Assessing Pretransplant and Posttransplant Therapy Response in Multiple Myeloma Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Settings
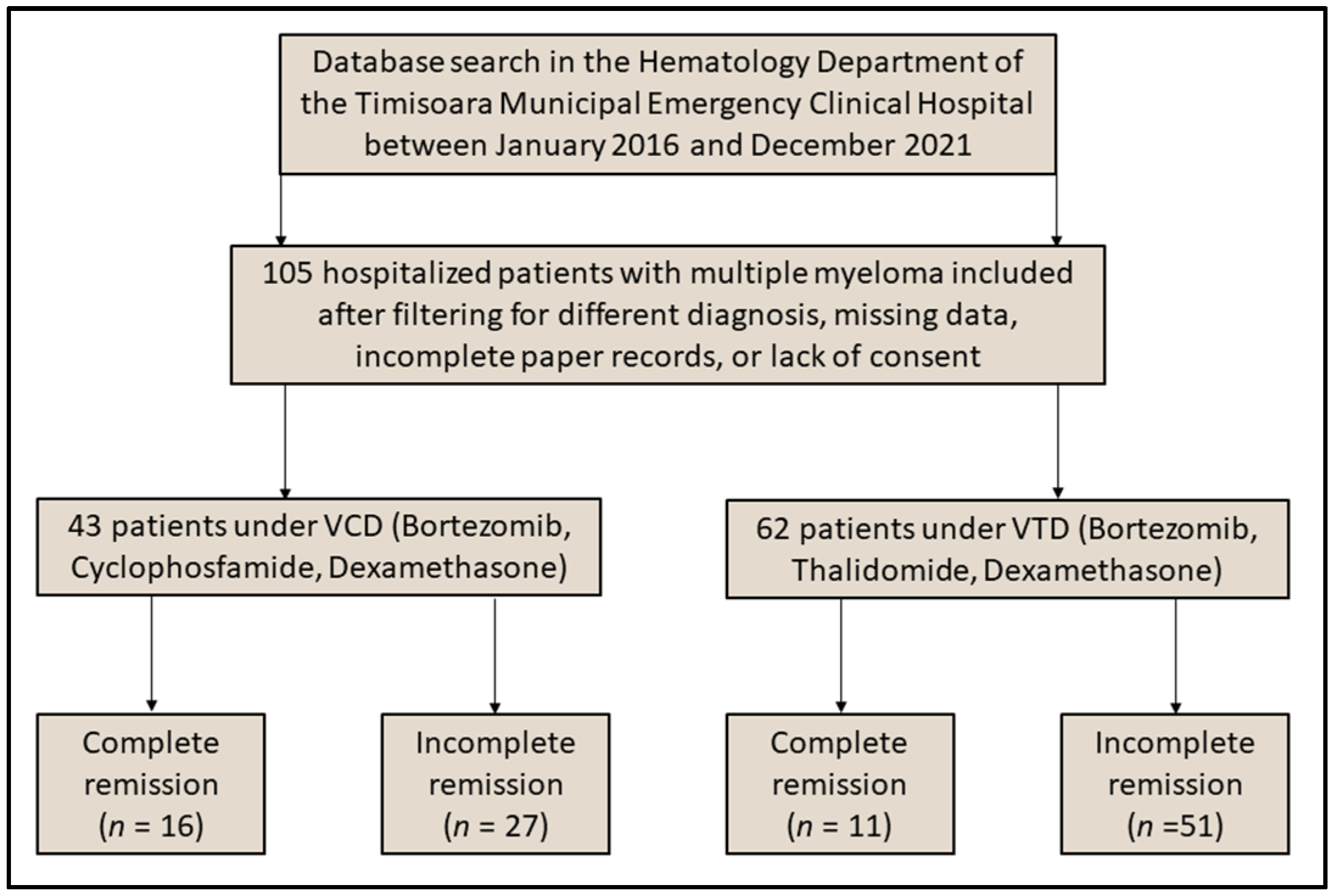
2.2. Participants and Protocols
2.3. Variables
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Multiple Myeloma Characteristics
4. Discussion
4.1. Literature Findings
4.2. Study Limitations and Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dima, D.; Jiang, D.; Singh, D.J.; Hasipek, M.; Shah, H.S.; Ullah, F.; Khouri, J.; Maciejewski, J.P.; Jha, B.K. Multiple Myeloma Therapy: Emerging Trends and Challenges. Cancers 2022, 14, 4082. [Google Scholar] [CrossRef]
- Boeriu, E.; Borda, A.; Vulcanescu, D.D.; Sarbu, V.; Arghirescu, S.T.; Ciorica, O.; Bratosin, F.; Marincu, I.; Horhat, F.G. Diagnosis and Management of Febrile Neutropenia in Pediatric Oncology Patients—A Systematic Review. Diagnostics 2022, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Kazandjian, D. Multiple myeloma epidemiology and survival: A unique malignancy. Semin. Oncol. 2016, 43, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Ghobrial, I.M. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: A review of the current understanding of epidemiology, biology, risk stratification, and management of myeloma precursor disease. Clin. Cancer Res. 2013, 19, 985–994. [Google Scholar] [CrossRef] [PubMed]
- Pinto, V.; Bergantim, R.; Caires, H.R.; Seca, H.; Guimarães, J.E.; Vasconcelos, M.H. Multiple Myeloma: Available Therapies and Causes of Drug Resistance. Cancers 2020, 12, 407. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, F.; Schreder, M.; Engelhardt, M.; Langer, C.; Wolleschak, D.; Mügge, L.O.; Dürk, H.; Schäfer-Eckart, K.; Blau, I.W.; Gramatzki, M.; et al. Kinetics of Renal Function during Induction in Newly Diagnosed Multiple Myeloma: Results of Two Prospective Studies by the German Myeloma Study Group DSMM. Cancers 2021, 13, 1322. [Google Scholar] [CrossRef] [PubMed]
- Aljama, M.A.; Sidiqi, M.H.; Lakshman, A.; Dispenzieri, A.; Jevremovic, D.; Gertz, M.A.; Lacy, M.Q.; Buadi, F.K.; Dingli, D.; Muchtar, E.; et al. Plasma cell proliferative index is an independent predictor of progression in smoldering multiple myeloma. Blood Adv. 2018, 2, 3149–3154. [Google Scholar] [CrossRef]
- Bird, S.A.; Boyd, K. Multiple myeloma: An overview of management. Palliat. Care Soc. Pract. 2019, 13, 1178224219868235. [Google Scholar] [CrossRef]
- Soh, K.T.; Tario JDJr Wallace, P.K. Diagnosis of Plasma Cell Dyscrasias and Monitoring of Minimal Residual Disease by Multiparametric Flow Cytometry. Clin. Lab. Med. 2017, 37, 821–853. [Google Scholar] [CrossRef]
