Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer
Abstract
:1. Introduction
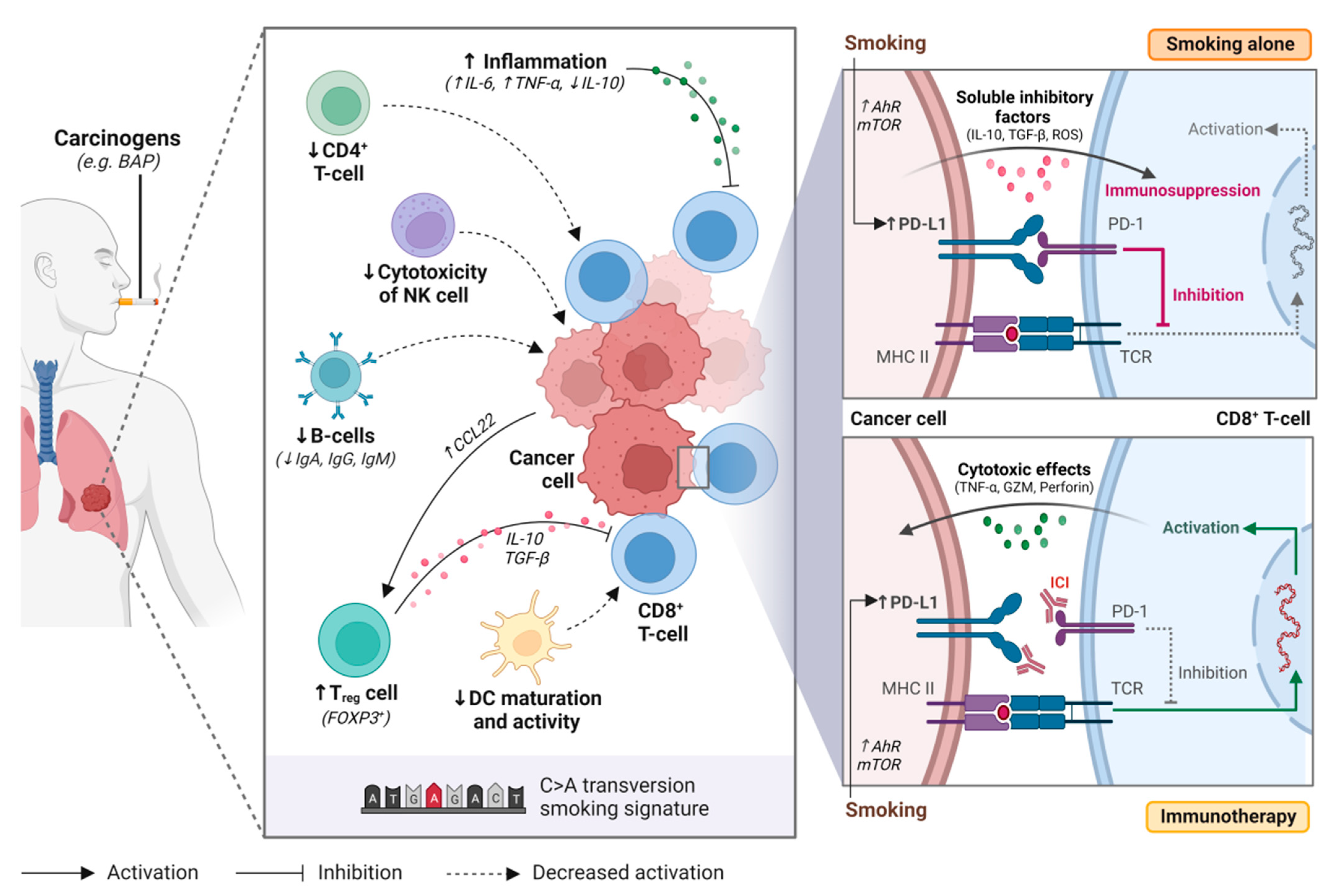
2. Tobacco Smoking and the Immune System
2.1. Smoking and Immune Cells
2.2. Smoking, Tumor Mutational Burden (TMB) and the Genomic Landscape
3. Moving into Clinic–Anti-PD(L)1 Checkpoint Inhibitors, Smoking and Outcomes
3.1. Previously Treated Advanced NSCLC
3.2. Treatment Naïve Advanced NSCLC
3.2.1. First Line Anti-PD(L)1 Monotherapy
3.2.2. Anti-PD(L)1 and Chemotherapy Combinations
3.2.3. Anti-PD(L)1 and Anti-CTLA-4 Combinations, with or without Chemotherapy
3.3. Anti-PD(L)1 Therapy in Non-Metastatic NSCLC
3.3.1. Locally Advanced NSCLC–Anti-PD-(L)1 Consolidation Therapy
3.3.2. Perioperative Anti-PD-(L)1 Therapy
3.4. Checkpoint Inhibitors and NSCLC with Oncogene Driven Tumors
3.5. Checkpoint Inhibitors and Small Cell Lung Cancer
4. Future Directions to Understanding Tobacco Use and ICI outcomes
Author Contributions
Funding
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Steven, A.; Fisher, S.A.; Robinson, B.W. Immunotherapy for lung cancer. Respirology 2016, 21, 821–833. [Google Scholar] [CrossRef] [PubMed]
- Ettinger, D.S.; Wood, D.E.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R.; D’Amico, T.A.; et al. NCCN Guidelines Insights: Non-Small Cell Lung Cancer, Version 2.2021. J. Natl. Compr. Canc. Netw. 2021, 19, 254–266. [Google Scholar] [CrossRef]
- Garon, E.B.; Hellmann, M.D.; Rizvi, N.A.; Carcereny, E.; Leighl, N.B.; Ahn, M.-J.; Eder, J.P.; Balmanoukian, A.S.; Aggarwal, C.; Horn, L.; et al. Five-Year Overall Survival for Patients with Advanced Non‒Small-Cell Lung Cancer Treated with Pembrolizumab: Results from the Phase I KEYNOTE-001 Study. J. Clin. Oncol. 2019, 37, 2518–2527. [Google Scholar] [CrossRef]
- Bodor, J.N.; Boumber, Y.; Borghaei, H. Biomarkers for immune checkpoint inhibition in non-small cell lung cancer (NSCLC). Cancer 2020, 126, 260–270. [Google Scholar] [CrossRef]
- Leighl, N.B. Meeting Immunotherapy Resistance in Lung Cancer. J. Thorac. Oncol. 2021, 16, 187–190. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef]
- Li, J.J.N.; Karim, K.; Sung, M.; Le, L.W.; Lau, S.C.M.; Sacher, A.; Leighl, N.B. Tobacco exposure and immunotherapy response in PD-L1 positive lung cancer patients. Lung Cancer 2020, 150, 159–163. [Google Scholar] [CrossRef] [PubMed]
- El-Osta, H.; Jafri, S. Predictors for clinical benefit of immune checkpoint inhibitors in advanced non-small-cell lung cancer: A meta-analysis. Immunotherapy 2019, 11, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Schiller, J.H.; Gazdar, A.F. Lung cancer in never smokers--a different disease. Nat. Rev. Cancer 2007, 7, 778–790. [Google Scholar] [CrossRef]
- Rodgman, A.; Perfetti, T. The Chemical Components of Tobacco and Tobacco Smoke; Routledge: Abingdon, UK, 2008. [Google Scholar]
- Gazdar, A.F. DNA Repair and Survival in Lung Cancer—The Two Faces of Janus. N. Eng. J. Med. 2007, 356, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Poulos, R.C.; Shah, A.; Beck, D.; Pimanda, J.E.; Wong, J.W. Differential DNA repair underlies mutation hotspots at active promoters in cancer genomes. Nature 2016, 532, 259–263. [Google Scholar] [CrossRef]
- Pfeifer, G.P.; Denissenko, M.F.; Olivier, M.; Tretyakova, N.; Hecht, S.S.; Hainaut, P. Tobacco smoke carcinogens, DNA damage and p53 mutations in smoking-associated cancers. Oncogene 2002, 21, 7435–7451. [Google Scholar] [CrossRef]
- Alexandrov, L.B.; Ju, Y.S.; Haase, K.; Van Loo, P.; Martincorena, I.; Nik-Zainal, S.; Totoki, Y.; Fujimoto, A.; Nakagawa, H.; Shibata, T.; et al. Mutational signatures associated with tobacco smoking in human cancer. Science 2016, 354, 618–622. [Google Scholar] [CrossRef]
