Preoperative Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio Can Predict Prognosis in Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Follow-up
2.3. Statistical Processing
3. Results
3.1. Patients’ Characteristics in Two Independent NSCLC Cohorts
3.2. Identifications of Optimal Cut-Off Value of TG/HDL-C Derived from the Training Group
3.3. Relation of the TG/HDL-C with Multiple Clinicopathologic Values
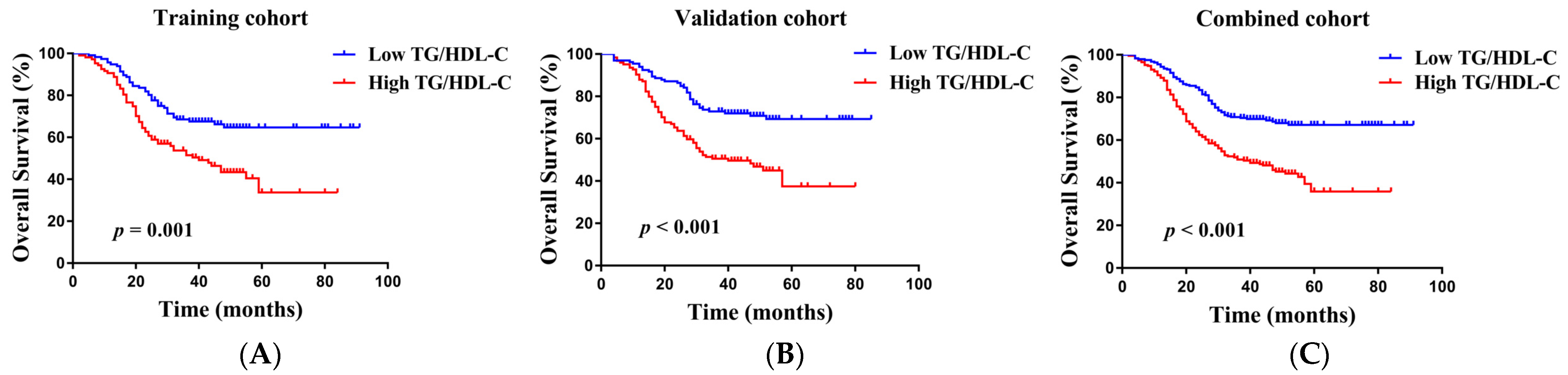
3.4. Prognostic Value of Preoperative TG/HDL-C in the Training Cohort
3.5. Verification of the TG/HDL-C in the Validation Group and Combined Group
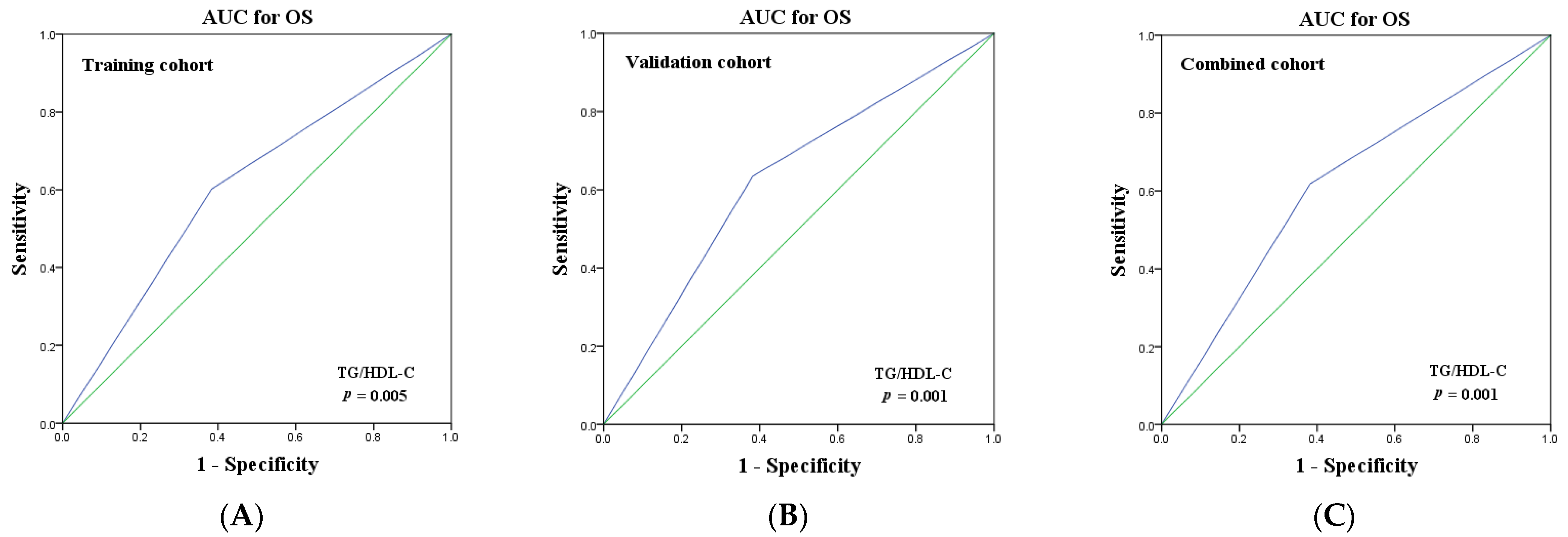
3.6. Predictive Performance of TG/HDL-C for NSCLC
4. Discussion
5. Strengths and Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zheng, R.; Baade, P.D.; Zhang, S.; Zeng, H.; Bray, F.; Jemal, A.; Yu, X.Q.; He, J. Cancer statistics in China, 2015. CA A Cancer J. Clin. 2016, 66, 115–132. [Google Scholar] [CrossRef] [PubMed]
- Molina, J.R.; Yang, P.; Cassivi, S.D.; Schild, S.E.; Adjei, A.A. Non-small cell lung cancer: Epidemiology, risk factors, treatment, and survivorship. Mayo Clin. Proc. 2008, 83, 584–594. [Google Scholar] [CrossRef]
- Hirsch, F.R.; Scagliotti, G.V.; Mulshine, J.L.; Kwon, R.; Curran, W.J., Jr.; Wu, Y.L.; Paz-Ares, L. Lung cancer: Current therapies and new targeted treatments. Lancet 2017, 389, 299–311. [Google Scholar] [CrossRef]
- Merino Salvador, M.; Gómez de Cedrón, M.; Moreno Rubio, J.; Falagán Martínez, S.; Sánchez Martínez, R.; Casado, E.; Ramírez de Molina, A.; Sereno, M. Lipid metabolism and lung cancer. Crit. Rev. Oncol. /Hematol. 2017, 112, 31–40. [Google Scholar] [CrossRef]
- Goldstraw, P.; Chansky, K.; Crowley, J.; Rami-Porta, R.; Asamura, H.; Eberhardt, W.E.; Nicholson, A.G.; Groome, P.; Mitchell, A.; Bolejack, V.; et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2016, 11, 39–51. [Google Scholar] [CrossRef]
- Planchard, D.; Popat, S.; Kerr, K.; Novello, S.; Smit, E.F.; Faivre-Finn, C.; Mok, T.S.; Reck, M.; Van Schil, P.E.; Hellmann, M.D.; et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2018, 29, iv192–iv237. [Google Scholar] [CrossRef]
- Liu, L.; Shi, M.; Wang, Z.; Lu, H.; Li, C.; Tao, Y.; Chen, X.; Zhao, J. A molecular and staging model predicts survival in patients with resected non-small cell lung cancer. BMC Cancer 2018, 18, 966. [Google Scholar] [CrossRef]