- Nunnelee, J.; Cottini, F.; Zhao, Q.; Faisal, M.S.; Elder, P.; Rosko, A.; Bumma, N.; Khan, A.; Devarakonda, S.; Benson, D.M.; et al. Improvement in Post-Autologous Stem Cell Transplant Survival of Multiple Myeloma Patients: A Long-Term Institutional Experience. Cancers 2022, 14, 2277. [Google Scholar] [CrossRef]
- Folescu, R.; Levai, C.M.; Grigoras, M.L.; Arghirescu, T.S.; Talpos, I.C.; Gindac, C.M.; Zamfir, C.L.; Poroch, V.; Anghel, M.D. Expression and significance of Ki-67 in lung cancer. Rom. J. Morphol. Embryol. 2018, 59, 227–233. [Google Scholar]
- Marincu, I.; Bratosin, F.; Curescu, M.; Suciu, O.; Turaiche, M.; Cerbu, B.; Vidican, I. Direct-Acting Antiviral Use for Genotype 1b Hepatitis C Patients with Associated Hematological Disorders from Romania. Medicina 2021, 57, 986. [Google Scholar] [CrossRef]
- Paquin, A.R.; Kumar, S.K.; Buadi, F.K.; Gertz, M.A.; Lacy, M.Q.; Dispenzieri, A.; Dingli, D.; Hwa, L.; Fonder, A.; Hobbs, M.; et al. Overall survival of transplant eligible patients with newly diagnosed multiple myeloma: Comparative effectiveness analysis of modern induction regimens on outcome. Blood Cancer J. 2018, 8, 125. [Google Scholar] [CrossRef]
- Rodríguez-Otero, P.; Mateos, M.V.; Martínez-López, J.; Hernández, M.T.; Ocio, E.M.; Rosiñol, L.; Martínez, R.; Teruel, A.I.; Gutiérrez, N.C.; Bargay, J.; et al. Predicting long-term disease control in transplant-ineligible patients with multiple myeloma: Impact of an MGUS-like signature. Blood Cancer J. 2019, 9, 36. [Google Scholar] [CrossRef]
- Hall, B.R.; Cannon, A.; Atri, P.; Wichman, C.S.; Smith, L.M.; Kumar, S.; Batra, S.K.; Wang, H.; Ganti, A.K.; Sasson, A.R.; et al. A Comparative Analysis of Survival and Funding Discrepancies in Cancers With High Mortality. Ann. Surg. 2020, 271, 296–302. [Google Scholar] [CrossRef]
- Jianu, C.; Goleț, I.; Stoin, D.; Cocan, I.; Bujancă, G.; Mișcă, C.; Mioc, M.; Mioc, A.; Șoica, C.; Lukinich-Gruia, A.T.; et al. Chemical Profile of Ruta graveolens, Evaluation of the Antioxidant and Antibacterial Potential of Its Essential Oil, and Molecular Docking Simulations. Appl. Sci. 2021, 11, 11753. [Google Scholar] [CrossRef]
- Ioan Faur, C.; Abu-Awwad, A.; Pop, D.L.; Zamfir, C.L.; Gurgus, D.; Hoinoiu, T.; Motoc, A.; Haivas, C.; Grigoraș, M.L.; Folescu, R. Liquid Nitrogen Efficiency in Treatment of Giant Cell Tumor of Bone and Prevention of Recurrence. Appl. Sci. 2020, 10, 6310. [Google Scholar] [CrossRef]