- Wang, G.Z.; Zhang, L.; Zhao, X.C.; Gao, S.H.; Qu, L.W.; Yu, H.; Fang, W.F.; Zhou, Y.C.; Liang, F.; Zhang, C.; et al. The Aryl hydrocarbon receptor mediates tobacco-induced PD-L1 expression and is associated with response to immunotherapy. Nat. Commun. 2019, 10, 1125. [Google Scholar] [CrossRef]
- Xiao, F.; Liu, Y.; Zhang, Z.; Wang, L.; Wang, T.; Wang, X. Tobacco extracts promote PD-L1 expression and enhance malignant biological differences via mTOR in gefitinib-resistant cell lines. Thorac. Cancer 2020, 11, 2237–2251. [Google Scholar] [CrossRef]
- Calles, A.; Liao, X.; Sholl, L.M.; Rodig, S.J.; Freeman, G.J.; Butaney, M.; Lydon, C.; Dahlberg, S.E.; Hodi, F.S.; Oxnard, G.R.; et al. Expression of PD-1 and Its Ligands, PD-L1 and PD-L2, in Smokers and Never Smokers with KRAS-Mutant Lung Cancer. J. Thorac. Oncol. 2015, 10, 1726–1735. [Google Scholar] [CrossRef] [Green Version]
- Masaki, T.; Okazawa, M.; Asano, R.; Inagaki, T.; Ishibashi, T.; Yamagishi, A.; Umeki-Mizushima, S.; Nishimura, M.; Manabe, Y.; Ishibashi-Ueda, H.; et al. Aryl hydrocarbon receptor is essential for the pathogenesis of pulmonary arterial hypertension. Proc. Natl. Acad. Sci. USA 2021, 118, e2023899118. [Google Scholar] [CrossRef] [PubMed]
- Qiu, F.; Liang, C.L.; Liu, H.; Zeng, Y.Q.; Hou, S.; Huang, S.; Lai, X.; Dai, Z. Impacts of cigarette smoking on immune responsiveness: Up and down or upside down? Oncotarget 2017, 8, 268–284. [Google Scholar] [CrossRef] [PubMed]
- Arimilli, S.; Schmidt, E.; Damratoski, B.E.; Prasad, G.L. Role of Oxidative Stress in the Suppression of Immune Responses in Peripheral Blood Mononuclear Cells Exposed to Combustible Tobacco Product Preparation. Inflammation 2017, 40, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Li, X.; Xie, F.; Yang, Z.; Pan, X.; Zhu, M.; Shang, P.; Nie, C.; Liu, H.; Xie, J. Immunomodulatory effects of cigarette smoke condensate in mouse macrophage cell line. Int. J. Immunopathol. Pharmacol. 2017, 30, 315–321. [Google Scholar] [CrossRef]
- Prado-Garcia, H.; Romero-Garcia, S.; Aguilar-Cazares, D.; Meneses-Flores, M.; Lopez-Gonzalez, J.S. Tumor-induced CD8+ T-cell dysfunction in lung cancer patients. Clin. Dev. Immunol. 2012, 2012, 741741. [Google Scholar] [CrossRef]
- Arnson, Y.; Shoenfeld, Y.; Amital, H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J. Autoimmun. 2010, 34, J258–J265. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Finn, O.J. T-cell death and cancer immune tolerance. Cell Death Differ. 2008, 15, 70–79. [Google Scholar] [CrossRef]
- Barry, M.; Bleackley, R.C. Cytotoxic T lymphocytes: All roads lead to death. Nat. Rev. Immunol. 2002, 2, 401–409. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef]
- Sato, E.; Olson, S.H.; Ahn, J.; Bundy, B.; Nishikawa, H.; Qian, F.; Jungbluth, A.A.; Frosina, D.; Gnjatic, S.; Ambrosone, C.; et al. Intraepithelial CD8+ tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 18538–18543. [Google Scholar] [CrossRef] [Green Version]
- Jackutė, J.; Žemaitis, M.; Pranys, D.; Šitkauskienė, B.; Miliauskas, S.; Bajoriūnas, V.; Sakalauskas, R. Distribution of CD4(+) and CD8(+) T cells in tumor islets and stroma from patients with non-small cell lung cancer in association with COPD and smoking. Medicina 2015, 51, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Saetta, M.; Baraldo, S.; Corbino, L.; Turato, G.; Braccioni, F.; Rea, F.; Cavallesco, G.; Tropeano, G.; Mapp, C.E.; Maestrelli, P.; et al. CD8+ve cells in the lungs of smokers with chronic obstructive pulmonary disease. Am. J Respir. Crit. Care Med. 1999, 160, 711–717. [Google Scholar] [CrossRef] [PubMed]
- Tian, C.; Lu, S.; Fan, Q.; Zhang, W.; Jiao, S.; Zhao, X.; Wu, Z.; Sun, L.; Wang, L. Prognostic significance of tumor-infiltrating CD8⁺ or CD3⁺ T lymphocytes and interleukin-2 expression in radically resected non-small cell lung cancer. Chin. Med. J. 2015, 128, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Alifano, M.; Mansuet-Lupo, A.; Lococo, F.; Roche, N.; Bobbio, A.; Canny, E.; Schussler, O.; Dermine, H.; Régnard, J.F.; Burroni, B.; et al. Systemic inflammation, nutritional status and tumor immune microenvironment determine outcome of resected non-small cell lung cancer. PLoS ONE 2014, 9, e106914. [Google Scholar] [CrossRef]
