 | 213 | 202 | |||
| Targeted therapy | 245 | 127 | 118 | |||
| Factor | Estimated Difference in Days | Standard Error | t Value | p-Value a | Unadjusted R2 Value |
|---|---|---|---|---|---|
| Timing of diagnosis | 0.008 | ||||
| Pre COVID-19 | Reference | -- | -- | -- | |
| During COVID-19 | −4.6 | 1.29 | −3.55 | 0.0004 | |
| Type of treatment | 0.013 | ||||
| I/O | Reference | -- | -- | -- | |
| Targeted therapy | −9.7 | 2.28 | −4.26 | <0.0001 | |
| Chemotherapy | −5.9 | 2.24 | −2.64 | 0.0084 | |
| Chemo + I/O combo | −6 | 1.64 | −3.69 | 0.0002 | |
| ECOG PS score | 0.001 | ||||
| 0 | Reference | -- | -- | -- | |
| 1 | 1.9 | 1.93 | 0.97 | 0.3308 | |
| 2 | 2.3 | 2.45 | 0.95 | 0.3414 | |
| 3 | −2.1 | 4.31 | −0.48 | 0.633 | |
| Not documented | 1.7 | 2.11 | 0.81 | 0.4158 | |
| Age (mean) | −0.1 | 0.06 | −1.6 | 0.1108 | 0.035 |
| Gender | 0.001 | ||||
| Male | Reference | -- | -- | -- | |
| Female | 0.7 | 1.31 | 0.53 | 0.5958 | |
| Not documented | −18.8 | 26.64 | −0.71 | 0.4807 | |
| Race | 0.003 | ||||
| White | Reference | -- | -- | -- | |
| Asian | −4.2 | 3.88 | −1.08 | 0.2808 | |
| Black | −2 | 2.31 | −0.87 | 0.3827 | |
| Other race | 3.2 | 5.5 | 0.59 | 0.5562 | |
| Not documented | −1.9 | 1.6 | −1.2 | 0.2308 | |
| Histology type | 0.0004 | ||||
| Nonsquamous | Reference | -- | -- | -- | |
| Squamous | −1.1 | 1.61 | −0.65 | 0.5138 | |
| Not documented | 1.05 | 2.96 | 0.35 | 0.7235 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhai, Y.; Chopra, P.; Kang, D.; Robert, N.J.; Zhang, W. Impact of COVID-19 Pandemic on Non-Small Cell Lung Cancer Care. Curr. Oncol. 2023, 30, 769-785. https://doi.org/10.3390/curroncol30010059
Zhai Y, Chopra P, Kang D, Robert NJ, Zhang W. Impact of COVID-19 Pandemic on Non-Small Cell Lung Cancer Care. Current Oncology. 2023; 30(1):769-785. https://doi.org/10.3390/curroncol30010059
Chicago/Turabian StyleZhai, YiYuan, Pooja Chopra, David Kang, Nicholas J. Robert, and Wei Zhang. 2023. "Impact of COVID-19 Pandemic on Non-Small Cell Lung Cancer Care" Current Oncology 30, no. 1: 769-785. https://doi.org/10.3390/curroncol30010059
APA StyleZhai, Y., Chopra, P., Kang, D., Robert, N. J., & Zhang, W. (2023). Impact of COVID-19 Pandemic on Non-Small Cell Lung Cancer Care. Current Oncology, 30(1), 769-785. https://doi.org/10.3390/curroncol30010059




