Radiation Therapy for Retroperitoneal Sarcomas: A Strass-Ful Situation
Abstract
1. Introduction
2. Preoperative Radiation Therapy in the Management of Retroperitoneal Sarcomas
3. Postoperative Radiation Therapy in the Management of Retroperitoneal Sarcomas
4. Intraoperative Radiation in the Management of Retroperitoneal Sarcomas
5. Radiation Modality
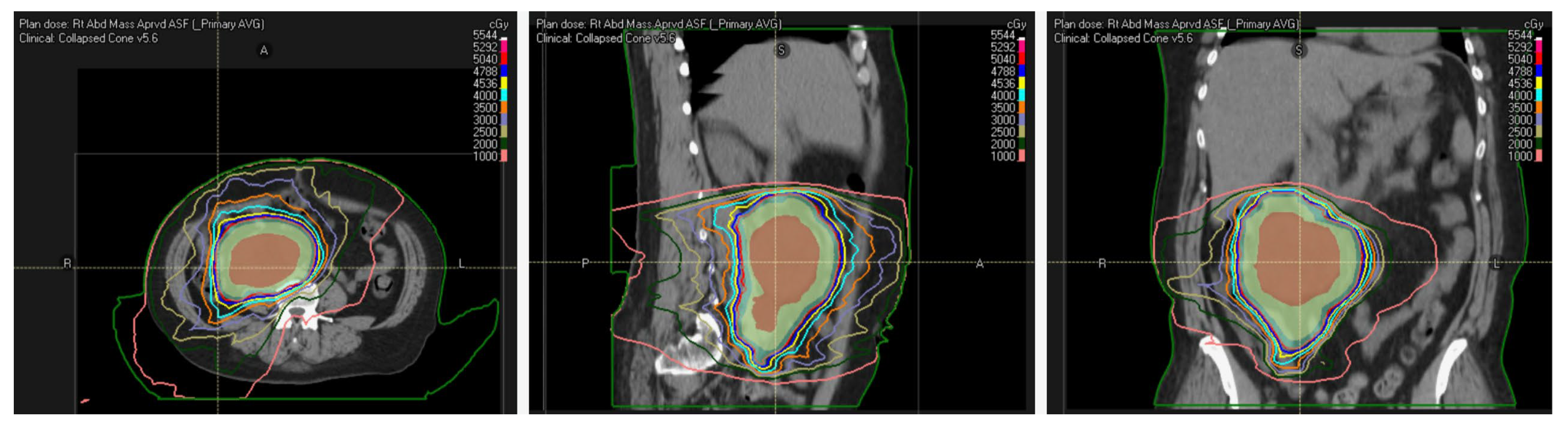
6. Radiation Considerations
7. Radiation and Immune Checkpoint Inhibition
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lawrence, W., Jr.; Donegan, W.L.; Natarajan, N.; Mettlin, C.; Beart, R.; Winchester, D. Adult soft tissue sarcomas. A pattern of care survey of the American College of Surgeons. Ann. Surg. 1987, 205, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Porter, G.A.; Baxter, N.N.; Pisters, P.W. Retroperitoneal sarcoma: A population-based analysis of epidemiology, surgery, and radiotherapy. Cancer 2006, 106, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Gronchi, A.; Casali, P.G.; Fiore, M.; Mariani, L.; Lo Vullo, S.; Bertulli, R.; Colecchia, M.; Lozza, L.; Olmi, P.; Santinami, M.; et al. Retroperitoneal soft tissue sarcomas: Patterns of recurrence in 167 patients treated at a single institution. Cancer 2004, 100, 2448–2455. [Google Scholar] [CrossRef] [PubMed]
- Lewis, J.J.; Leung, D.; Woodruff, J.M.; Brennan, M.F. Retroperitoneal soft-tissue sarcoma: Analysis of 500 patients treated and followed at a single institution. Ann. Surg. 1998, 228, 355–365. [Google Scholar] [CrossRef]
- Stoeckle, E.; Coindre, J.M.; Bonvalot, S.; Kantor, G.; Terrier, P.; Bonichon, F.; Nguyen Bui, B.; French Federation of Cancer Centers Sarcoma Group. Prognostic factors in retroperitoneal sarcoma: A multivariate analysis of a series of 165 patients of the French Cancer Center Federation Sarcoma Group. Cancer 2001, 92, 359–368. [Google Scholar] [CrossRef]
- Lee, A.T.J.; Thway, K.; Huang, P.H.; Jones, R.L. Clinical and Molecular Spectrum of Liposarcoma. J. Clin. Oncol. 2018, 36, 151–159. [Google Scholar] [CrossRef]
- Binh, M.B.; Sastre-Garau, X.; Guillou, L.; de Pinieux, G.; Terrier, P.; Lagace, R.; Aurias, A.; Hostein, I.; Coindre, J.M. MDM2 and CDK4 immunostainings are useful adjuncts in diagnosing well-differentiated and dedifferentiated liposarcoma subtypes: A comparative analysis of 559 soft tissue neoplasms with genetic data. Am. J. Surg. Pathol. 2005, 29, 1340–1347. [Google Scholar] [CrossRef]
- Singer, S.; Corson, J.M.; Demetri, G.D.; Healey, E.A.; Marcus, K.; Eberlein, T.J. Prognostic factors predictive of survival for truncal and retroperitoneal soft-tissue sarcoma. Ann. Surg. 1995, 221, 185–195. [Google Scholar] [CrossRef]
- Singer, S.; Antonescu, C.R.; Riedel, E.; Brennan, M.F. Histologic subtype and margin of resection predict pattern of recurrence and survival for retroperitoneal liposarcoma. Ann. Surg. 2003, 238, 358–370. [Google Scholar] [CrossRef]