- Hiraoka, K.; Miyamoto, M.; Cho, Y.; Suzuoki, M.; Oshikiri, T.; Nakakubo, Y.; Itoh, T.; Ohbuchi, T.; Kondo, S.; Katoh, H. Concurrent infiltration by CD8+ T cells and CD4+ T cells is a favourable prognostic factor in non-small-cell lung carcinoma. Br. J. Cancer 2006, 94, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Trojan, A.; Urosevic, M.; Dummer, R.; Giger, R.; Weder, W.; Stahel, R.A. Immune activation status of CD8+ T cells infiltrating non-small cell lung cancer. Lung Cancer 2004, 44, 143–147. [Google Scholar] [CrossRef]
- Vassallo, R.; Walters, P.R.; Lamont, J.; Kottom, T.J.; Yi, E.S.; Limper, A.H. Cigarette smoke promotes dendritic cell accumulation in COPD; a Lung Tissue Research Consortium study. Respir. Res. 2010, 11, 45. [Google Scholar] [CrossRef]
- Botelho, F.M.; Nikota, J.K.; Bauer, C.M.; Morissette, M.C.; Iwakura, Y.; Kolbeck, R.; Finch, D.; Humbles, A.A.; Stämpfli, M.R. Cigarette smoke-induced accumulation of lung dendritic cells is interleukin-1α-dependent in mice. Respir. Res. 2012, 13, 81. [Google Scholar] [CrossRef]
- Sharma, S.; Stolina, M.; Yang, S.C.; Baratelli, F.; Lin, J.F.; Atianzar, K.; Luo, J.; Zhu, L.; Lin, Y.; Huang, M.; et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin. Cancer Res. 2003, 9, 961–968. [Google Scholar] [PubMed]
- Robbins, C.S.; Franco, F.; Mouded, M.; Cernadas, M.; Shapiro, S.D. Cigarette smoke exposure impairs dendritic cell maturation and T cell proliferation in thoracic lymph nodes of mice. J. Immunol. 2008, 180, 6623–6628. [Google Scholar] [CrossRef] [Green Version]
- Givi, M.E.; Folkerts, G.; Wagenaar, G.T.M.; Redegeld, F.A.; Mortaz, E. Cigarette smoke differentially modulates dendritic cell maturation and function in time. Respir. Res. 2015, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Baxevanis, C.N.; Voutsas, I.F.; Tsitsilonis, O.E.; Gritzapis, A.D.; Sotiriadou, R.; Papamichail, M. Tumor-specific CD4+ T lymphocytes from cancer patients are required for optimal induction of cytotoxic T cells against the autologous tumor. J. Immunol. 2000, 164, 3902–3912. [Google Scholar] [CrossRef] [PubMed]
- Kitz, A.; Singer, E.; Hafler, D. Regulatory T Cells: From Discovery to Autoimmunity. Cold Spring Harb. Perspect. Med. 2018, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Tanaka, J.; Kjaergaard, J.; Shu, S. Depletion of CD4+ CD25+ regulatory cells augments the generation of specific immune T cells in tumor-draining lymph nodes. J. Immunother. 2002, 25, 207–217. [Google Scholar] [CrossRef]
- Dannull, J.; Su, Z.; Rizzieri, D.; Yang, B.K.; Coleman, D.; Yancey, D.; Zhang, A.; Dahm, P.; Chao, N.; Gilboa, E.; et al. Enhancement of vaccine-mediated antitumor immunity in cancer patients after depletion of regulatory T cells. J. Clin. Investig. 2005, 115, 3623–3633. [Google Scholar] [CrossRef]
- Kinoshita, T.; Muramatsu, R.; Fujita, T.; Nagumo, H.; Sakurai, T.; Noji, S.; Takahata, E.; Yaguchi, T.; Tsukamoto, N.; Kudo-Saito, C.; et al. Prognostic value of tumor-infiltrating lymphocytes differs depending on histological type and smoking habit in completely resected non-small-cell lung cancer. Ann. Oncol. 2016, 27, 2117–2123. [Google Scholar] [CrossRef]
- Forsslund, H.; Mikko, M.; Karimi, R.; Grunewald, J.; Wheelock, Å.M.; Wahlström, J.; Sköld, C.M. Distribution of T-cell subsets in BAL fluid of patients with mild to moderate COPD depends on current smoking status and not airway obstruction. Chest 2014, 145, 711–722. [Google Scholar] [CrossRef]
- Sato, K.; Mimaki, S.; Yamashita, R.; Togashi, Y.; Naito, T.; Udagawa, H.; Katsumata, S.; Nakasone, S.; Miyoshi, T.; Tane, K.; et al. Association between the mutational smoking signature and the immune microenvironment in lung adenocarcinoma. Lung Cancer 2020, 147, 12–20. [Google Scholar] [CrossRef]
