Spinal Lesions as Clinical Manifestations of Plasma Cell Neoplasia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Methods
2.2. Statistics
2.3. Ethics Approval
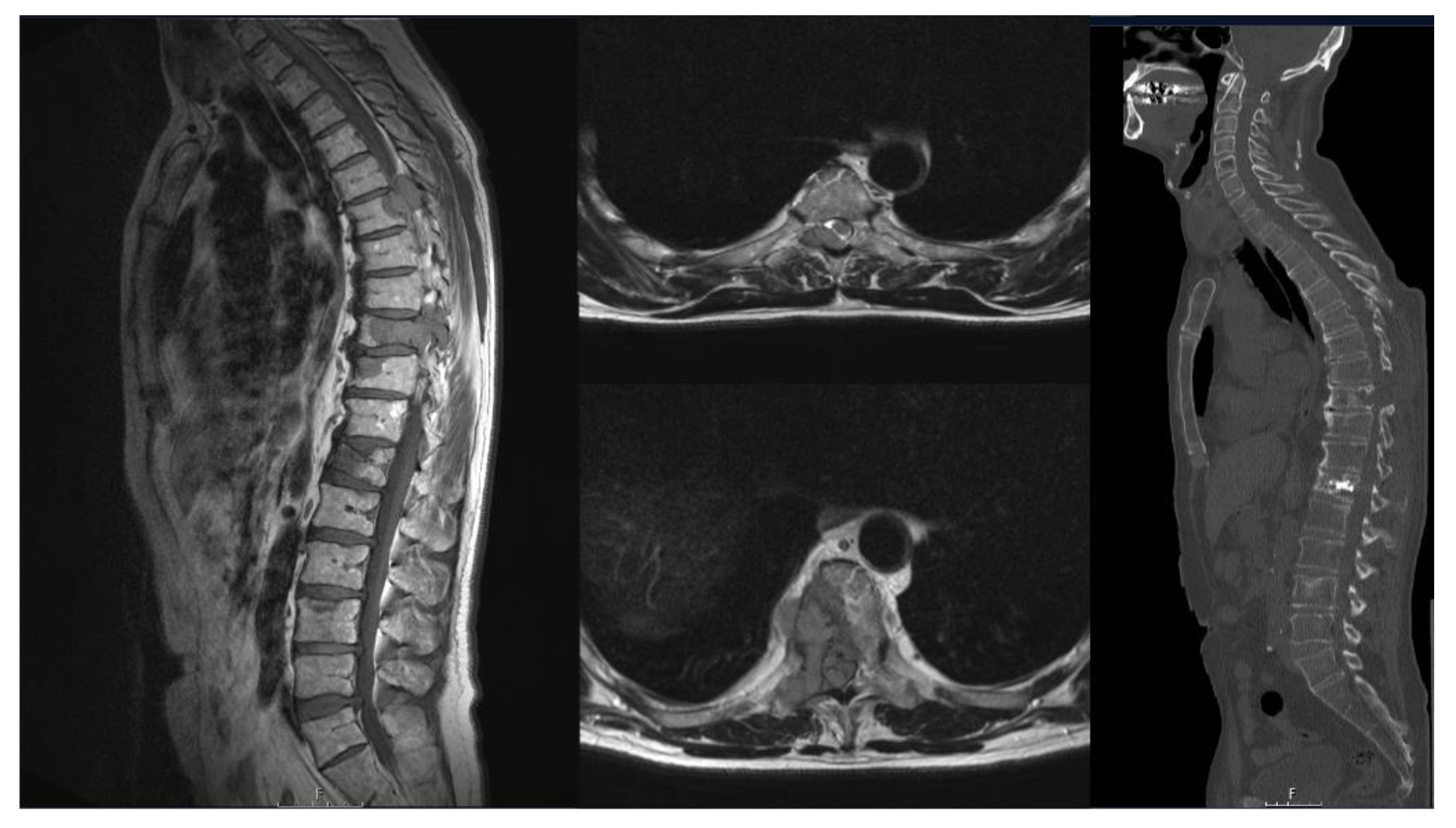
3. Results
3.1. Overall Survival
3.1.1. Univariate Analysis
3.1.2. Multivariate Analysis
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef]
- Palumbo, A.; Anderson, K. Multiple myeloma. N. Engl. J. Med. 2011, 364, 1046–1060. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Dimopoulos, M.A.; Palumbo, A.; Blade, J.; Merlini, G.; Mateos, M.-V.; Kumar, S.; Hillengass, J.; Kastritis, E.; Richardson, P.; et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014, 15, e538–e548. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; Liu, C.; Chen, C.; Yan, J. Solitary Plasmacytoma of Bone of the Spine: Results From Surveillance, Epidemiology, and End Results (SEER) Registry. Spine 2019, 44, E117–E225. [Google Scholar] [CrossRef]
- Landgren, O.; Weiss, B.M. Patterns of monoclonal gammopathy of undetermined significance and multiple myeloma in various ethnic/racial groups: Support for genetic factors in pathogenesis. Leukemia. 2009, 23, 1691–1697. [Google Scholar] [CrossRef]
- Ludwig, H.; Durie, B.G.M.; Bolejack, V.; Turesson, I.; Kyle, R.A.; Blade, J.; Fonseca, R.; Dimopoulos, M.; Shimizu, K.; Miguel, J.S.; et al. Myeloma in patients younger than age 50 years presents with more favorable features and shows better survival: An analysis of 10,549 patients from the International Myeloma Working Group. Blood 2008, 111, 4039–4047. [Google Scholar] [CrossRef]
- Langseth, Ø.; Myklebust, T.; Johannesen, T.B.; Hjertner, Ø.; Waage, A. Incidence and survival of multiple myeloma: A population-based study of 10,524 patients diagnosed 1982–2017. Br. J. Haematol. 2020, 191, 418–425. [Google Scholar] [CrossRef]
- Howlader, N.N.A.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; Chen, H.S.; et al. (Eds.) SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 1 April 2019).
- Attal, M.; Lauwers-Cances, V.; Hulin, C.; Leleu, X.; Caillot, D.; Escoffre, M.; Arnulf, B.; Macro, M.; Belhadj, K.; Garderet, L.; et al. Lenalidomide, Bortezomib, and Dexamethasone with Transplantation for Myeloma. N. Engl. J. Med. 2017, 376, 1311–1320. [Google Scholar] [CrossRef]
- Durie, B.G.M.; Hoering, A.; Abidi, M.H.; Rajkumar, S.V.; Epstein, J.; Kahanic, S.P.; Thakuri, M.; Reu, F.; Reynolds, C.M.; Sexton, R.; et al. Bortezomib with lenalidomide and dexamethasone versus lenalidomide and dexamethasone alone in patients with newly diagnosed myeloma without intent for immediate autologous stem-cell transplant (SWOG S0777): A randomised, open-label, phase 3 trial. Lancet 2017, 389, 519–527. [Google Scholar] [CrossRef]