- Parkes, A.; Urquiola, E.; Bhosale, P.; Lin, H.; Watson, K.; Wang, W.L.; Feig, B.; Torres, K.; Roland, C.L.; Conley, A.P.; et al. PET/CT Imaging as a Diagnostic Tool in Distinguishing Well-Differentiated versus Dedifferentiated Liposarcoma. Sarcoma 2020, 2020, 8363986. [Google Scholar] [CrossRef]
- van Houdt, W.J.; Raut, C.P.; Bonvalot, S.; Swallow, C.J.; Haas, R.; Gronchi, A. New research strategies in retroperitoneal sarcoma. The case of TARPSWG, STRASS and RESAR: Making progress through collaboration. Curr. Opin. Oncol. 2019, 31, 310–316. [Google Scholar] [CrossRef]
- Raut, C.P.; Bonvalot, S.; Gronchi, A. A call to action: Why sarcoma surgery needs to be centralized. Cancer 2018, 124, 4452–4454. [Google Scholar] [CrossRef]
- Salerno, K.E.; Alektiar, K.M.; Baldini, E.H.; Bedi, M.; Bishop, A.J.; Bradfield, L.; Chung, P.; DeLaney, T.F.; Folpe, A.; Kane, J.M.; et al. Radiation Therapy for Treatment of Soft Tissue Sarcoma in Adults: Executive Summary of an ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2021, 11, 339–351. [Google Scholar] [CrossRef]
- Swallow, C.J.; Strauss, D.C.; Bonvalot, S.; Rutkowski, P.; Desai, A.; Gladdy, R.A.; Gonzalez, R.; Gyorki, D.E.; Fairweather, M.; van Houdt, W.J.; et al. Management of Primary Retroperitoneal Sarcoma (RPS) in the Adult: An Updated Consensus Approach from the Transatlantic Australasian RPS Working Group. Ann. Surg. Oncol. 2021, 28, 7873–7888. [Google Scholar] [CrossRef]
- Avances, C.; Mottet, N.; Mahatmat, A.; Chapuis, E.; Serre, I.; Culine, S. Prognostic factors for first recurrence in patients with retroperitoneal sarcoma. Urol. Oncol. 2006, 24, 94–96. [Google Scholar] [CrossRef]
- Catton, C.N.; O’Sullivan, B.; Kotwall, C.; Cummings, B.; Hao, Y.; Fornasier, V. Outcome and prognosis in retroperitoneal soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 1994, 29, 1005–1010. [Google Scholar] [CrossRef]
- Gronchi, A.; Miceli, R.; Allard, M.A.; Callegaro, D.; Le Pechoux, C.; Fiore, M.; Honore, C.; Sanfilippo, R.; Coppola, S.; Stacchiotti, S.; et al. Personalizing the approach to retroperitoneal soft tissue sarcoma: Histology-specific patterns of failure and postrelapse outcome after primary extended resection. Ann. Surg. Oncol. 2015, 22, 1447–1454. [Google Scholar] [CrossRef]
- Pisters, P.W.; Harrison, L.B.; Leung, D.H.; Woodruff, J.M.; Casper, E.S.; Brennan, M.F. Long-term results of a prospective randomized trial of adjuvant brachytherapy in soft tissue sarcoma. J. Clin. Oncol. 1996, 14, 859–868. [Google Scholar] [CrossRef]
- Rosenberg, S.A.; Tepper, J.; Glatstein, E.; Costa, J.; Baker, A.; Brennan, M.; DeMoss, E.V.; Seipp, C.; Sindelar, W.F.; Sugarbaker, P.; et al. The treatment of soft-tissue sarcomas of the extremities: Prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann. Surg. 1982, 196, 305–315. [Google Scholar] [CrossRef]
