Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Treatment
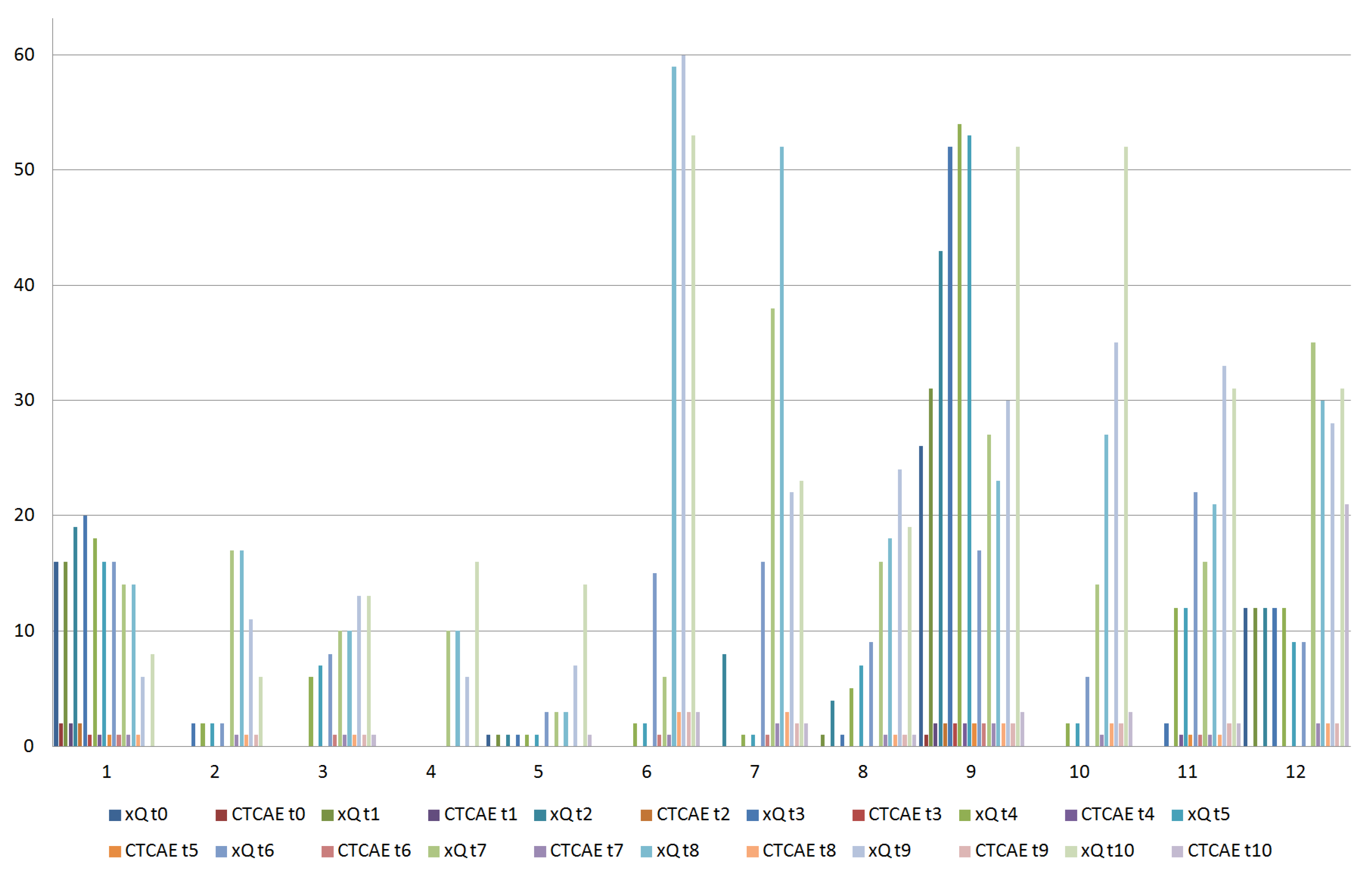
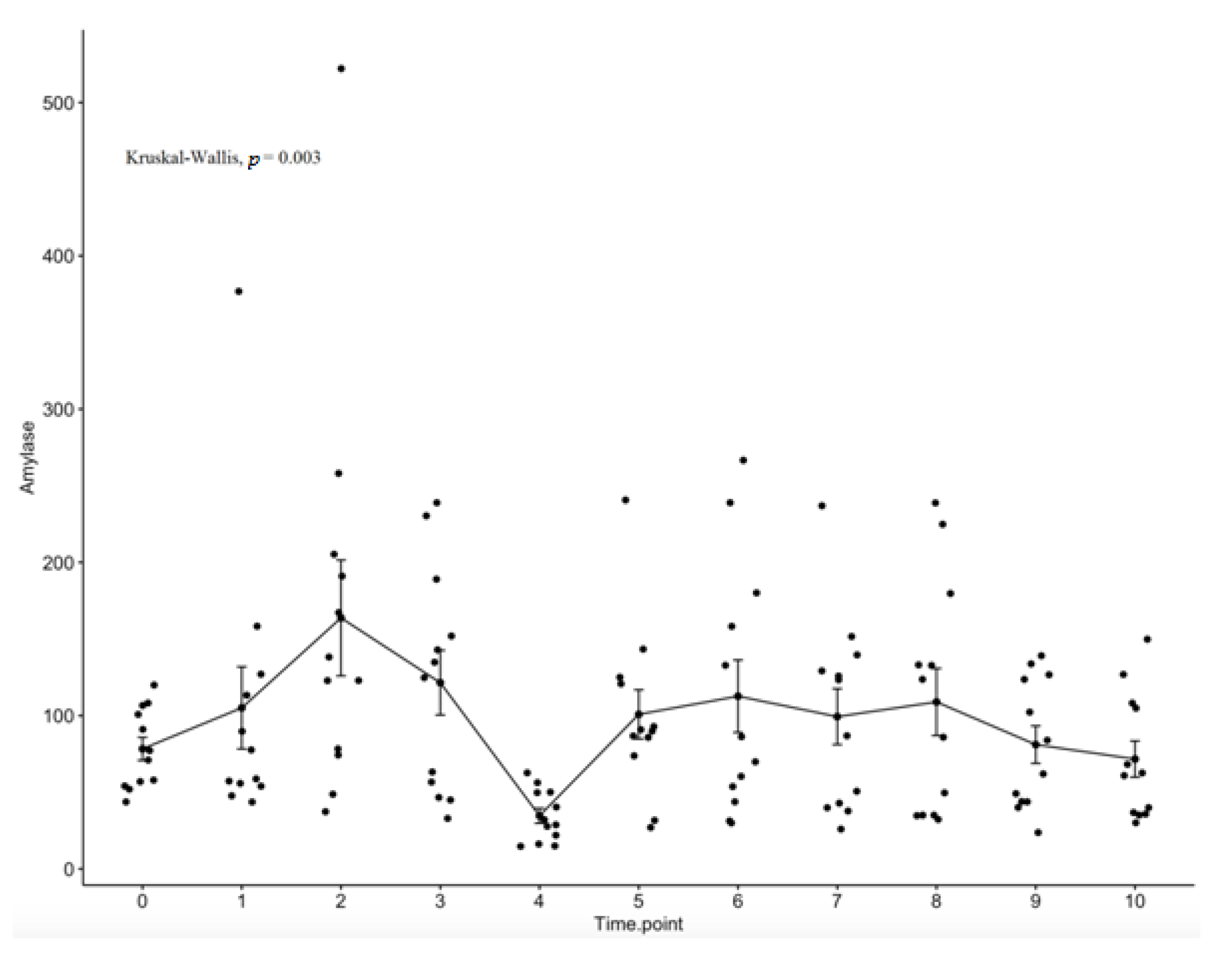
2.3. Salivary Amylase Evaluation
2.4. Xerostomia Evaluation
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology (NCCN Guidelines). Head and Neck Cancers, Version 2. 2022. Available online: https://www.nccn.org (accessed on 19 August 2022).