- Rajkumar, S.V. Treatment of multiple myeloma. Nat. Rev. Clin. Oncol. 2011, 8, 479–491. [Google Scholar] [CrossRef]
- Poczta, A.; Rogalska, A.; Marczak, A. Treatment of Multiple Myeloma and the Role of Melphalan in the Era of Modern Therapies—Current Research and Clinical Approaches. J. Clin. Med. 2021, 10, 1841. [Google Scholar] [CrossRef]
- Branagan, A.; Lei, M.; Lou, U.; Raje, N. Current Treatment Strategies for Multiple Myeloma. JCO Oncol. Pract. 2020, 16, 5–14. [Google Scholar] [CrossRef]
- D’Agostino, M.; Bertamini, L.; Oliva, S.; Boccadoro, M.; Gay, F. Pursuing a Curative Approach in Multiple Myeloma: A Review of New Therapeutic Strategies. Cancers 2019, 11, 2015. [Google Scholar] [CrossRef] [PubMed]
- Jeryczynski, G.; Bolomsky, A.; Agis, H.; Krauth, M.-T. Stratification for RRMM and Risk-Adapted Therapy: Sequencing of Therapies in RRMM. Cancers 2021, 13, 5886. [Google Scholar] [CrossRef] [PubMed]
- Hanbali, A.; Hassanein, M.; Rasheed, W.; Aljurf, M.; Alsharif, F. The Evolution of Prognostic Factors in Multiple Myeloma. Adv. Hematol. 2017, 2017, 4812637. [Google Scholar] [CrossRef] [PubMed]
- Chari, A.; Martinez-Lopez, J.; Mateos, M.V.; Bladé, J.; Benboubker, L.; Oriol, A.; Arnulf, B.; Rodriguez-Otero, P.; Pineiro, L.; Jakubowiak, A.; et al. Daratumumab plus carfilzomib and dexamethasone in patients with relapsed or refractory multiple myeloma. Blood 2019, 134, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Gengenbach, L.; Graziani, G.; Reinhardt, H.; Rösner, A.; Braun, M.; Möller, M.D.; Greil, C.; Wäsch, R.; Engelhardt, M. Choosing the Right Therapy for Patients with Relapsed/Refractory Multiple Myeloma (RRMM) in Consideration of Patient-, Disease- and Treatment-Related Factors. Cancers 2021, 13, 4320. [Google Scholar] [CrossRef]
- Rajkumar, S.V.; Kumar, S. Multiple myeloma current treatment algorithms. Blood Cancer J. 2020, 10, 94. [Google Scholar] [CrossRef]
- Sonneveld, P.; Broijl, A. Treatment of relapsed and refractory multiple myeloma. Haematologica 2016, 101, 396–406. [Google Scholar] [CrossRef]
- Kim, D.S.; Yu, E.S.; Kang, K.W.; Lee, S.R.; Park, Y.; Sung, H.J.; Choi, C.W.; Kim, B.S. Myeloma prognostic index at diagnosis might be a prognostic marker in patients newly diagnosed with multiple myeloma. Korean J. Intern. Med. 2017, 32, 711–721. [Google Scholar] [CrossRef]
- World Health Organization. ICD-10: International Statistical Classification of Diseases and Related Health Problems: Tenth Revision, 2nd ed.; World Health Organization: Geneva, Switzerland, 2004; Available online: https://apps.who.int/iris/handle/10665/42980 (accessed on 24 July 2022).