- Larmonier, N.; Marron, M.; Zeng, Y.; Cantrell, J.; Romanoski, A.; Sepassi, M.; Thompson, S.; Chen, X.; Andreansky, S.; Katsanis, E. Tumor-derived CD4+CD25+ regulatory T cell suppression of dendritic cell function involves TGF-β and IL-10. Cancer Immunol. Immunother. 2007, 56, 48–59. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Hellmann, M.D.; Snyder, A.; Kvistborg, P.; Makarov, V.; Havel, J.J.; Lee, W.; Yuan, J.; Wong, P.; Ho, T.S.; et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 2015, 348, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Desrichard, A.; Kuo, F.; Chowell, D.; Lee, K.-W.; Riaz, N.; Wong, R.J.; Chan, T.A.; Morris, L.G.T. Tobacco Smoking-Associated Alterations in the Immune Microenvironment of Squamous Cell Carcinomas. JNCI J. Nat. Cancer Inst. 2018, 110, 1386–1392. [Google Scholar] [CrossRef] [PubMed]
- Nagahashi, M.; Sato, S.; Yuza, K.; Shimada, Y.; Ichikawa, H.; Watanabe, S.; Takada, K.; Okamoto, T.; Okuda, S.; Lyle, S.; et al. Common driver mutations and smoking history affect tumor mutation burden in lung adenocarcinoma. J. Surg. Res. 2018, 230, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ricciuti, B.; Nguyen, T.; Li, X.; Rabin, M.S.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Association between Smoking History and Tumor Mutation Burden in Advanced Non-Small Cell Lung Cancer. Cancer Res. 2021, 81, 2566–2573. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.-W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Cho, B.C.; Reinmuth, N.; Lee, K.H.; Luft, A.; Ahn, M.-J.; van den Heuvel, M.M.; Cobo, M.; Vicente, D.; Smolin, A.; et al. Durvalumab With or Without Tremelimumab vs. Standard Chemotherapy in First-line Treatment of Metastatic Non–Small Cell Lung Cancer: The MYSTIC Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020, 6, 661–674. [Google Scholar] [CrossRef]
- Nik-Zainal, S.; Kucab, J.E.; Morganella, S.; Glodzik, D.; Alexandrov, L.B.; Arlt, V.M.; Weninger, A.; Hollstein, M.; Stratton, M.R.; Phillips, D.H. The genome as a record of environmental exposure. Mutagenesis 2015, 30, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Carbone, D.P.; Reck, M.; Paz-Ares, L.; Creelan, B.; Horn, L.; Steins, M.; Felip, E.; van den Heuvel, M.M.; Ciuleanu, T.-E.; Badin, F.; et al. First-Line Nivolumab in Stage IV or Recurrent Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2017, 376, 2415–2426. [Google Scholar] [CrossRef]
- DerSimonian, R.; Laird, N. Meta-analysis in clinical trials. Control. Clin. Trials 1986, 7, 177–188. [Google Scholar] [CrossRef]
- Deeks, J.J.; Higgins, J.P.T.; Altman, D.G. Analysing data and undertaking meta-analyses. In Cochrane Handbook for Systematic Reviews of Interventions; Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, W.A., Eds.; John Wiley & Sons, Ltd.: Chichester, UK, 2006. [Google Scholar]
- Herbst, R.S.; Garon, E.B.; Kim, D.-W.; Cho, B.C.; Gervais, R.; Perez-Gracia, J.L.; Han, J.-Y.; Majem, M.; Forster, M.D.; Monnet, I.; et al. Five Year Survival Update From KEYNOTE-010: Pembrolizumab Versus Docetaxel for Previously Treated, Programmed Death-Ligand 1-Positive Advanced NSCLC. J. Thorac. Oncol. 2021, 16, 1718–1732. [Google Scholar] [CrossRef]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.M.; Burgio, M.A.; Carpeno, J.d.C.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-Year Outcomes from the Randomized, Phase III Trials CheckMate 017 and 057: Nivolumab Versus Docetaxel in Previously Treated Non–Small-Cell Lung Cancer. J. Clin. Oncol. 2021, 39, 723–733. [Google Scholar] [CrossRef]