- Machiels, J.P.; René Leemans, C.; Golusinski, W.; Grau, C.; Licitra, L.; Gregoire, V. EHNS Executive Board. Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020, 31, 1462–1475. [Google Scholar] [CrossRef] [PubMed]
- Nutting, C.M.; Morden, J.P.; Harrington, K.J.; Urbano, T.G.; Bhide, S.A.; Clark, C.; Miles, E.A.; Miah, A.B.; Newbold, K.; Tanay, M.; et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): A phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011, 12, 127–136. [Google Scholar] [CrossRef]
- Richards, T.M.; Hurley, T.; Grove, L.; Harrington, K.J.; Carpenter, G.H.; Proctor, G.B.; Nutting, C.M. The effect of parotid gland-sparing intensity-modulated radiotherapy on salivary composition, flow rate and xerostomia measures. Oral Dis. 2017, 23, 990–1000. [Google Scholar] [CrossRef] [PubMed]
- Becciolini, A.; Porciani, S.; Lanini, A.; Chiavacci, A.; Cellai, E. Effects of irradiation with conventional and multiple daily fractionation on serum amylase activity. Acta Oncol. 1987, 26, 139–142. [Google Scholar] [CrossRef] [PubMed]
- Leslie, M.D.; Dische, S. Changes in serum and salivary amylase during radiotherapy for head and neck cancer: A comparison of conventionally fractionated radiotherapy with CHART. Radiother. Oncol. 1992, 24, 27–31. [Google Scholar] [CrossRef]
- Lydiatt, W.M.; Patel, S.G.; O’Sullivan, B.; Brandwein, M.S.; Ridge, J.A.; Migliacci, J.C.; Loomis, A.M.; Shah, J.P. Head and Neck cancers-major changes in the American Joint Committee on cancer eighth edition cancer staging manual. CA Cancer J. Clin. 2017, 67, 122–137. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; Musio, D.; Terenzi, V.; Valentini, V.; Cassoni, A.; Tombolini, M.; De Vincentiis, M.; Tombolini, V. Treatment improvement and better patient care: Which is the most important one in oral cavity cancer? Radiat. Oncol. 2014, 9, 263. [Google Scholar] [CrossRef] [PubMed]
- De Felice, F.; de Vincentiis, M.; Valentini, V.; Musio, D.; Mezi, S.; Lo Mele, L.; Terenzi, V.; D’Aguanno, V.; Cassoni, A.; Di Brino, M.; et al. Follow-up program in head and neck cancer. Crit. Rev. Oncol. Hematol. 2017, 113, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Eisbruch, A.; Kim, H.M.; Terrell, J.E.; Marsh, L.H.; Dawson, L.A.; Ship, J.A. Xerostomia and its predictors following parotid-sparing irradiation of head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys. 2001, 50, 695–704. [Google Scholar] [CrossRef]
- De Felice, F.; Tombolini, M.; Musella, A.; Marampon, F.; Tombolini, V.; Musio, D. Radiation therapy and serum salivary amylase in head and neck cancer. Oncotarget 2017, 8, 90496–90500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashima, H.K.; Kirkham, W.R.; Andrews, J.R. Postirradiation sialadenitis: A study of the clinical features, histopathologic changes and serum enzyme variations following irradiation of human salivary glands. Am. J. Roentgenol. 1965, 94, 271–291. [Google Scholar]
- Borok, T.L.; Cooper, J.S. Time course and significance of acute hyperamylasemia in patients receiving fractionated therapeutic radiation to the parotid gland region. Int. J. Radiat. Oncol. Biol. Phys. 1982, 8, 1449–1451. [Google Scholar] [CrossRef]