- Yang, J.C.; Chang, A.E.; Baker, A.R.; Sindelar, W.F.; Danforth, D.N.; Topalian, S.L.; DeLaney, T.; Glatstein, E.; Steinberg, S.M.; Merino, M.J.; et al. Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J. Clin. Oncol. 1998, 16, 197–203. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, B.; Davis, A.M.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Kandel, R.; Goddard, K.; Sadura, A.; et al. Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: A randomised trial. Lancet 2002, 359, 2235–2241. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.J.; Catton, C.N.; O’Sullivan, B.; Couture, J.; Heisler, R.L.; Kandel, R.A.; Swallow, C.J. Initial results of a trial of preoperative external-beam radiation therapy and postoperative brachytherapy for retroperitoneal sarcoma. Ann. Surg. Oncol. 2002, 9, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Pisters, P.W.; Ballo, M.T.; Fenstermacher, M.J.; Feig, B.W.; Hunt, K.K.; Raymond, K.A.; Burgess, M.A.; Zagars, G.K.; Pollock, R.E.; Benjamin, R.S.; et al. Phase I trial of preoperative concurrent doxorubicin and radiation therapy, surgical resection, and intraoperative electron-beam radiation therapy for patients with localized retroperitoneal sarcoma. J. Clin. Oncol. 2003, 21, 3092–3097. [Google Scholar] [CrossRef]
- Pawlik, T.M.; Pisters, P.W.; Mikula, L.; Feig, B.W.; Hunt, K.K.; Cormier, J.N.; Ballo, M.T.; Catton, C.N.; Jones, J.J.; O’Sullivan, B.; et al. Long-term results of two prospective trials of preoperative external beam radiotherapy for localized intermediate- or high-grade retroperitoneal soft tissue sarcoma. Ann. Surg. Oncol. 2006, 13, 508–517. [Google Scholar] [CrossRef]
- Hull, M.A.; Molina, G.; Niemierko, A.; Haynes, A.B.; Jacobson, A.; Bernstein, K.A.; Chen, Y.L.; DeLaney, T.F.; Mullen, J.T. Improved local control with an aggressive strategy of preoperative (with or without intraoperative) radiation therapy combined with radical surgical resection for retroperitoneal sarcoma. J. Surg. Oncol. 2017, 115, 746–751. [Google Scholar] [CrossRef]
- Kelly, K.J.; Yoon, S.S.; Kuk, D.; Qin, L.X.; Dukleska, K.; Chang, K.K.; Chen, Y.L.; Delaney, T.F.; Brennan, M.F.; Singer, S. Comparison of Perioperative Radiation Therapy and Surgery Versus Surgery Alone in 204 Patients With Primary Retroperitoneal Sarcoma: A Retrospective 2-Institution Study. Ann. Surg. 2015, 262, 156–162. [Google Scholar] [CrossRef]
- Nussbaum, D.P.; Rushing, C.N.; Lane, W.O.; Cardona, D.M.; Kirsch, D.G.; Peterson, B.L.; Blazer, D.G., 3rd. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: A case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016, 17, 966–975. [Google Scholar] [CrossRef]
- ASGOG Protocol Z9031: A Phase III Randomized Study of Preoperative.Radiation Plus Surgery Versus Surgery Alone for Patients with Retroperitoneal Sarcomas (RPS). Available online: https://clinicaltrials.gov/ct2/show/NCT00091351 (accessed on 18 September 2022).