- Russell, S.J.; Rajkumar, S.V. Multiple myeloma and the road to personalised medicine. Lancet Oncol. 2011, 12, 617–619. [Google Scholar] [CrossRef]
- Vu, T.; Gonsalves, W.I.; Kumar, S.; Dispenzieri, A.; Lacy, M.Q.; Buadi, F.K.; Gertz, M.A.; Rajkumar, S.V. Characteristics of exceptional responders to lenalidomide-based therapy in multiple myeloma. Blood Cancer J. 2015, 5, e363. [Google Scholar] [CrossRef]
- Durie, B.G.; Salmon, S.E. A clinical staging system for multiple myeloma. Correlation of measured myeloma cell mass with presenting clinical features, response to treatment, and survival. Cancer 1975, 36, 842–854. [Google Scholar] [CrossRef]
- Greipp, P.R.; Miguel, J.S.; Durie, B.G.; Crowley, J.J.; Barlogie, B.; Bladé, J.; Boccadoro, M.; Child, J.A.; Avet-Loiseau, H.; Kyle, R.A.; et al. International staging system for multiple myeloma. J. Clin. Oncol. 2005, 23, 3412–3420. [Google Scholar] [CrossRef]
- Hari, P.N.; Zhang, M.-J.; Roy, V.; Pérez, W.S.; Bashey, A.; To, L.B.; Elfenbein, G.; Freytes, C.O.; Gale, R.P.; Gibson, J.; et al. Is the International Staging System superior to the Durie-Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia 2009, 23, 1528–1534. [Google Scholar] [CrossRef]
- Palumbo, A.; Avet-Loiseau, H.; Oliva, S.; Lokhorst, H.M.; Goldschmidt, H.; Rosinol, L.; Richardson, P.; Caltagirone, S.; Lahuerta, J.J.; Facon, T.; et al. Revised International Staging System for Multiple Myeloma: A Report From International Myeloma Working Group. J. Clin. Oncol. 2015, 33, 2863–2869. [Google Scholar] [CrossRef]
- Kumar, S.K.; Mikhael, J.R.; Buadi, F.K.; Dingli, D.; Dispenzieri, A.; Fonseca, R.; Gertz, M.A.; Greipp, P.R.; Hayman, S.R.; Kyle, R.A.; et al. Management of newly diagnosed symptomatic multiple myeloma: Updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo. Clin. Proc. 2009, 84, 1095–1110. [Google Scholar] [CrossRef]
- Kumar, S.; Paiva, B.; Anderson, K.C.; Durie, B.; Landgren, O.; Moreau, P.; Munshi, N.; Lonial, S.; Bladé, J.; Mateos, M.-V.; et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016, 17, e328–e346. [Google Scholar] [CrossRef]
- Abbott, K.C.; Agodoa, L.Y. Multiple myeloma and light chain-associated nephropathy at end-stage renal disease in the United States: Patient characteristics and survival. Clin. Nephrol. 2001, 56, 207–210. [Google Scholar]
- Bellomo, R.; Ronco, C.; Kellum, J.A.; Mehta, R.L.; Palevsky, P.; Workgroup ADQI. Acute renal failure—Definition, outcome measures, animal models, fluid therapy and information technology needs: The Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit. Care. 2004, 8, R204–R212. [Google Scholar] [CrossRef]