- Grosu, H.B.; Manzanera, A.; Shivakumar, S.; Sun, S.; Noguras Gonzalez, G.; Ost, D.E. Survival disparities following surgery among patients with different histological types of non-small cell lung cancer. Lung Cancer 2020, 140, 55–58. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Packard, C.J.; Chapman, M.J.; Borén, J.; Aguilar-Salinas, C.A.; Averna, M.; Ference, B.A.; Gaudet, D.; Hegele, R.A.; Kersten, S.; et al. Triglyceride-rich lipoproteins and their remnants: Metabolic insights, role in atherosclerotic cardiovascular disease, and emerging therapeutic strategies-a consensus statement from the European Atherosclerosis Society. Eur. Heart J. 2021, 42, 4791–4806. [Google Scholar] [CrossRef]
- Johnson, K.E.; Siewert, K.M.; Klarin, D.; Damrauer, S.M.; Program, V.A.M.V.; Chang, K.M.; Tsao, P.S.; Assimes, T.L.; Maxwell, K.N.; Voight, B.F. The relationship between circulating lipids and breast cancer risk: A Mendelian randomization study. PLoS Med. 2020, 17, e1003302. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Zhou, J.; Hooi, S.C.; Jiang, Y.M.; Lu, G.D. Fatty acid activation in carcinogenesis and cancer development: Essential roles of long-chain acyl-CoA synthetases. Oncol. Lett. 2018, 16, 1390–1396. [Google Scholar] [CrossRef] [PubMed]
- Balaban, S.; Lee, L.S.; Schreuder, M.; Hoy, A.J. Obesity and cancer progression: Is there a role of fatty acid metabolism? Biomed Res. Int. 2015, 2015, 274585. [Google Scholar] [CrossRef] [PubMed]
- Pih, G.Y.; Gong, E.J.; Choi, J.Y.; Kim, M.J.; Ahn, J.Y.; Choe, J.; Bae, S.E.; Chang, H.S.; Na, H.K.; Lee, J.H.; et al. Associations of Serum Lipid Level with Gastric Cancer Risk, Pathology, and Prognosis. Cancer Res. Treat. 2021, 53, 445–456. [Google Scholar] [CrossRef]
- Kuzu, O.F.; Noory, M.A.; Robertson, G.P. The Role of Cholesterol in Cancer. Cancer Res. 2016, 76, 2063–2070. [Google Scholar] [CrossRef]
- Norata, G.D.; Pirillo, A.; Ammirati, E.; Catapano, A.L. Emerging role of high density lipoproteins as a player in the immune system. Atherosclerosis 2012, 220, 11–21. [Google Scholar] [CrossRef]
- Lin, Q.; Liu, W.; Xu, S.; Sun, L. Associations of preoperative serum high-density lipoprotein cholesterol and low-density lipoprotein cholesterol levels with the prognosis of ovarian cancer. Arch. Gynecol. Obstet. 2022, 305, 683–691. [Google Scholar] [CrossRef]
- Ma, M.Z.; Yuan, S.Q.; Chen, Y.M.; Zhou, Z.W. Preoperative apolipoprotein B/apolipoprotein A1 ratio: A novel prognostic factor for gastric cancer. OncoTargets Ther. 2018, 11, 2169–2176. [Google Scholar] [CrossRef]
- Shen, J.G.; Jin, L.D.; Dong, M.J.; Wang, L.B.; Zhao, W.H.; Shen, J. Low level of serum high-density lipoprotein cholesterol in gastric cancer correlates with cancer progression but not survival. Transl. Cancer Res. 2020, 9, 6206–6213. [Google Scholar] [CrossRef]