- Bonvalot, S.; Gronchi, A.; Le Pechoux, C.; Swallow, C.J.; Strauss, D.; Meeus, P.; van Coevorden, F.; Stoldt, S.; Stoeckle, E.; Rutkowski, P.; et al. Preoperative radiotherapy plus surgery versus surgery alone for patients with primary retroperitoneal sarcoma (EORTC-62092: STRASS): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1366–1377. [Google Scholar] [CrossRef]
- Chowdhary, M.; Spraker, M.B. Preoperative radiotherapy for retroperitoneal sarcoma. Lancet Oncol. 2021, 22, e2. [Google Scholar] [CrossRef]
- DeLaney, T.; Mullen, J.T.; Wang, D.; Goldberg, S.I.; Kirsch, D.G. Preoperative radiotherapy for retroperitoneal sarcoma. Lancet Oncol. 2021, 22, e1. [Google Scholar] [CrossRef]
- Callegaro, D.; Raut, C.P.; Ajayi, T.; Strauss, D.; Bonvalot, S.; Ng, D.; Stoeckle, E.; Fairweather, M.; Rutkowski, P.; van Houdt, W.J.; et al. Preoperative Radiotherapy in Patients With Primary Retroperitoneal Sarcoma: EORTC-62092 Trial (STRASS) Versus Off-trial (STREXIT) Results. Ann. Surg. 2022. [Google Scholar] [CrossRef]
- Haas, R.; Stelmes, J.J.; Zaffaroni, F.; Sauve, N.; Clementel, E.; Bar-Deroma, R.; Le Pechoux, C.; Litiere, S.; Marreaud, S.; Alyamani, N.; et al. Critical impact of radiotherapy protocol compliance and quality in the treatment of retroperitoneal sarcomas: Results from the EORTC 62092-22092 STRASS trial. Cancer 2022, 128, 2796–2805. [Google Scholar] [CrossRef]
- Bishop, A.J.; Roland, C.L. Is quality related to quantity: Interpreting the results of STRASS in the context of noncompliant radiotherapy. Cancer 2022, 128, 2701–2703. [Google Scholar] [CrossRef]
- Bishop, A.J.; Zagars, G.K.; Torres, K.E.; Hunt, K.K.; Cormier, J.N.; Feig, B.W.; Guadagnolo, B.A. Combined Modality Management of Retroperitoneal Sarcomas: A Single-Institution Series of 121 Patients. Int. J. Radiat. Oncol. Biol. Phys. 2015, 93, 158–165. [Google Scholar] [CrossRef]
- Davis, A.M.; O’Sullivan, B.; Turcotte, R.; Bell, R.; Catton, C.; Chabot, P.; Wunder, J.; Hammond, A.; Benk, V.; Kandel, R.; et al. Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother. Oncol. 2005, 75, 48–53. [Google Scholar] [CrossRef]
- Zagars, G.K.; Ballo, M.T.; Pisters, P.W.; Pollock, R.E.; Patel, S.R.; Benjamin, R.S. Preoperative vs. postoperative radiation therapy for soft tissue sarcoma: A retrospective comparative evaluation of disease outcome. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 482–488. [Google Scholar] [CrossRef]
- Li, X.; Wu, T.; Xiao, M.; Wu, S.; Min, L.; Luo, C. Adjuvant therapy for retroperitoneal sarcoma: A meta-analysis. Radiat. Oncol. 2021, 16, 196. [Google Scholar] [CrossRef]
- Kim, H.J.; Koom, W.S.; Cho, J.; Kim, H.S.; Suh, C.O. Efficacy of Postoperative Radiotherapy Using Modern Techniques in Patients with Retroperitoneal Soft Tissue Sarcoma. Yonsei Med. J. 2018, 59, 1049–1056. [Google Scholar] [CrossRef]
- Haas, R.L.; Floot, B.G.J.; Scholten, A.N.; van der Graaf, W.T.A.; van Houdt, W.; Schrage, Y.; van de Ven, M.; Bovee, J.; van Coevorden, F.; Vens, C. Cellular Radiosensitivity of Soft Tissue Sarcoma. Radiat. Res. 2021, 196, 23–30. [Google Scholar] [CrossRef]