- Fehrenbacher, L.; von Pawel, J.; Park, K.; Rittmeyer, A.; Gandara, D.R.; Ponce Aix, S.; Han, J.-Y.; Gadgeel, S.M.; Hida, T.; Cortinovis, D.L.; et al. Updated Efficacy Analysis Including Secondary Population Results for OAK: A Randomized Phase III Study of Atezolizumab versus Docetaxel in Patients with Previously Treated Advanced Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2018, 13, 1156–1170. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Özgüroğlu, M.; Vansteenkiste, J.; Spigel, D.; Yang, J.C.H.; Ishii, H.; Garassino, M.; de Marinis, F.; Szczesna, A.; Polychronis, A.; et al. Avelumab Versus Docetaxel in Patients With Platinum-Treated Advanced NSCLC: 2-Year Follow-Up From the JAVELIN Lung 200 Phase 3 Trial. J Thorac Oncol 2021, 16, 1369–1378. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Five-Year Outcomes with Pembrolizumab Versus Chemotherapy for Metastatic Non-Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score ≥ 50%. J. Clin. Oncol. 2021, 39, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Reck, M.; Rodríguez–Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater. J. Clin. Oncol. 2019, 37, 537–546. [Google Scholar] [CrossRef]
- Mok, T.S.K.; Wu, Y.L.; Kudaba, I.; Kowalski, D.M.; Cho, B.C.; Turna, H.Z.; Castro, G., Jr.; Srimuninnimit, V.; Laktionov, K.K.; Bondarenko, I.; et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): A randomised, open-label, controlled, phase 3 trial. Lancet 2019, 393, 1819–1830. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Eng. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Jassem, J.; de Marinis, F.; Giaccone, G.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Oprean, C.; Kim, Y.C.; Andric, Z.; et al. Updated Overall Survival Analysis from IMpower110: Atezolizumab Versus Platinum-Based Chemotherapy in Treatment-Naive Programmed Death-Ligand 1-Selected NSCLC. J. Thorac. Oncol. 2021, 16, 1872–1882. [Google Scholar] [CrossRef]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Abreu, D.; Powell, S.F.; Hochmair, M.J.; Gadgeel, S.; Esteban, E.; Felip, E.; Speranza, G.; De Angelis, F.; Dómine, M.; Cheng, S.Y.; et al. Pemetrexed plus platinum with or without pembrolizumab in patients with previously untreated metastatic nonsquamous NSCLC: Protocol-specified final analysis from KEYNOTE-189. Ann. Oncol. 2021, 32, 881–895. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Jotte, R.; Cappuzzo, F.; Vynnychenko, I.; Stroyakovskiy, D.; Rodríguez-Abreu, D.; Hussein, M.; Soo, R.; Conter, H.J.; Kozuki, T.; Huang, K.C.; et al. Atezolizumab in Combination with Carboplatin and Nab-Paclitaxel in Advanced Squamous NSCLC (IMpower131): Results from a Randomized Phase III Trial. J. Thorac. Oncol. 2020, 15, 1351–1360. [Google Scholar] [CrossRef] [PubMed]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Eng. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Socinski, M.A.; Nishio, M.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; et al. IMpower150 Final Overall Survival Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in First-Line Metastatic Nonsquamous NSCLC. J. Thorac. Oncol. 2021, 16, 1909–1924. [Google Scholar] [CrossRef] [PubMed]
- Gogishvili, M.; Melkadze, T.; Makharadze, T.; Giorgadze, D.; Dvorkin, M.; Penkov, K.D.; Laktionov, K.; Nemsadze, G.; Nechaeva, M.; Rozhkova, I.; et al. LBA51 EMPOWER-Lung 3: Cemiplimab in combination with platinum doublet chemotherapy for first-line (1L) treatment of advanced non-small cell lung cancer (NSCLC). Ann. Oncol. 2021, 32, S1328. [Google Scholar] [CrossRef]
- Ren, S.; Chen, J.; Xu, X.; Jiang, T.; Cheng, Y.; Chen, G.; Pan, Y.; Fang, Y.; Wang, Q.; Huang, Y.; et al. Camrelizumab Plus Carboplatin and Paclitaxel as First-Line Treatment for Advanced Squamous NSCLC (CameL-Sq): A Phase 3 Trial. J. Thorac. Oncol. 2022, 17, 544–557. [Google Scholar] [CrossRef]