- Kho, P.F.; Amant, F.; Annibali, D.; Ashton, K.; Attia, J.; Auer, P.L.; Beckmann, M.W.; Black, A.; Brinton, L.; Buchanan, D.D.; et al. Mendelian randomization analyses suggest a role for cholesterol in the development of endometrial cancer. Int. J. Cancer 2021, 148, 307–319. [Google Scholar] [CrossRef]
- Orho-Melander, M.; Hindy, G.; Borgquist, S.; Schulz, C.A.; Manjer, J.; Melander, O.; Stocks, T. Blood lipid genetic scores, the HMGCR gene and cancer risk: A Mendelian randomization study. Int. J. Epidemiol. 2018, 47, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.S.; Weng, D.S.; Jiang, L.; Zhang, Y.J.; Pan, K.; Pan, Q.Z.; Chen, C.L.; Zhao, J.J.; Zhang, X.F.; Zhang, H.X.; et al. The clinical significance of preoperative serum cholesterol and high-density lipoprotein-cholesterol levels in hepatocellular carcinoma. J. Cancer 2016, 7, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.T.; Chen, M.Y.; Guo, X.; Guo, L.; Mo, H.Y.; Qian, C.N.; Wen, B.X.; Hong, M.H.; Huang, P.Y. Association between Pretreatment Serum High-density Lipoprotein Cholesterol and Treatment Outcomes in Patients with Locoregionally Advanced Nasopharyngeal Carcinoma Treated with Chemoradiotherapy: Findings from a Randomised Trial. J. Cancer 2019, 10, 3618–3623. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Z.Q.; Wang, F.H.; Lei, X.F.; Yan, S.M.; Wang, D.S.; Zhang, F.; Xu, R.H.; Wang, L.Y.; Li, Y.H. Predictive value of chemotherapy-related high-density lipoprotein cholesterol (HDL) elevation in patients with colorectal cancer receiving adjuvant chemotherapy: An exploratory analysis of 851 cases. Oncotarget 2016, 7, 57290–57300. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wang, X.; Guo, J.; Liu, P. Prognostic significance of preoperative serum triglycerides and high-density lipoproteins cholesterol in patients with non-small cell lung cancer: A retrospective study. Lipids Health Dis. 2021, 20, 69. [Google Scholar] [CrossRef]
- Luo, F.; Zeng, K.M.; Cao, J.X.; Zhou, T.; Lin, S.X.; Ma, W.J.; Yang, Y.P.; Zhang, Z.H.; Lu, F.T.; Huang, Y.; et al. Predictive value of a reduction in the level of high-density lipoprotein-cholesterol in patients with non-small-cell lung cancer undergoing radical resection and adjuvant chemotherapy: A retrospective observational study. Lipids Health Dis. 2021, 20, 109. [Google Scholar] [CrossRef]
- Chi, P.D.; Liu, W.; Chen, H.; Zhang, J.P.; Lin, Y.; Zheng, X.; Liu, W.; Dai, S. High-density lipoprotein cholesterol is a favorable prognostic factor and negatively correlated with C-reactive protein level in non-small cell lung carcinoma. PLoS ONE 2014, 9, e91080. [Google Scholar] [CrossRef]