- Reitan, J.B.; Kaalhus, O. Radiotherapy of liposarcomas. Br. J. Radiol. 1980, 53, 969–975. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, W.F.; Kinsella, T.J.; Chen, P.W.; DeLaney, T.F.; Tepper, J.E.; Rosenberg, S.A.; Glatstein, E. Intraoperative radiotherapy in retroperitoneal sarcomas. Final results of a prospective, randomized, clinical trial. Arch. Surg. 1993, 128, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Alektiar, K.M.; Hu, K.; Anderson, L.; Brennan, M.F.; Harrison, L.B. High-dose-rate intraoperative radiation therapy (HDR-IORT) for retroperitoneal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2000, 47, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Dziewirski, W.; Rutkowski, P.; Nowecki, Z.I.; Salamacha, M.; Morysinski, T.; Kulik, A.; Kawczynska, M.; Kasprowicz, A.; Lyczek, J.; Ruka, W. Surgery combined with intraoperative brachytherapy in the treatment of retroperitoneal sarcomas. Ann. Surg. Oncol. 2006, 13, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Krempien, R.; Roeder, F.; Oertel, S.; Weitz, J.; Hensley, F.W.; Timke, C.; Funk, A.; Lindel, K.; Harms, W.; Buchler, M.W.; et al. Intraoperative electron-beam therapy for primary and recurrent retroperitoneal soft-tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 773–779. [Google Scholar] [CrossRef]
- Petersen, I.A.; Haddock, M.G.; Donohue, J.H.; Nagorney, D.M.; Grill, J.P.; Sargent, D.J.; Gunderson, L.L. Use of intraoperative electron beam radiotherapy in the management of retroperitoneal soft tissue sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2002, 52, 469–475. [Google Scholar] [CrossRef]
- Gieschen, H.L.; Spiro, I.J.; Suit, H.D.; Ott, M.J.; Rattner, D.W.; Ancukiewicz, M.; Willett, C.G. Long-term results of intraoperative electron beam radiotherapy for primary and recurrent retroperitoneal soft tissue sarcoma. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 127–131. [Google Scholar] [CrossRef]
- Pierie, J.P.; Betensky, R.A.; Choudry, U.; Willett, C.G.; Souba, W.W.; Ott, M.J. Outcomes in a series of 103 retroperitoneal sarcomas. Eur. J. Surg. Oncol. 2006, 32, 1235–1241. [Google Scholar] [CrossRef]
- Roeder, F.; Ulrich, A.; Habl, G.; Uhl, M.; Saleh-Ebrahimi, L.; Huber, P.E.; Schulz-Ertner, D.; Nikoghosyan, A.V.; Alldinger, I.; Krempien, R.; et al. Clinical phase I/II trial to investigate preoperative dose-escalated intensity-modulated radiation therapy (IMRT) and intraoperative radiation therapy (IORT) in patients with retroperitoneal soft tissue sarcoma: Interim analysis. BMC Cancer 2014, 14, 617. [Google Scholar] [CrossRef]
- Purdy, J.A. 3D treatment planning and intensity-modulated radiation therapy. Oncology 1999, 13, 155–168. [Google Scholar]
- Paumier, A.; Le Pechoux, C.; Beaudre, A.; Negretti, L.; Ferreira, I.; Roberti, E.; Brahim, J.; Lefkopoulos, D.; Daly-Schweitzer, N.; Bourhis, J.; et al. IMRT or conformal radiotherapy for adjuvant treatment of retroperitoneal sarcoma? Radiother. Oncol. 2011, 99, 73–78. [Google Scholar] [CrossRef]