- Yang, Y.; Sun, J.; Wang, Z.; Fang, J.; Yu, Q.; Han, B.; Cang, S.; Chen, G.; Mei, X.; Yang, Z.; et al. Updated Overall Survival Data and Predictive Biomarkers of Sintilimab Plus Pemetrexed and Platinum as First-Line Treatment for Locally Advanced or Metastatic Nonsquamous NSCLC in the Phase 3 ORIENT-11 Study. J. Thorac. Oncol. 2021, 16, 2109–2120. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Ciuleanu, T.-E.; Cobo, M.; Schenker, M.; Zurawski, B.; Menezes, J.; Richardet, E.; Bennouna, J.; Felip, E.; Juan-Vidal, O.; et al. First-line nivolumab plus ipilimumab combined with two cycles of chemotherapy in patients with non-small-cell lung cancer (CheckMate 9L.A): An international, randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 198–211. [Google Scholar] [CrossRef]
- Johnson, M.; Cho, B.C.; Luft, A.; Alatorre-Alexander, J.; Geater, S.L.; Laktionov, K.; Vasiliev, A.; Trukhin, D.; Kim, S.; Ursol, G.; et al. Durvalumab ± Tremelimumab + Chemotherapy as First-line Treatment for mNSCLC: Results from the Phase 3 POSEIDON study. In Proceedings of the World Conference on Lung Cancer, Virtual, 26–29 January 2021. [Google Scholar]
- Leighl, N.B.; Laurie, S.A.; Goss, G.D.; Hughes, B.G.M.; Stockler, M.; Tsao, M.S.; Hwang, D.M.; Joubert, P.; Kulkarni, S.; Blais, N.; et al. CCTG BR34: A Randomized Phase 2 Trial of Durvalumab and Tremelimumab with or Without Platinum-Based Chemotherapy in Patients with Metastatic NSCLC. J. Thorac. Oncol. 2022, 17, 434–445. [Google Scholar] [CrossRef]
- Faivre-Finn, C.; Vicente, D.; Kurata, T.; Planchard, D.; Paz-Ares, L.; Vansteenkiste, J.F.; Spigel, D.R.; Garassino, M.C.; Reck, M.; Senan, S.; et al. Four-Year Survival with Durvalumab After Chemoradiotherapy in Stage III NSCLC—An Update from the PACIFIC Trial. J. Thorac. Oncol. 2021, 16, 860–867. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB–IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Paz-Ares, L.; O’Brien, M.E.R.; Mauer, M.; Dafni, U.; Oselin, K.; Havel, L.; Esteban Gonzalez, E.; Isla, D.; Martinez-Marti, A.; Faehling, M.; et al. VP3-2022: Pembrolizumab (pembro) versus placebo for early-stage non-small cell lung cancer (NSCLC) following complete resection and adjuvant chemotherapy (chemo) when indicated: Randomized, triple-blind, phase III EORTC-1416-LCG/ETOP 8-15—PEARLS/KEYNOTE-091 study. Ann. Oncol. 2022, 33, 451–453. [Google Scholar] [CrossRef]
- Forde, P.M.; Spicer, J.; Lu, S.; Provencio, M.; Mitsudomi, T.; Awad, M.M.; Felip, E.; Broderick, S.R.; Brahmer, J.R.; Swanson, S.J.; et al. Neoadjuvant Nivolumab plus Chemotherapy in Resectable Lung Cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non-Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- Mazieres, J.; Drilon, A.; Lusque, A.; Mhanna, L.; Cortot, A.B.; Mezquita, L.; Thai, A.A.; Mascaux, C.; Couraud, S.; Veillon, R.; et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: Results from the IMMUNOTARGET registry. Ann. Oncol. 2019, 30, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Zhang, H.; Hai, J.; Socinski, M.A.; Lim, E.; Chen, H.; Stebbing, J. Impact of PD-L1 expression, driver mutations and clinical characteristics on survival after anti-PD-1/PD-L1 immunotherapy versus chemotherapy in non-small-cell lung cancer: A meta-analysis of randomized trials. Oncoimmunology 2018, 7, e1396403. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.L.; Liu, Y.; Dimou, A.; Patil, T.; Aisner, D.L.; Dong, Z.; Jiang, T.; Su, C.; Wu, C.; Ren, S.; et al. Predictive value of oncogenic driver subtype, programmed death-1 ligand (PD-L1) score, and smoking status on the efficacy of PD-1/PD-L1 inhibitors in patients with oncogene-driven non-small cell lung cancer. Cancer 2019, 125, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Garassino, M.C.; Gelibter, A.J.; Grossi, F.; Chiari, R.; Soto Parra, H.; Cascinu, S.; Cognetti, F.; Turci, D.; Blasi, L.; Bengala, C.; et al. Italian Nivolumab Expanded Access Program in Nonsquamous Non–Small Cell Lung Cancer Patients: Results in Never-Smokers and EGFR-Mutant Patients. J. Thorac. Oncol. 2018, 13, 1146–1155. [Google Scholar] [CrossRef] [PubMed]