- Bladé, J.; Rosiñol, L. Renal, hematologic and infectious complications in multiple myeloma. Best Pract. Res. Clin. Haematol. 2005, 18, 635–652. [Google Scholar] [CrossRef]
- Wallington, M.; Mendis, S.; Premawardhana, U.; Sanders, P.; Shahsavar-Haghighi, K. Local control and survival in spinal cord compression from lymphoma and myeloma. Radiother. Oncol. 1997, 42, 43–47. [Google Scholar] [CrossRef]
- Cai, W.; Yan, W.; Huang, Q.; Huang, W.; Yin, H.; Xiao, J. Surgery for plasma cell neoplasia patients with spinal instability or neurological impairment caused by spinal lesions as the first clinical manifestation. Eur. Spine J. 2015, 24, 1761–1767. [Google Scholar] [CrossRef]
- Amelot, A.; Moles, A.; Cristini, J.; Salaud, C.; Touzeau, C.; Hamel, O.; Bord, E.; Buffenoir, K. Predictors of survival in patients with surgical spine multiple myeloma metastases. Surg. Oncol. 2016, 25, 178–183. [Google Scholar] [CrossRef]
- Tomita, K.; Kawahara, N.; Kobayashi, T.; Yoshida, A.; Murakami, H.; Akamaru, T. Surgical strategy for spinal metastases. Spine 2001, 26, 298–306. [Google Scholar] [CrossRef]
- Association GAotWM. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. J. Am. Coll. Dent. 2014, 81, 14–18. [Google Scholar]
- Fourney, D.R.; Frangou, E.M.; Ryken, T.C.; DiPaola, C.P.; Shaffrey, C.I.; Berven, S.H.; Bilsky, M.H.; Harrop, J.S.; Fehlings, M.G.; Boriani, S.; et al. Spinal instability neoplastic score: An analysis of reliability and validity from the spine oncology study group. J. Clin. Oncol. 2011, 29, 3072–3077. [Google Scholar] [CrossRef]
- Jónsson, B.; Sjöström, L.; Jónsson, H.; Karlström, G. Surgery for multiple myeloma of the spine. A retrospective analysis of 12 patients. Acta Orthop. Scand. 1992, 63, 192–194. [Google Scholar] [CrossRef]
- Short, K.D.; Rajkumar, S.V.; Larson, D.; Buadi, F.; Hayman, S.; Dispenzieri, A.; Gertz, M.; Kumar, S.; Mikhael, J.; Roy, V.; et al. Incidence of extramedullary disease in patients with multiple myeloma in the era of novel therapy, and the activity of pomalidomide on extramedullary myeloma. Leukemia 2011, 25, 906–908. [Google Scholar] [CrossRef]
- Kyriakou, C.; Molloy, S.; Vrionis, F.; Alberico, R.; Bastian, L.; Zonder, J.A.; Giralt, S.; Raje, N.; Kyle, R.A.; Roodman, D.G.D.; et al. The role of cement augmentation with percutaneous vertebroplasty and balloon kyphoplasty for the treatment of vertebral compression fractures in multiple myeloma: A consensus statement from the International Myeloma Working Group (IMWG). Blood Cancer J. 2019, 9, 27. [Google Scholar] [CrossRef]