- Ludwig, H.; Novis Durie, S.; Meckl, A.; Hinke, A.; Durie, B. Multiple Myeloma Incidence and Mortality Around the Globe; Interrelations Between Health Access and Quality, Economic Resources, and Patient Empowerment. Oncologist 2020, 25, e1406–e1413. [Google Scholar] [CrossRef]
- Hari, P.N.; Zhang, M.J.; Roy, V.; Pérez, W.S.; Bashey, A.; To, L.B.; Elfenbein, G.; Freytes, C.O.; Gale, R.P.; Gibson, J.; et al. Is the International Staging System superior to the Durie-Salmon staging system? A comparison in multiple myeloma patients undergoing autologous transplant. Leukemia 2009, 23, 1528–1534. [Google Scholar] [CrossRef]
- Lee, Y.J.; Moon, J.H.; Sohn, S.K.; Kim, S.J.; Jung, S.H.; Lee, J.J.; Jo, J.C.; Shin, H.J.; Lee, W.S.; Lee, J.H.; et al. Benefits of additional cycles of bortezomib/thalidomide/dexamethasone (VTD) induction therapy compared to four cycles of VTD for newly diagnosed multiple myeloma. Bone Marrow Transpl. 2019, 54, 2051–2059. [Google Scholar] [CrossRef]
- Paul, B.; Lipe, B.; Ocio, E.M.; Usmani, S.Z. Induction Therapy for Newly Diagnosed Multiple Myeloma. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e176–e186. [Google Scholar] [CrossRef]
- Giri, S.; Grimshaw, A.; Bal, S.; Godby, K.N.; Kharel, P.; Djulbegovic, B.; Usmani, S.Z.; Facon, T.; Moreau, P.; Dimopoulos, M.A.; et al. Efficacy of daratumumab in the treatment of multiple myeloma with high-risk cytogenetics: Meta-analysis of randomized phase III trials. J. Clin. Oncol. 2020, 38, 8540. [Google Scholar] [CrossRef]
- Cazaubiel, T.; Mulas, O.; Montes, L.; Schavgoulidze, A.; Avet-Loiseau, H.; Corre, J.; Perrot, A. Risk and Response-Adapted Treatment in Multiple Myeloma. Cancers 2020, 12, 3497. [Google Scholar] [CrossRef]
- Moreau, P.; Hulin, C.; Macro, M.; Caillot, D.; Chaleteix, C.; Roussel, M.; Garderet, L.; Royer, B.; Brechignac, S.; Tiab, M.; et al. VTD is superior to VCD prior to intensive therapy in multipelt myeloma: Results of the prospective IFM2013-04 trial. Blood 2016, 127, 2569–2574. [Google Scholar] [CrossRef]
- Yarlagadda, L.; Gundarlapalli, S.; Parikh, R.; Landes, R.D.; Kottarathara, M.; Ogunsesan, Y.; Hoque, S.; Mitma, A.A.; Bailey, C.; Hill, K.M.; et al. Salvage Autologous Stem Cell Transplantation in Daratumumab-Refractory Multiple Myeloma. Cancers 2021, 13, 4019. [Google Scholar] [CrossRef]