- Calles, A.; Riess, J.W.; Brahmer, J.R. Checkpoint Blockade in Lung Cancer with Driver Mutation: Choose the Road Wisely. Am. Soc. Clin. Oncol. Educ. Book 2020, 40, 372–384. [Google Scholar] [CrossRef]
- Chung, H.C.; Piha-Paul, S.A.; Lopez-Martin, J.; Schellens, J.H.M.; Kao, S.; Miller, W.H., Jr.; Delord, J.-P.; Gao, B.; Planchard, D.; Gottfried, M.; et al. Pembrolizumab After Two or More Lines of Previous Therapy in Patients with Recurrent or Metastatic SCLC: Results From the KEYNOTE-028 and KEYNOTE-158 Studies. J. Thorac. Oncol. 2020, 15, 618–627. [Google Scholar] [CrossRef] [Green Version]
- Spigel, D.R.; Vicente, D.; Ciuleanu, T.E.; Gettinger, S.; Peters, S.; Horn, L.; Audigier-Valette, C.; Pardo Aranda, N.; Juan-Vidal, O.; Cheng, Y.; et al. Second-line nivolumab in relapsed small-cell lung cancer: CheckMate 331. Ann. Oncol 2021, 32, 631–641. [Google Scholar] [CrossRef]
- Paz-Ares, L.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab plus platinum–etoposide versus platinum–etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): A randomised, controlled, open-label, phase 3 trial. Lancet 2019, 394, 1929–1939. [Google Scholar] [CrossRef]
- Liu, S.V.; Reck, M.; Mansfield, A.S.; Mok, T.; Scherpereel, A.; Reinmuth, N.; Garassino, M.C.; De Castro Carpeno, J.; Califano, R.; Nishio, M.; et al. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients with Extensive-Stage Small-Cell Lung Cancer Treated with Atezolizumab, Carboplatin, and Etoposide (IMpower133). J. Clin. Oncol. 2021, 39, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Rudin, C.M.; Awad, M.M.; Navarro, A.; Gottfried, M.; Peters, S.; Csőszi, T.; Cheema, P.K.; Rodriguez-Abreu, D.; Wollner, M.; Yang, J.C.; et al. Pembrolizumab or Placebo Plus Etoposide and Platinum as First-Line Therapy for Extensive-Stage Small-Cell Lung Cancer: Randomized, Double-Blind, Phase III KEYNOTE-604 Study. J. Clin. Oncol. 2020, 38, 2369–2379. [Google Scholar] [CrossRef] [PubMed]
- Parra, E.R.; Behrens, C.; Rodriguez-Canales, J.; Lin, H.; Mino, B.; Blando, J.; Zhang, J.; Gibbons, D.L.; Heymach, J.V.; Sepesi, B.; et al. Image Analysis-based Assessment of PD-L1 and Tumor-Associated Immune Cells Density Supports Distinct Intratumoral Microenvironment Groups in Non-small Cell Lung Carcinoma Patients. Clin. Cancer Res. 2016, 22, 6278–6289. [Google Scholar] [CrossRef] [PubMed]
- Muppa, P.; Parrilha Terra, S.B.S.; Sharma, A.; Mansfield, A.S.; Aubry, M.-C.; Bhinge, K.; Asiedu, M.K.; de Andrade, M.; Janaki, N.; Murphy, S.J.; et al. Immune Cell Infiltration May Be a Key Determinant of Long-Term Survival in Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 1286–1295. [Google Scholar] [CrossRef]
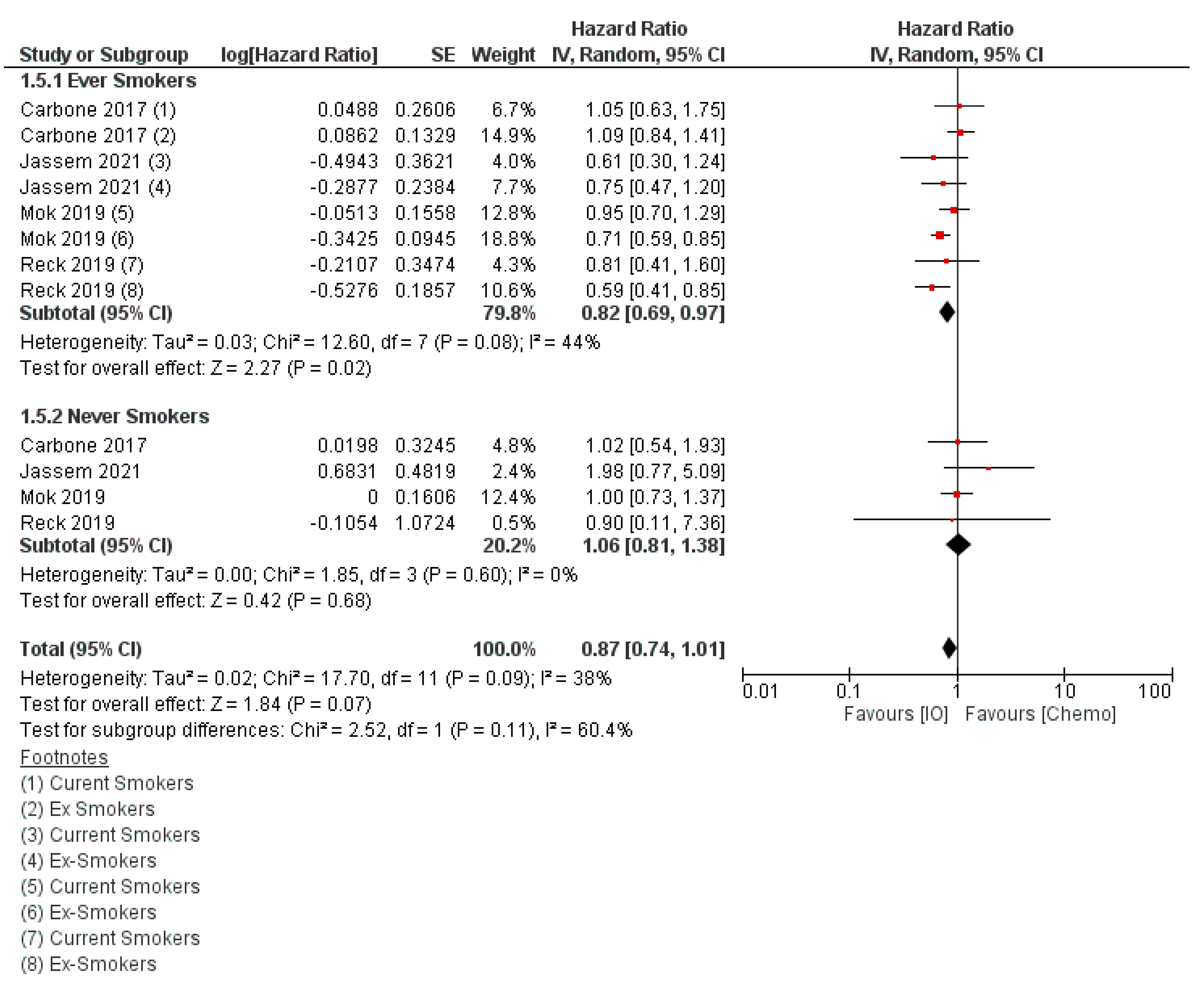
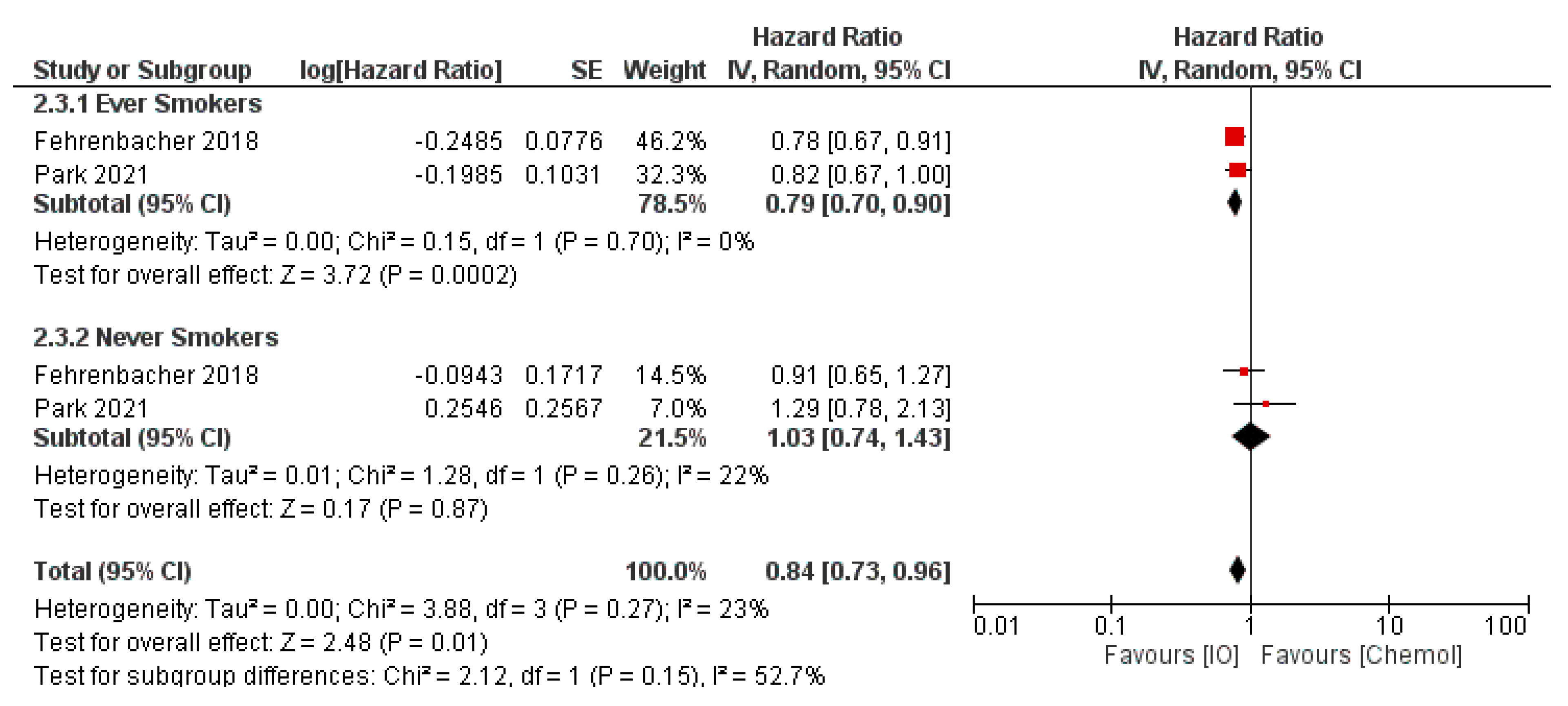
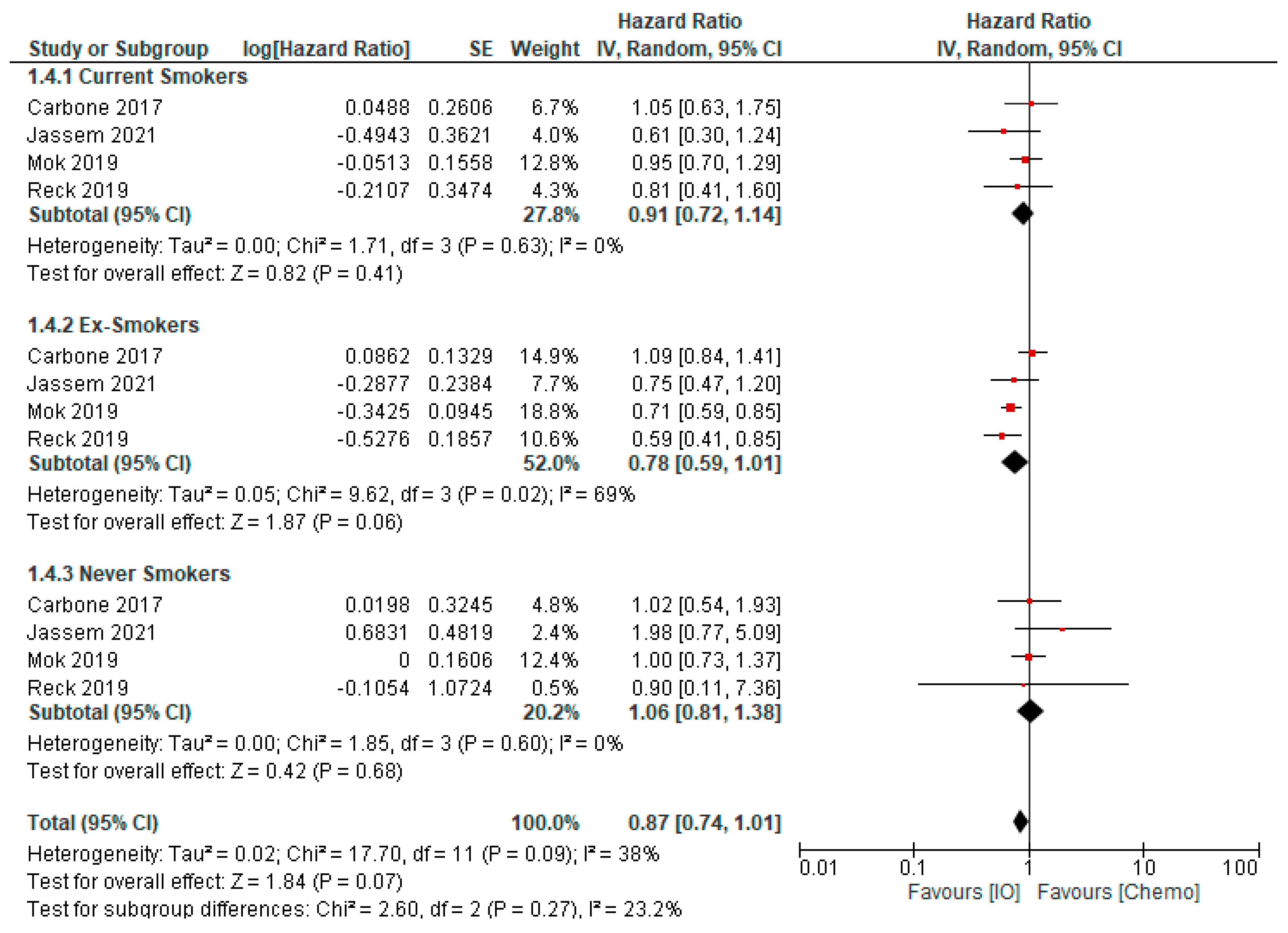
- Dai, L.; Jin, B.; Liu, T.; Chen, J.; Li, G.; Dang, J. The effect of smoking status on efficacy of immune checkpoint inhibitors in metastatic non-small cell lung cancer: A systematic review and meta-analysis. EClinicalMedicine 2021, 38, 100990. [Google Scholar] [CrossRef]
- Zhao, W.; Jiang, W.; Wang, H.; He, J.; Su, C.; Yu, Q. Impact of Smoking History on Response to Immunotherapy in Non-Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2021, 11, 703143. [Google Scholar] [CrossRef]
- Cortellini, A.; De Giglio, A.; Cannita, K.; Cortinovis, D.L.; Cornelissen, R.; Baldessari, C.; Giusti, R.; D’Argento, E.; Grossi, F.; Santoni, M.; et al. Smoking status during first-line immunotherapy and chemotherapy in NSCLC patients: A case–control matched analysis from a large multicenter study. Thor. Cancer 2021, 12, 880–889. [Google Scholar] [CrossRef]