- Bossi, A.; De Wever, I.; Van Limbergen, E.; Vanstraelen, B. Intensity modulated radiation-therapy for preoperative posterior abdominal wall irradiation of retroperitoneal liposarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2007, 67, 164–170. [Google Scholar] [CrossRef]
- Taggar, A.S.; Graham, D.; Kurien, E.; Grafe, J.L. Volumetric-modulated arc therapy versus intensity-modulated radiotherapy for large volume retroperitoneal sarcomas: A comparative analysis of dosimetric and treatment delivery parameters. J. Appl. Clin. Med. Phys. 2018, 19, 276–281. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155–R209. [Google Scholar] [CrossRef]
- Chung, C.; Trofimov, A.; Adams, J.; Kung, J.; Kirsch, D.G.; Yoon, S.; Doppke, K.; Bortfeld, T.; Delaney, T.F. Comparison of 3D Conformal Proton Therapy, Intensity-Modulated Proton Therapy, and Intensity-Modulated Photon Therapy for Retroperitoneal Sarcoma. Sarcoma 2022, 2022, 5540615. [Google Scholar] [CrossRef]
- Guttmann, D.M.; Frick, M.A.; Carmona, R.; Deville, C., Jr.; Levin, W.P.; Berman, A.T.; Chinniah, C.; Hahn, S.M.; Plastaras, J.P.; Simone, C.B., 2nd. A prospective study of proton reirradiation for recurrent and secondary soft tissue sarcoma. Radiother. Oncol. 2017, 124, 271–276. [Google Scholar] [CrossRef]
- Kanai, T.; Endo, M.; Minohara, S.; Miyahara, N.; Koyama-ito, H.; Tomura, H.; Matsufuji, N.; Futami, Y.; Fukumura, A.; Hiraoka, T.; et al. Biophysical characteristics of HIMAC clinical irradiation system for heavy-ion radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 1999, 44, 201–210. [Google Scholar] [CrossRef]
- Malouff, T.D.; Mahajan, A.; Krishnan, S.; Beltran, C.; Seneviratne, D.S.; Trifiletti, D.M. Carbon Ion Therapy: A Modern Review of an Emerging Technology. Front. Oncol. 2020, 10, 82. [Google Scholar] [CrossRef]
- Serizawa, I.; Kagei, K.; Kamada, T.; Imai, R.; Sugahara, S.; Okada, T.; Tsuji, H.; Ito, H.; Tsujii, H. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 1105–1110. [Google Scholar] [CrossRef]
- Seidensaal, K.; Kieser, M.; Hommertgen, A.; Jaekel, C.; Harrabi, S.B.; Herfarth, K.; Mechtesheimer, G.; Lehner, B.; Schneider, M.; Nienhueser, H.; et al. Neoadjuvant irradiation of retroperitoneal soft tissue sarcoma with ions (Retro-Ion): Study protocol for a randomized phase II pilot trial. Trials 2021, 22, 134. [Google Scholar] [CrossRef]
- Schmitz, R.; Adam, M.A.; Blazer, D.G., 3rd. Overcoming a travel burden to high-volume centers for treatment of retroperitoneal sarcomas is associated with improved survival. World J. Surg. Oncol. 2019, 17, 180. [Google Scholar] [CrossRef] [PubMed]