- Munshi, N.C.; Anderson, L.D., Jr.; Shah, N.; Madduri, D.; Berdeja, J.; Lonial, S.; Raje, N.; Lin, Y.; Siegel, D.; Oriol, A.; et al. Idecabtagene Vicleucel in Relapsed and Refractory Multiple Myeloma. N. Engl. J. Med. 2021, 384, 705–716. [Google Scholar] [CrossRef]
- Gandhi, U.H.; Cornell, R.F.; Lakshman, A.; Gahvari, Z.J.; McGehee, E.; Jagosky, M.H.; Gupta, R.; Varnado, W.; Fiala, M.A.; Chhabra, S.; et al. Outcomes of patients with multiple myeloma refractory to CD38-targeted monoclonal antibody therapy. Leukemia 2019, 33, 2266–2275. [Google Scholar] [CrossRef]
- Lonial, S.; Dimopoulos, M.; Palumbo, A.; White, D.; Grosicki, S.; Spicka, I.; Walter-Croneck, A.; Moreau, P.; Mateos, M.V.; Magen, H.; et al. Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N. Engl. J. Med. 2015, 373, 621–631. [Google Scholar] [CrossRef]
- Dimopoulos, M.A.; Dytfeld, D.; Grosicki, S.; Moreau, P.; Takezako, N.; Hori, M.; Leleu, X.; LeBlanc, R.; Suzuki, K.; Raab, M.S.; et al. Elotuzumab plus Pomalidomide and Dexamethasone for Multiple Myeloma. N. Engl. J. Med. 2018, 379, 1811–1822. [Google Scholar] [CrossRef] [PubMed]
- Jackson, G.H.; Davies, F.E.; Pawlyn, C.; Cairns, D.A.; Striha, A.; Collett, C.; Waterhouse, A.; Jones, J.R.; Kishore, B.; Garg, M.; et al. Lenalidomide before and after autologous stem cell transplantation for transplant-eligible patients of all ages in the randomized, phase III, Myeloma XI trial. Haematologica 2021, 106, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.P.; Barosi, G. Is lenalidomide the standard-of-care after an autotransplant for plasma cell myeloma? Leukemia 2019, 33, 588–596. [Google Scholar]
- Uyl-de Groot, C.A.; Ramsden, R.; Lee, D.; Boersma, J.; Zweegman, S.; Dhanasiri, S. Lenalidomide as maintenance treatment for patients with multiple myeloma after autologous stem cell transplantation: A pharmaco-economic assessment. Eur. J. Haematol. 2020, 105, 635–645. [Google Scholar] [CrossRef]
- Attal, M.; Harousseau, J.L.; Leyvraz, S.; Doyen, C.; Hulin, C.; Benboubker, L.; Agha, I.Y.; Bourhis, J.; Garderet, L.; Pegourie, B.; et al. Maintenance therapy with thalidomide improves survival in patients with multiple myeloma. Blood 2006, 108, 3289–3294. [Google Scholar] [CrossRef]