- Sun, H.; Huang, X.; Wang, Z.; Zhang, G.; Mei, Y.; Wang, Y.; Nie, Z.; Wang, S. Triglyceride-to-high density lipoprotein cholesterol ratio predicts clinical outcomes in patients with gastric cancer. J. Cancer 2019, 10, 6829–6836. [Google Scholar] [CrossRef]
- Dai, D.; Chen, B.; Wang, B.; Tang, H.; Li, X.; Zhao, Z.; Li, X.; Xie, X.; Wei, W. Pretreatment TG/HDL-C Ratio Is Superior to Triacylglycerol Level as an Independent Prognostic Factor for the Survival of Triple Negative Breast Cancer Patients. J. Cancer 2016, 7, 1747–1754. [Google Scholar] [CrossRef]
- Lv, Y.; Ding, X.S.; Li, Y.; An, X.; Miao, L.Y. High BMI and low HDL-C predict the chemotherapy-related hepatic dysfunction in Chinese advanced NSCLC patients. Cancer Biomark. Sect. A Dis. Markers 2016, 16, 89–97. [Google Scholar] [CrossRef]
- Lofterod, T.; Mortensen, E.S.; Nalwoga, H.; Wilsgaard, T.; Frydenberg, H.; Risberg, T.; Eggen, A.E.; McTiernan, A.; Aziz, S.; Wist, E.A.; et al. Impact of pre-diagnostic triglycerides and HDL-cholesterol on breast cancer recurrence and survival by breast cancer subtypes. BMC Cancer 2018, 18, 654. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Q.; Wu, P.W.; Liu, D.H.; Yan, S.J.; Shen, X.M.; Yang, L.Y. Prognostic significance of high triglyceride and apolipoprotein B levels in patients with stage III and high-risk stage II colorectal cancer undergoing curative surgery. Oncol. Lett. 2020, 20, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Wallace, M.; Metallo, C.M. Tracing insights into de novo lipogenesis in liver and adipose tissues. Semin. Cell Dev. Biol. 2020, 108, 65–71. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, T.; Reaven, G.; Abbasi, F.; Lamendola, C.; Saad, M.; Waters, D.; Simon, J.; Krauss, R.M. Is there a simple way to identify insulin-resistant individuals at increased risk of cardiovascular disease? Am. J. Cardiol. 2005, 96, 399–404. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and tumor progression: Signaling pathways and targeted intervention. Signal Transduct. Target. Ther. 2021, 6, 263. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Arthur, R.; Moller, H.; Garmo, H.; Holmberg, L.; Stattin, P.; Malmstrom, H.; Lambe, M.; Hammar, N.; Walldius, G.; Robinson, D.; et al. Association between baseline serum glucose, triglycerides and total cholesterol, and prostate cancer risk categories. Cancer Med. 2016, 5, 1307–1318. [Google Scholar] [CrossRef]