- Zadnik, P.L.; Goodwin, C.R.; Karami, K.J.; Mehta, A.I.; Amin, A.G.; Groves, M.L.; Wolinsky, J.-P.; Witham, T.F.; Bydon, A.; Gokaslan, Z.L.; et al. Outcomes following surgical intervention for impending and gross instability caused by multiple myeloma in the spinal column. J. Neurosurg. Spine 2015, 22, 301–309. [Google Scholar] [CrossRef]
- Dispenzieri, A.; Kyle, R.A. Neurological aspects of multiple myeloma and related disorders. Best Pract. Res. Clin. Haematol. 2005, 18, 673–688. [Google Scholar] [CrossRef]
- Cawley, D.T.; Butler, J.S.; Benton, A.; Altaf, F.; Rezajooi, K.; Kyriakou, C.; Selvadurai, S.; Molloy, S. Managing the cervical spine in multiple myeloma patients. Hematol. Oncol. 2019, 37, 129–135. [Google Scholar] [CrossRef]
- Patchell, R.A.; Tibbs, P.A.; Walsh, J.W.; Dempsey, R.J.; Maruyama, Y.; Kryscio, R.J.; Markesbery, W.R.; Macdonald, J.S.; Young, B. A randomized trial of surgery in the treatment of single metastases to the brain. N. Engl. J. Med. 1990, 322, 494–500. [Google Scholar] [CrossRef]
- Qian, J.; Jing, J.; Tian, D.; Yang, H. Partial tumor resection combined with chemotherapy for multiple myeloma spinal cord compression. Ann. Surg. Oncol. 2014, 21, 3661–3667. [Google Scholar] [CrossRef]
- Rao, G.; Ha, C.S.; Chakrabarti, I.; Feiz-Erfan, I.; Mendel, E.; Rhines, L.D. Multiple myeloma of the cervical spine: Treatment strategies for pain and spinal instability. J. Neurosurg. Spine 2006, 5, 140–145. [Google Scholar] [CrossRef]
- Ma, X.; An, H.S.; Zhang, Y.; Brown, N.M.; Chen, Z.; Zhang, G.; Xiang, H.; Hu, Y.; Chen, B. A radical procedure of circumferential spinal cord decompression through a modified posterior approach for thoracic myelopathy caused by severely impinging anterior ossification. Spine J. 2014, 14, 651–658. [Google Scholar] [CrossRef]
- Soutar, R.; Lucraft, H.; Jackson, G.; Reece, A.; Bird, J.; Low, E.; Samson, D. Guidelines on the diagnosis and management of solitary plasmacytoma of bone and solitary extramedullary plasmacytoma. Clin. Oncol. 2004, 16, 405–413. [Google Scholar] [CrossRef]
- Hu, K.; Yahalom, J. Radiotherapy in the management of plasma cell tumors. Oncology 2000, 14, 101–108+111; discussion 111–112+115. [Google Scholar]
- Liebross, R.H.; Ha, C.S.; Cox, J.D.; Weber, D.; Delasalle, K.; Alexanian, R. Solitary bone plasmacytoma: Outcome and prognostic factors following radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1998, 41, 1063–1067. [Google Scholar] [CrossRef]
- Galieni, P.; Cavo, M.; Pulsoni, A.; Avvisati, G.; Bigazzi, C.; Neri, S.; Caliceti, U.; Benni, M.; Ronconi, S.; Lauria, F. Clinical outcome of extramedullary plasmacytoma. Haematologica 2000, 85, 47–51. [Google Scholar]