- Spencer, A.; Prince, H.M.; Roberts, A.W.; Prosser, I.W.; Bradstock, K.F.; Coyle, L.; Gill, D.S.; Horvath, N.; Reynolds, J.; Kennedy, N. Consolidation therapy with low-dose thalidomide and prednisolone prolongs the survival of multiple myeloma patients undergoing a single autologous stem-cell transplantation procedure. J. Clin. Oncol. 2009, 27, 1788–1793. [Google Scholar] [CrossRef][Green Version]
- Ludwig, H.; Bolejack, V.; Crowley, J.; Bladé, J.; Miguel, J.S.; Kyle, R.A.; Rajkumar, S.V.; Shimizu, K.; Turesson, I.; Westin, J.; et al. Survival and Years of Life Lost in Different Age Cohorts of Patients with Multiple Myeloma. J. Clin. Oncol. 2010, 28, 1599–1605. [Google Scholar] [CrossRef]
| Variables | VTD (n = 62) | VCD (n = 43) | p-Value * |
|---|---|---|---|
| Age | 0.935 | ||
| 18–64 years | 10 (16.1%) | 6 (14.0%) | |
| 65–75 years | 25 (40.3%) | 17 (39.5%) | |
| >75 years | 27 (43.5%) | 20 (46.5%) | |
| Sex | 0.816 | ||
| Men | 39 (62.9%) | 28 (65.1%) | |
| Women | 23 (37.1%) | 15 (34.9%) | |
| BMI | 0.107 | ||
| Underweight (<18.5 kg/m2) | 8 (12.9%) | 6 (14.0%) | |
| Normal weight (18.5–25.0 kg/m2) | 32 (51.6%) | 20 (46.5%) | |
| Overweight (>25.0 kg/m2) | 22 (35.5%) | 17 (39.5%) | |
| Area of residence (urban) | 34 (54.8%) | 26 (60.5%) | 0.566 |
| Relationship status (married) | 57 (91.9%) | 40 (93.0%) | 0.836 |
| Occupation (retired) | 52 (83.9%) | 37 (86.0%) | 0.760 |
| Substance use behavior | |||
| Frequent alcohol consumption | 11 (17.7%) | 9 (20.9%) | 0.682 |
| Frequent smoker | 20 (32.3%) | 16 (37.2%) | 0.599 |
| Chronic comorbidities ** | |||
| High blood pressure | 38 (61.3%) | 24 (55.8%) | 0.574 |
| Lung | 10 (16.1%) | 6 (14.0%) | 0.760 |
| Metabolic | 13 (21.0%) | 9 (20.9%) | 0.996 |
| Cerebrovascular | 16 (25.8%) | 15 (34.9%) | 0.315 |
| Digestive & liver | 6 (9.7%) | 4 (9.3%) | 0.948 |
| Depression | 7 (11.3%) | 4 (9.3%) | 0.743 |
| Other | 5 (8.1%) | 3 (7.0%) | 0.836 |
| Variables | VTD (n = 62) | VCD (n = 43) | p-Value * |
|---|---|---|---|
| Salmon-Durie staging | 0.124 | ||
| 1 | 5 (8.1%) | 3 (7.0%) | |
| 2 | 12 (19.4%) | 16 (37.2%) | |
| 3 | 45 (72.6%) | 24 (55.8%) | |