- Sekine, Y.; Koike, H.; Nakano, T.; Nakajima, K.; Suzuki, K. Remnant lipoproteins stimulate proliferation and activate MAPK and Akt signaling pathways via G protein-coupled receptor in PC-3 prostate cancer cells. Clin. Chim. Acta; Int. J. Clin. Chem. 2007, 383, 78–84. [Google Scholar] [CrossRef]
- Koohestani, N.; Chia, M.C.; Pham, N.A.; Tran, T.T.; Minkin, S.; Wolever, T.M.; Bruce, W.R. Aberrant crypt focus promotion and glucose intolerance: Correlation in the rat across diets differing in fat, n-3 fatty acids and energy. Carcinogenesis 1998, 19, 1679–1684. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Corr, J.G.; Thaler, H.T.; Tao, Y.; Fair, W.R.; Heston, W.D. Decreased growth of established human prostate LNCaP tumors in nude mice fed a low-fat diet. J. Natl. Cancer Inst. 1995, 87, 1456–1462. [Google Scholar] [CrossRef] [PubMed]
- Hao, B.; Yu, M.; Sang, C.; Bi, B.; Chen, J. Dyslipidemia and non-small cell lung cancer risk in Chinese population: A case-control study. Lipids Health Dis. 2018, 17, 278. [Google Scholar] [CrossRef] [PubMed]
- Fox, E.M.; Andrade, J.; Shupnik, M.A. Novel actions of estrogen to promote proliferation: Integration of cytoplasmic and nuclear pathways. Steroids 2009, 74, 622–627. [Google Scholar] [CrossRef]
- Asavasupreechar, T.; Chan, M.S.M.; Saito, R.; Miki, Y.; Boonyaratanakornkit, V.; Sasano, H. Sex steroid metabolism and actions in non-small cell lung carcinoma. J. Steroid Biochem. Mol. Biol. 2019, 193, 105440. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.D.; Kim, C.; Zhang, Y.; Rindhe, S.; Cobb, M.H.; Yu, Y. Cholesterol Regulates the Tumor Adaptive Resistance to MAPK Pathway Inhibition. J. Proteome Res. 2021, 20, 5379–5391. [Google Scholar] [CrossRef]
- Revilla, G.; Cedo, L.; Tondo, M.; Moral, A.; Perez, J.I.; Corcoy, R.; Lerma, E.; Fuste, V.; Reddy, S.T.; Blanco-Vaca, F.; et al. LDL, HDL and endocrine-related cancer: From pathogenic mechanisms to therapies. Semin. Cancer Biol. 2021, 73, 134–157. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Ricciuti, B.; Rader, D.J.; Catapano, A.L.; Sahebkar, A.; Banach, M. High density lipoprotein cholesterol and cancer: Marker or causative? Prog. Lipid Res. 2018, 71, 54–69. [Google Scholar] [CrossRef]
- Ferretti, G.; Bacchetti, T.; Busni, D.; Rabini, R.A.; Curatola, G. Protective effect of paraoxonase activity in high-density lipoproteins against erythrocyte membranes peroxidation: A comparison between healthy subjects and type 1 diabetic patients. J. Clin. Endocrinol. Metab. 2004, 89, 2957–2962. [Google Scholar] [CrossRef]