- Venigalla, S.; Nead, K.T.; Sebro, R.; Guttmann, D.M.; Sharma, S.; Simone, C.B., 2nd; Levin, W.P.; Wilson, R.J., 2nd; Weber, K.L.; Shabason, J.E. Association Between Treatment at High-Volume Facilities and Improved Overall Survival in Soft Tissue Sarcomas. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Keung, E.Z.; Chiang, Y.J.; Cormier, J.N.; Torres, K.E.; Hunt, K.K.; Feig, B.W.; Roland, C.L. Treatment at low-volume hospitals is associated with reduced short-term and long-term outcomes for patients with retroperitoneal sarcoma. Cancer 2018, 124, 4495–4503. [Google Scholar] [CrossRef] [PubMed]
- Baldini, E.H.; Abrams, R.A.; Bosch, W.; Roberge, D.; Haas, R.L.M.; Catton, C.N.; Indelicato, D.J.; Olsen, J.R.; Deville, C.; Chen, Y.L.; et al. Retroperitoneal Sarcoma Target Volume and Organ at Risk Contour Delineation Agreement Among NRG Sarcoma Radiation Oncologists. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, C.W.; Fiveash, J.B.; Popple, R.A.; Arnoletti, J.P.; Russo, S.M.; Urist, M.M.; Bland, K.I.; Heslin, M.J. Preoperative radiation therapy with selective dose escalation to the margin at risk for retroperitoneal sarcoma. Cancer 2006, 107, 371–379. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Chen, Y.L.; Baldini, E.H.; Wang, D.; Adams, J.; Hickey, S.B.; Yeap, B.Y.; Hahn, S.M.; De Amorim Bernstein, K.; Nielsen, G.P.; et al. Phase 1 trial of preoperative image guided intensity modulated proton radiation therapy with simultaneously integrated boost to the high risk margin for retroperitoneal sarcomas. Adv. Radiat. Oncol. 2017, 2, 85–93. [Google Scholar] [CrossRef]
- DeLaney, T.F.; Mullen, J.T.; Chen, Y.-L.; Petersen, I.A.; Bishop, A.J.; Yoon, S.S.; Haynes, A.B.; Roland, C.L.; Cohen, S.; Choy, E.; et al. Preliminary results of phase 2 trial of preoperative image guided intensity modulated proton radiation therapy (IMPT) with simultaneously integrated boost (SIB) to the high-risk margin for retroperitoneal sarcomas (RPS). J. Clin. Oncol. 2021, 39, 11550. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Burgess, M.; Bolejack, V.; Van Tine, B.A.; Schuetze, S.M.; Hu, J.; D’Angelo, S.; Attia, S.; Riedel, R.F.; Priebat, D.A.; et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): A multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 1493–1501. [Google Scholar] [CrossRef]
- Keung, E.Z.; Lazar, A.J.; Torres, K.E.; Wang, W.L.; Cormier, J.N.; Ashleigh Guadagnolo, B.; Bishop, A.J.; Lin, H.; Hunt, K.K.; Bird, J.; et al. Phase II study of neoadjuvant checkpoint blockade in patients with surgically resectable undifferentiated pleomorphic sarcoma and dedifferentiated liposarcoma. BMC Cancer 2018, 18, 913. [Google Scholar] [CrossRef]
- Roland, C.L.; Keung, E.Z.-Y.; Lazar, A.J.; Torres, K.E.; Wang, W.-L.; Guadagnolo, A.; Bishop, A.J.; Lin, H.Y.; Hunt, K.; Feig, B.W. Preliminary Results of a Phase II Study of Neoadjuvant Checkpoint Blockade for Surgically Resectable Undifferentiated Pleomorphic Sarcoma (UPS) and Dedifferentiated Liposarcoma (DDLPS). J. Clin. Oncol. 2020, 38, 11505. [Google Scholar] [CrossRef]