| Translocation t(4;14) | 9 (14.5%) | 7 (16.3%) | 0.804 |
| Chromosome 17p deletion | 5 (8.1%) | 5 (11.6%) | 0.540 |
| High GEP 70 risk score at diagnosis | 18 (29.0%) | 14 (32.6%) | 0.699 |
| High cytogenic risk | 17 (27.4%) | 12 (27.9%) | 0.956 |
| Karnosfky performance status ≥ 90 | 34 (54.8%) | 26 (60.5%) | 0.566 |
| Myeloma type | 0.887 | ||
| IgA | 13 (20.3%) | 10 (22.7%) | |
| IgG | 36 (56.3%) | 21 (47.7%) | |
| IgD | 4 (6.3%) | 4 (9.1%) | |
| Light Chain | 7 (10.9%) | 4 (9.1%) | |
| Nonsecretory | 2 (3.1%) | 2 (4.5%) | |
| Others | 2 (3.1%) | 3 (6.8%) | |
| Positive urine immunofixation | 34 (54.8%) | 25 (58.1%) | 0.737 |
| Variables | VTD (n = 62) | p-Value * | VCD (n = 43) | p-Value * | ||
|---|---|---|---|---|---|---|
| % Outside Normal Range | Pretreatment | Posttreatment | Pretreatment | Posttreatment | ||
| Albumin, g/L | 22 (35.5%) | 20 (32.3%) | 0.704 | 14 (32.6%) | 11 (25.6%) | 0.476 |
| Total proteins | 39 (62.9%) | 28 (45.2%) | 0.047 | 30 (69.8%) | 21 (48.8%) | 0.048 |
| Creatinine level, mmol/L | 32 (51.6%) | 17 (27.4%) | 0.005 | 12 (27.9%) | 16 (37.2%) | 0.357 |
| BUN (mmol/L) | 14 (22.6%) | 28 (45.2%) | 0.007 | 9 (20.9%) | 16 (37.2%) | 0.096 |
| GFR | 7 (11.3%) | 13 (21.0%) | 0.142 | 11 (25.6%) | 17 (39.5%) | 0.167 |
| Hemoglobin level, g/dL | 18 (29.0%) | 16 (25.8%) | 0.687 | 9 (20.9%) | 17 (39.5%) | 0.060 |
| Calcium level, mmol/L | 29 (46.8%) | 25 (40.3%) | 0.468 | 22 (51.2%) | 13 (30.2%) | 0.048 |
| RBC (millions/mm3) | 16 (25.8%) | 15 (24.2%) | 0.835 | 10 (23.3%) | 9 (20.9%) | 0.794 |
| PLT (thousands/mm3) | 17 (27.4%) | 13 (21.0%) | 0.401 | 10 (23.3%) | 8 (18.6%) | 0.596 |
| WBC (thousands/mm3) | 37 (59.7%) | 24 (38.7%) | 0.019 | 28 (65.1%) | 18 (41.9%) | 0.030 |
| ALT (U/L) | 21 (33.9%) | 33 (53.2%) | 0.029 | 13 (30.2%) | 23 (53.5%) | 0.028 |
| AST (U/L) | 19 (30.6%) | 30 (48.4%) | 0.043 | 14 (32.6%) | 23 (53.5%) | 0.049 |
| Variables | VTD (n = 62) | VCD (n = 43) | p-Value * |
|---|---|---|---|
| Drug complications | |||
| Neutropenia | 11 (17.7%) | 15 (34.9%) | 0.045 |
| Thrombocytopenia | 3 (4.8%) | 5 (11.6%) | 0.197 |
| Anemia | 4 (6.5%) | 10 (22.7%) | 0.012 |
| Pancytopenia | 4 (6.5%) | 4 (9.3%) | 0.588 |
| Neuropathy | 11 (17.7%) | 3 (7.0%) | 0.110 |