- Child, J.A.; Morgan, G.J.; Davies, F.E.; Owen, R.G.; Bell, S.E.; Hawkins, K.; Brown, J.; Drayson, M.T.; Selby, P.J.; Medical Research Council Adult Leukaemia Working Party. High-dose chemotherapy with hematopoietic stem-cell rescue for multiple myeloma. N. Engl. J. Med. 2003, 348, 1875–1883. [Google Scholar] [CrossRef] [PubMed]
- Lenhoff, S.; Hjorth, M.; Holmberg, E.; Turesson, I.; Westin, J.; Nielsen, J.L.; Wislöff, F.; Brinch, L.; Carlson, K.; Carlsson, M.; et al. Impact on survival of high-dose therapy with autologous stem cell support in patients younger than 60 years with newly diagnosed multiple myeloma: A population-based study. Nordic Myeloma Study Group. Blood 2000, 95, 7–11. [Google Scholar] [PubMed]
- Singhal, S.; Mehta, J.; Desikan, R.; Ayers, D.; Roberson, P.; Eddlemon, P.; Munshi, N.; Anaissie, E.; Wilson, C.; Dhodapkar, M.; et al. Antitumor activity of thalidomide in refractory multiple myeloma. N. Engl. J. Med. 1999, 341, 1565–1571. [Google Scholar] [CrossRef] [PubMed]
- Rajkumar, S.V.; Blood, E.; Vesole, D.; Fonseca, R.; Greipp, P.R. Phase III clinical trial of thalidomide plus dexamethasone compared with dexamethasone alone in newly diagnosed multiple myeloma: A clinical trial coordinated by the Eastern Cooperative Oncology Group. J. Clin. Oncol. 2006, 24, 431–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, P.G.G.; Barlogie, B.; Berenson, J.; Singhal, S.; Jagannath, S.; Irwin, D.; Rajkumar, S.V.; Hideshima, T.; Xiao, H.; Esseltine, D.; et al. Clinical factors predictive of outcome with bortezomib in patients with relapsed, refractory multiple myeloma. Blood 2005, 106, 2977–2981. [Google Scholar] [CrossRef]
- Cavo, M.; Zamagni, E.; Tosi, P.; Tacchetti, P.; Cellini, C.; Cangini, D.; De Vivo, A.; Testoni, N.; Nicci, C.; Terragna, C.; et al. Superiority of thalidomide and dexamethasone over vincristine-doxorubicindexamethasone (VAD) as primary therapy in preparation for autologous transplantation for multiple myeloma. Blood 2005, 106, 35–39. [Google Scholar] [CrossRef]

| Demographics n (%) or Mean/Median | Multiple Myeloma | Solitary Bone Plasmocytoma | Total |
|---|---|---|---|
| Age | 64.29 years | 64.18 years | 64.27 years |
| Sex | 62 m/32 f | 14 m/6 f | 76 m/38 f |
| Clinical presentation preoperative | |||
| KPSS | 80% | 90% | 80% |
| ASIA A | 0 | 0 | 0 |
| ASIA B | 6 (6.38%) | 2 (10.00%) | 8 (7.02%) |
| ASIA C | 9 (9.57%) | 2 (10.00%) | 11 (9.65%) |
| ASIA D | 20 (21.28%) | 6 (30.00%) | 26 (22.81%) |
| ASIA E | 59 (62.77%) | 10 (50.00%) | 69 (60.53%) |
| SINS, n (%) | |||
| Median | 8 | 8 | 8 |
| Mean | 8 | 8 | 8 |