- Keung, E.Z.-Y.; Nassif, E.F.; Lin, H.Y.; Lazar, A.J.; Torres, K.E.; Wang, W.-L.; Guadagnolo, B.A.; Bishop, A.J.; Hunt, K.; Feig, B.W.; et al. Randomized phase II study of neoadjuvant checkpoint blockade for surgically resectable undifferentiated pleomorphic sarcoma (UPS) and dedifferentiated liposarcoma (DDLPS): Survival results after 2 years of follow-up and intratumoral B-cell receptor (BCR) correlates. J. Clin. Oncol. 2022, 40, LBA11501. [Google Scholar] [CrossRef]
| Study | Number of Patients | Radiation Treatment Dose and Technique | Chemotherapy | Outcomes | Toxicities |
|---|---|---|---|---|---|
| MD Anderson Single-Arm Prospective Trial | 35 | 18–50.4 Gy EBRT followed by intraoperative boost with electrons | Concurrent doxorubicin (4 mg/m2) | R0/R1 resections were performed in 26 of 29 (90%) evaluable patients | Grade 3–4 hematologic toxicities in 27% of patients |
| University of Toronto Single-Arm Prospective Trial | 55 | 42–50 Gy EBRT followed by postoperative brachytherapy in half of the patients to doses ranging between 7.3 and 30 Gy | No | 2-year OS and DFS for resected RPS patients was 88% and 80%, respectively | Postoperative Grade 3–4 toxicities in 39.1% of patients |
| STRASS Randomized, Prospective Phase III Trial | 266 | 50.4 Gy in 28 fractions with EBRT | No | Median ARFS 4.5 years with RT vs. 5.0 years surgery alone (p = 0.95) | Serious adverse events in 24% of patients in RT group vs. 10% in surgery alone |
| Study | Number of Patients | Radiation Dose and Technique | Chemotherapy | Outcomes | Toxicities |
|---|---|---|---|---|---|
| NCI Prospective, Randomized Trial | 35 | 20 Gy IORT + 35–40 Gy PORT vs. 50–55 Gy PORT alone | Doxorubicin and Cyclophosphamide for a subset | Median OS 45 months in IORT group vs. 52 months PORT. Fewer local recurrences (6/15) in IORT arm vs. PORT-alone arm (16/20) | Peripheral neuropathy in 60% of IORT patients, vs. 5% in the PORT alone arm |
| German Prospective, one-armed, single center Phase I/II trial | 27 | Preoperative 45–50 Gy of EBRT followed by Surgery + IORT to a total dose of 10–12 Gy | No | 5-year LRFS 72%, PFS 40%, and OS 74%. | 33% had severe postoperative complications (9 out of 27 patients) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farooqi, A.S.; Guadagnolo, B.A.; Mitra, D.; Bishop, A.J. Radiation Therapy for Retroperitoneal Sarcomas: A Strass-Ful Situation. Curr. Oncol. 2023, 30, 598-609. https://doi.org/10.3390/curroncol30010047
Farooqi AS, Guadagnolo BA, Mitra D, Bishop AJ. Radiation Therapy for Retroperitoneal Sarcomas: A Strass-Ful Situation. Current Oncology. 2023; 30(1):598-609. https://doi.org/10.3390/curroncol30010047
Chicago/Turabian StyleFarooqi, Ahsan S., B. Ashleigh Guadagnolo, Devarati Mitra, and Andrew J. Bishop. 2023. "Radiation Therapy for Retroperitoneal Sarcomas: A Strass-Ful Situation" Current Oncology 30, no. 1: 598-609. https://doi.org/10.3390/curroncol30010047
APA StyleFarooqi, A. S., Guadagnolo, B. A., Mitra, D., & Bishop, A. J. (2023). Radiation Therapy for Retroperitoneal Sarcomas: A Strass-Ful Situation. Current Oncology, 30(1), 598-609. https://doi.org/10.3390/curroncol30010047