- Wang, X.; Ricciuti, B.; Alessi, J.V.; Nguyen, T.; Awad, M.M.; Lin, X.; Johnson, B.E.; Christiani, D.C. Smoking History as a Potential Predictor of Immune Checkpoint Inhibitor Efficacy in Metastatic Non-Small Cell Lung Cancer. JNCI J. Nat. Cancer Inst. 2021, 113, 1761–1769. [Google Scholar] [CrossRef]
- Mo, J.; Hu, X.; Gu, L.; Chen, B.; Khadaroo, P.A.; Shen, Z.; Dong, L.; Lv, Y.; Chitumba, M.N.; Liu, J. Smokers or non-smokers: Who benefits more from immune checkpoint inhibitors in treatment of malignancies? An up-to-date meta-analysis. World J. Surg. Oncol. 2020, 18, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.C.; Lord, S.J.; Kasherman, L.; Marschner, I.; Stockler, M.; Gralla, R.; Yang, J.C.-H.; Mok, T.; Lee, C.K. The impact of smoking on the effectiveness of immune checkpoint inhibitors—A systematic review and meta-analysis. Acta Oncol. 2020, 59, 96–100. [Google Scholar] [CrossRef] [PubMed]
- Eng, L.; Brual, J.; Nagee, A.; Mok, S.; Fazelzad, R.; Truscott, R.; Mittmann, N.; Chaiton, M.; Saunders, D.; Liu, G.; et al. Reporting of tobacco use and impact on outcomes in cancer cooperative group clinical trials: A systematic scoping review. J. Clin. Oncol. 2021, 39, 40. [Google Scholar] [CrossRef]
- Land, S.R.; Warren, G.W.; Crafts, J.L.; Hatsukami, D.K.; Ostroff, J.S.; Willis, G.B.; Chollette, V.Y.; Mitchell, S.A.; Folz, J.N.; Gulley, J.L.; et al. Cognitive testing of tobacco use items for administration to patients with cancer and cancer survivors in clinical research. Cancer 2016, 122, 1728–1734. [Google Scholar] [CrossRef]
- Walter, A.W.; Lee, J.-W.; Gareen, I.F.; Kircher, S.M.; Herman, B.A.; Streck, J.M.; Kumar, S.; Mayer, I.A.; Saba, N.F.; Neal, J.W.; et al. Neighborhood socioeconomic disadvantage, tobacco use, and cessation indicators among adults with cancer in the United States: Results from 10 ECOG-ACRIN trials. J. Clin. Oncol. 2022, 40, 6514. [Google Scholar] [CrossRef]
- Warren, G.W.; Kasza, K.A.; Reid, M.E.; Cummings, K.M.; Marshall, J.R. Smoking at diagnosis and survival in cancer patients. Int. J. Cancer 2013, 132, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, F.A.; Rodrigues Pereira, J.; Ciuleanu, T.; Tan, E.H.; Hirsh, V.; Thongprasert, S.; Campos, D.; Maoleekoonpiroj, S.; Smylie, M.; Martins, R.; et al. Erlotinib in Previously Treated Non–Small-Cell Lung Cancer. N. Eng. J. Med. 2005, 353, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Van der Bol, J.M.; Mathijssen, R.H.; Loos, W.J.; Friberg, L.E.; van Schaik, R.H.; de Jonge, M.J.; Planting, A.S.T.; Verweij, J.; Sparreboom, A.; de Jong, F.A. Cigarette smoking and irinotecan treatment: Pharmacokinetic interaction and effects on neutropenia. J. Clin. Oncol. 2007, 25, 2719–2726. [Google Scholar] [CrossRef] [PubMed]
- Keizman, D.; Gottfried, M.; Ish-Shalom, M.; Maimon, N.; Peer, A.; Neumann, A.; Hammers, H.; Eisenberger, M.A.; Sinibaldi, V.; Pili, R.; et al. Active smoking may negatively affect response rate, progression-free survival, and overall survival of patients with metastatic renal cell carcinoma treated with sunitinib. Oncologist 2014, 19, 51–60. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corke, L.K.; Li, J.J.N.; Leighl, N.B.; Eng, L. Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer. Curr. Oncol. 2022, 29, 6260-6276. https://doi.org/10.3390/curroncol29090492
Corke LK, Li JJN, Leighl NB, Eng L. Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer. Current Oncology. 2022; 29(9):6260-6276. https://doi.org/10.3390/curroncol29090492
Chicago/Turabian StyleCorke, Lucy K., Janice J. N. Li, Natasha B. Leighl, and Lawson Eng. 2022. "Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer" Current Oncology 29, no. 9: 6260-6276. https://doi.org/10.3390/curroncol29090492
APA StyleCorke, L. K., Li, J. J. N., Leighl, N. B., & Eng, L. (2022). Tobacco Use and Response to Immune Checkpoint Inhibitor Therapy in Non-Small Cell Lung Cancer. Current Oncology, 29(9), 6260-6276. https://doi.org/10.3390/curroncol29090492