- Van den Brenk, H.A.; Hurley, R.A.; Gomez, C.; Richter, W. Serum amylase as a measure of salivary gland radiation damage. Hyperamylasaemia following fractionated exposure to 4 MV X rays delivered in high pressure oxygen, and effects of certain steroids on this response. Br. J. Radiol. 1969, 42, 688–700. [Google Scholar] [CrossRef] [PubMed]
- Valstar, M.H.; de Bakker, B.S.; Steenbakkers, R.J.H.M.; de Jong, K.H.; Smit, L.A.; Klein Nulent, T.J.W.; van Es, R.J.J.; Hofland, I.; de Keizer, B.; Jasperse, B.; et al. The tubarial salivary glands: A potential new organ at risk for radiotherapy. Radiother. Oncol. 2021, 154, 292–298. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Patient (%) |
|---|---|
| Age (years) | 58.5 |
| median (SD) | 8.5 |
| Gender | |
| male | 10 (83.3) |
| female | 2 (16.7) |
| Smoker | |
| never | 3 (25.0) |
| former | 2 (16.7) |
| current | 7 (58.3) |
| ≤10 pack/years | 2 (16.7) |
| >10 pack/years | 7 (58.3) |
| Alcohol consumption | |
| never | 6 (50.0) |
| former | 1 (8.3) |
| current | 5 (41.7) |
| ≤21 units/week | 5 (41.7) |
| >21 units/week | 1 (8.3) |
| Tumor localization | |
| oropharynx | 6 (50.0) |
| HPV-positive | 2 (33.3) |
| HPV-negative | 4 (66.7) |
| larynx | 2 (16.7) |
| nasopharynx | 2 (16.7) |
| hypopharynx | 1 (8.3) |
| oral cavity | 1 (8.3) |
| Clinical tumor stage (cT) | |
| 2 | 6 (50.0) |
| 3 | 2 (16.7) |
| 4a | 4 (33.3) |
| Clinical node stage (cN) | |
| 1 | 3 (25.0) |
| 2 | 7 (58.3) |
| 3 | 2 (16.7) |
| Treatment | |
| definitive CRT | 11 (91.7) |
| adjuvant CRT | 1 (8.3) |
| Mean Dose (Gy) | Irradiated Volume (cc) | |
|---|---|---|
| Ipsilateral parotid gland | 30.0 (±5.7) | 0.9 (±1.1) |
| Contralateral parotid gland | 24.2 (±3.3) | 0.6 (±0.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Felice, F.; Scarabelli, M.G.; De Pietro, R.; Chiarello, G.; Di Giammarco, F.; Cattaneo, C.G.; Lombardo, G.; Montinaro, F.R.; Tomaciello, M.; Tombolini, M.; et al. Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study. Curr. Oncol. 2022, 29, 6564-6572. https://doi.org/10.3390/curroncol29090516
De Felice F, Scarabelli MG, De Pietro R, Chiarello G, Di Giammarco F, Cattaneo CG, Lombardo G, Montinaro FR, Tomaciello M, Tombolini M, et al. Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study. Current Oncology. 2022; 29(9):6564-6572. https://doi.org/10.3390/curroncol29090516
Chicago/Turabian StyleDe Felice, Francesca, Maria Giulia Scarabelli, Raffaella De Pietro, Giuseppina Chiarello, Federico Di Giammarco, Carlo Guglielmo Cattaneo, Giuliana Lombardo, Francesca Romana Montinaro, Miriam Tomaciello, Mario Tombolini, and et al. 2022. "Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study" Current Oncology 29, no. 9: 6564-6572. https://doi.org/10.3390/curroncol29090516
APA StyleDe Felice, F., Scarabelli, M. G., De Pietro, R., Chiarello, G., Di Giammarco, F., Cattaneo, C. G., Lombardo, G., Montinaro, F. R., Tomaciello, M., Tombolini, M., Messineo, D., Di Paolo, P. L., Marchetti, C., Musio, D., & Tombolini, V. (2022). Relationship between Salivary Amylase and Xerostomia in Intensity-Modulated Radiation Therapy for Head and Neck Cancer: A Prospective Pilot Study. Current Oncology, 29(9), 6564-6572. https://doi.org/10.3390/curroncol29090516