- Rohatgi, A.; Westerterp, M.; von Eckardstein, A.; Remaley, A.; Rye, K.A. HDL in the 21st Century: A Multifunctional Roadmap for Future HDL Research. Circulation 2021, 143, 2293–2309. [Google Scholar] [CrossRef]
| Characteristics | Training Cohort | Validation Cohort | Combined Cohort | |||
|---|---|---|---|---|---|---|
| N = 223 | % | N = 256 | % | N = 479 | % | |
| Age (years) | 60.80 ± 10.75 | 59.91 ± 10.94 | 60.32 ± 10.85 | |||
| Gender | ||||||
| Male | 156 | 69.96 | 173 | 67.58 | 329 | 68.68 |
| Female | 67 | 30.04 | 83 | 32.42 | 150 | 31.32 |
| Smoking history | ||||||
| Yes | 152 | 68.16 | 159 | 62.11 | 311 | 64.93 |
| No | 71 | 31.84 | 97 | 37.89 | 168 | 35.07 |
| Stage | ||||||
| I-II | 153 | 68.61 | 155 | 60.55 | 308 | 64.30 |
| IIIA | 70 | 31.39 | 101 | 39.45 | 171 | 35.70 |
| Pathological tumor classification(pT) | ||||||
| pT1-2 | 196 | 87.89 | 216 | 84.38 | 412 | 86.01 |
| pT3-4 | 27 | 12.11 | 40 | 15.63 | 67 | 13.99 |
| Pathological lymph node stage(pN) | ||||||
| pN0 | 134 | 60.09 | 128 | 50.00 | 262 | 54.70 |
| pN1-2 | 89 | 39.91 | 128 | 50.00 | 217 | 45.30 |
| Histological type | ||||||
| Squamous cell carcinoma | 71 | 31.84 | 76 | 29.69 | 147 | 30.69 |
| Adenocarcinoma | 137 | 61.43 | 154 | 60.16 | 291 | 60.75 |
| Large cell carcinoma | 15 | 6.73 | 26 | 10.16 | 41 | 8.56 |
| Surgery type | ||||||
| Lobectomy | 170 | 76.23 | 174 | 67.97 | 344 | 71.82 |
| Pneumonectomy | 41 | 18.39 | 62 | 24.22 | 103 | 21.50 |
| Other | 12 | 5.38 | 20 | 7.81 | 32 | 6.68 |
| Adjuvant chemotherapy | ||||||
| Yes | 116 | 52.02 | 161 | 62.89 | 277 | 57.83 |
| No | 107 | 47.98 | 95 | 37.11 | 202 | 42.17 |
| TG/HDL-C | 1.16 ± 0.93 | 1.27 ± 0.87 | 1.22 ± 0.90 | |||
| Characteristics | TG/HDL-C | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Training Cohort (N = 223) | Validation Cohort (N = 256) | Combined Cohort (N = 479) | |||||||
| Low | High | p Value | Low | High | p Value | Low | High | p Value | |
| Age (years) | 0.500 | 0.059 | 0.357 | ||||||
| ≤60 | 49 | 50 | 72 | 53 | 121 | 103 | |||
| >60 | 67 | 57 | 60 | 71 | 127 | 128 | |||
| Gender | 0.404 | 0.455 | 0.264 | ||||||
| Male | 84 | 72 | 92 | 81 | 176 | 153 | |||
| Female | 32 | 35 | 40 | 43 | 72 | 78 | |||
| Smoking history | 0.985 | 0.437 | 0.568 | ||||||
| Yes | 79 | 73 | 85 | 74 | 164 | 147 | |||
| No | 37 | 34 | 47 | 50 | 84 | 84 | |||
| Stage | 0.064 | 0.431 | 0.069 | ||||||
| I-II | 86 | 67 | 83 | 72 | 169 | 139 | |||
| IIIA | 30 | 40 | 49 | 52 | 79 | 92 | |||
| Pathological tumor classification(pT) | 0.985 | 0.636 | 0.730 | ||||||
| pT1-2 | 102 | 94 | 110 | 106 | 212 | 200 | |||
| pT3-4 | 14 | 13 | 22 | 18 | 36 | 31 | |||