| Kidney damage | 5 (8.1%) | 11 (25.6%) | 0.014 |
| Elevated liver enzymes | 13 (21.0%) | 10 (22.7%) | 0.780 |
| Others | 5 (8.1%) | 5 (11.6%) | 0.540 |
| Disease-related complications | |||
| Infection | 18 (29.0%) | 11 (25.6%) | 0.697 |
| Fractures | 4 (6.5%) | 2 (4.7%) | 0.695 |
| Anemia | 18 (29.0%) | 13 (30.2%) | 0.894 |
| Reduced kidney function | 32 (51.6%) | 12 (27.9%) | 0.015 |
| Others | 5 (8.1%) | 2 (4.7%) | 0.490 |
| Treatment response | 0.033 | ||
| CR | 11 (17.7%) | 16 (37.2%) | |
| PR | 12 (19.4%) | 9 (20.9%) | |
| SD | 12 (19.4%) | 10 (23.3%) | |
| PS | 27 (43.5%) | 8 (18.6%) | |
| ICU admissions | 4 (6.5%) | 9 (20.9%) | 0.026 |
| Death | 3 (4.8%) | 4 (9.3%) | 0.367 |
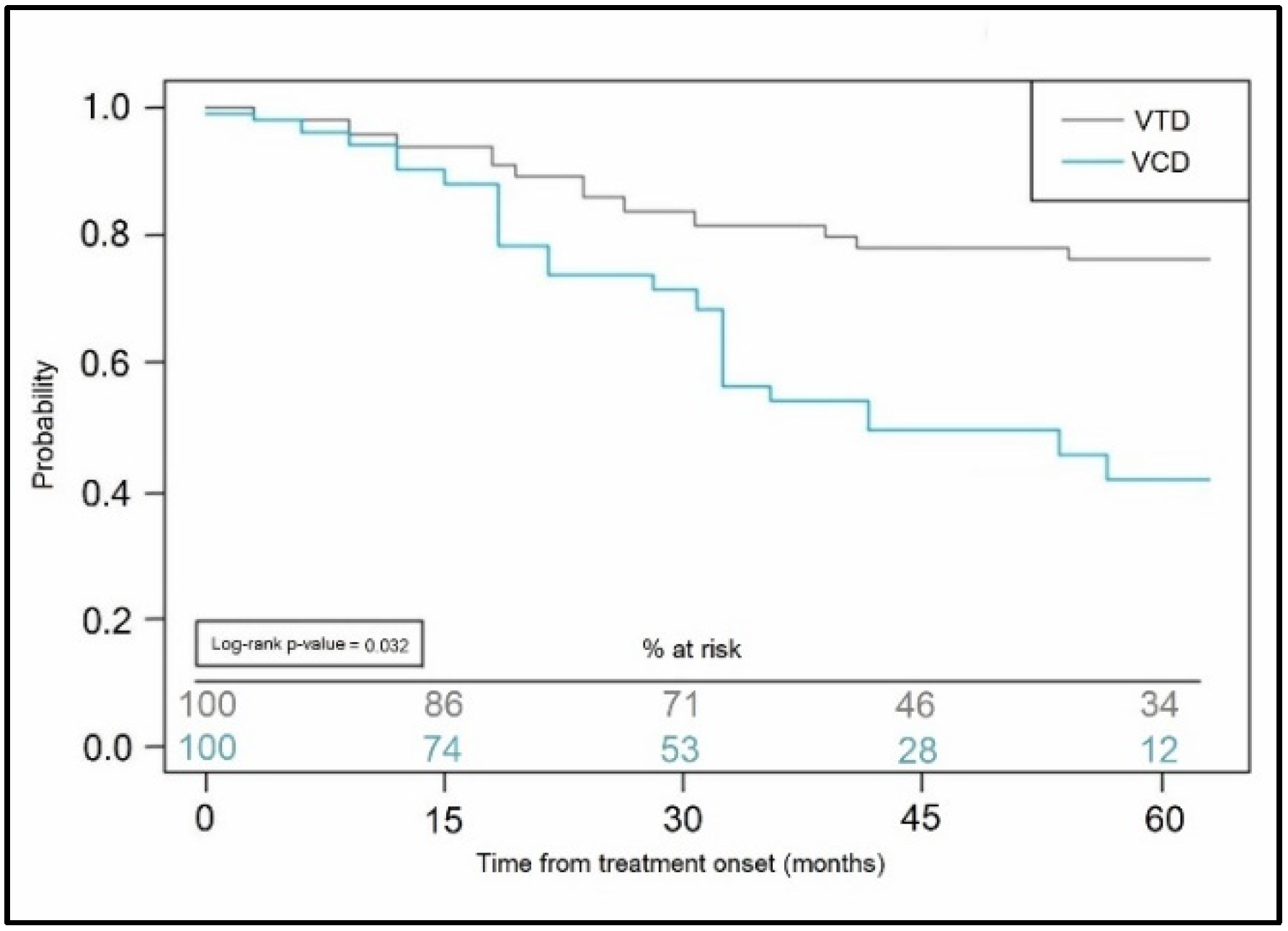
| Mean progression-free survival, (months) | 31.6 ± 9.9 | 27.2 ± 10.4 | 0.030 |
| Maintenance after ASCT | Thalidomide (n = 11) | Lenalidomide (n = 16) | |
| Mean progression-free survival, (months) | 35.5 ± 10.3 | 46.1 ± 11.8 | 0.023 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Potre, C.; Borsi, E.; Potre, O.; Samfireag, M.; Costachescu, D.; Cerbu, B.; Bratosin, F.; Secosan, C.; Negrean, R.A. Assessing Pretransplant and Posttransplant Therapy Response in Multiple Myeloma Patients. Curr. Oncol. 2022, 29, 8501-8512. https://doi.org/10.3390/curroncol29110670
Potre C, Borsi E, Potre O, Samfireag M, Costachescu D, Cerbu B, Bratosin F, Secosan C, Negrean RA. Assessing Pretransplant and Posttransplant Therapy Response in Multiple Myeloma Patients. Current Oncology. 2022; 29(11):8501-8512. https://doi.org/10.3390/curroncol29110670
Chicago/Turabian StylePotre, Cristina, Ema Borsi, Ovidiu Potre, Miruna Samfireag, Dan Costachescu, Bianca Cerbu, Felix Bratosin, Cristina Secosan, and Rodica Anamaria Negrean. 2022. "Assessing Pretransplant and Posttransplant Therapy Response in Multiple Myeloma Patients" Current Oncology 29, no. 11: 8501-8512. https://doi.org/10.3390/curroncol29110670
APA StylePotre, C., Borsi, E., Potre, O., Samfireag, M., Costachescu, D., Cerbu, B., Bratosin, F., Secosan, C., & Negrean, R. A. (2022). Assessing Pretransplant and Posttransplant Therapy Response in Multiple Myeloma Patients. Current Oncology, 29(11), 8501-8512. https://doi.org/10.3390/curroncol29110670