| Stable | 20 (21.27%) | 4 (20.00%) | 24 (21.05%) |
| Indeterminate | 68 (72.35%) | 15 (75.00%) | 83 (72.81%) |
| Instable | 6 (6.38%) | 1 (5.00%) | 7 (6.14%) |
| Clinical presentation postoperative | |||
| KPSS | 80% | 90% | 80% |
| ASIA A | 0 | 0 | 0 |
| ASIA B | 1 (1.06%) | 0 | 1 (0.88%) |
| ASIA C | 9 (9.57%) | 4 (20.00%) | 13 (11.40%) |
| ASIA D | 22 (23.40%) | 6 (30.00%) | 28 (24.56%) |
| ASIA E | 62 (65.96%) | 10 (50.00%) | 72 (63.16%) |
| Location n (%) or Mean/Median | Multiple Myeloma | Solitary Bone Plasmocytoma | Total |
|---|---|---|---|
| Cervical | 12 (12.77%) | 5 (25.00%) | 17 (14.91%) |
| Thoracic | 26 (27.66%) | 9 (45.00%) | 35 (30.70%) |
| Lumbar | 11 (11.70%) | 1 (5.00%) | 12 (10.53%) |
| Sacral | 2 (2.13%) | 1 (5.00%) | 3 (2.63%) |
| Cervico-thoracic | 17 (18.09%) | 3 (15.00%) | 20 (17.54%) |
| Thoraco-lumbar | 22 (23.40%) | 1 (5.00%) | 23 (20.18%) |
| Lumbo-sacral | 1 (1.06%) | 0 | 1 (0.88%) |
| Thoraco-lumbo-sacral | 2 (2.13%) | 0 | 2 (1.75%) |
| Cervical and lumbar | 1 (1.06%) | 0 | 1 (0.88%) |
| Adjuvant Treatment * n (%) or Mean/Median | Multiple Myeloma | Solitary Bone Plasmocytoma | Total |
|---|---|---|---|
| Chemoimmune therapy alone | 27 (28.72%) | 2 (10.00%) | 29 (25.44%) |
| Radiotherapy alone | 18 (19.15%) | 9 (45.00%) | 27 (23.68%) |
| Chemoimmune therapy+ radiotherapy | 27 (28.72%) | 3 (15.00%) | 30 (26.32%) |
| Unknown | 21 (22.34%) | 6 (30.00%) | 27 (23.68%) |
| Antibody therapy | 1 (1.06%) | 0 | 1 (0.88%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgart, L.; Barz, M.; Delbridge, C.; Aftahy, A.K.; Janssen, I.K.; Jost, P.J.; Ryang, Y.-M.; Meyer, B.; Gempt, J. Spinal Lesions as Clinical Manifestations of Plasma Cell Neoplasia. Curr. Oncol. 2022, 29, 6236-6244. https://doi.org/10.3390/curroncol29090490
Baumgart L, Barz M, Delbridge C, Aftahy AK, Janssen IK, Jost PJ, Ryang Y-M, Meyer B, Gempt J. Spinal Lesions as Clinical Manifestations of Plasma Cell Neoplasia. Current Oncology. 2022; 29(9):6236-6244. https://doi.org/10.3390/curroncol29090490
Chicago/Turabian StyleBaumgart, Lea, Melanie Barz, Claire Delbridge, Amir Kaywan Aftahy, Insa Katrin Janssen, Philipp J. Jost, Yu-Mi Ryang, Bernhard Meyer, and Jens Gempt. 2022. "Spinal Lesions as Clinical Manifestations of Plasma Cell Neoplasia" Current Oncology 29, no. 9: 6236-6244. https://doi.org/10.3390/curroncol29090490
APA StyleBaumgart, L., Barz, M., Delbridge, C., Aftahy, A. K., Janssen, I. K., Jost, P. J., Ryang, Y.-M., Meyer, B., & Gempt, J. (2022). Spinal Lesions as Clinical Manifestations of Plasma Cell Neoplasia. Current Oncology, 29(9), 6236-6244. https://doi.org/10.3390/curroncol29090490