| Pathological lymph node stage(pN) | 0.002 | 0.006 | <0.001 | ||||||
| pN0 | 81 | 53 | 77 | 51 | 158 | 104 | |||
| pN1-2 | 35 | 54 | 55 | 73 | 90 | 127 | |||
| Histological type | 0.038 | 0.609 | 0.331 | ||||||
| Squamous cell carcinoma | 45 | 26 | 36 | 40 | 81 | 66 | |||
| Adenocarcinoma | 62 | 75 | 81 | 73 | 143 | 148 | |||
| Large cell carcinoma | 9 | 6 | 15 | 11 | 24 | 17 | |||
| Surgery type | 0.496 | 0.348 | 0.179 | ||||||
| Lobectomy | 92 | 78 | 94 | 80 | 186 | 158 | |||
| Pneumonectomy | 18 | 23 | 27 | 35 | 45 | 58 | |||
| Other | 6 | 6 | 11 | 9 | 17 | 15 | |||
| Adjuvant chemotherapy | 0.152 | 0.194 | 0.054 | ||||||
| Yes | 55 | 61 | 78 | 83 | 133 | 144 | |||
| No | 61 | 46 | 54 | 41 | 115 | 87 | |||
| Characteristics | Training Cohort | Validation Cohort | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | Univariate Analysis | Multivariate Analysis | |||||||||
| HR | 95%CI | p Value | HR | 95%CI | p Value | HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age (years) | 0.127 | 0.412 | ||||||||||
| ≤60 | 1.000 | Reference | 1.000 | Reference | ||||||||
| >60 | 1.370 | 0.914–2.054 | 1.176 | 0.798–1.732 | ||||||||
| Gender | 0.281 | 0.802 | ||||||||||
| Male | 1.000 | Reference | 1.000 | Reference | ||||||||
| Female | 0.781 | 0.499–1.224 | 0.949 | 0.628–1.434 | ||||||||
| Smoking history | 0.783 | 0.417 | ||||||||||
| Yes | 1.000 | Reference | 1.000 | Reference | ||||||||
| No | 0.942 | 0.615–1.442 | 0.846 | 0.564–1.268 | ||||||||
| Stage | <0.001 | 0.003 | <0.001 | 0.029 | ||||||||
| I-II | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||
| IIIA | 4.289 | 2.854–6.444 | 2.734 | 1.401–5.334 | 3.553 | 2.380–5.303 | 2.196 | 1.086–4.439 | ||||
| Pathological tumor classification(pT) | 0.002 | 0.840 | 0.118 | |||||||||
| pT1-2 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||||
| pT3-4 | 2.269 | 1.357–3.795 | 1.071 | 0.552–2.076 | 1.477 | 0.906–2.409 | ||||||
| Pathological lymph node stage(pN) | <0.001 | 0.001 | <0.001 | <0.001 | ||||||||
| pN0 | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||
| pN1-2 | 3.531 | 2.341–5.326 | 3.360 | 1.608–7.021 | 4.519 | 2.878–7.096 | 4.894 | 2.386–8.039 | ||||
| Histological type | 0.184 | 0.367 | ||||||||||
| Squamous cell carcinoma | 1.000 | Reference | 1.000 | Reference | ||||||||
| Adenocarcinoma | 1.008 | 0.647–1.571 | 0.899 | 0.581–1.391 | ||||||||
| Large cell carcinoma | 1.920 | 0.906–4.068 | 1.404 | 0.718–2.743 | ||||||||
| Surgery type | <0.001 | 0.086 | <0.001 | 0.109 | ||||||||
| Lobectomy | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||
| Pneumonectomy | 3.364 | 2.146–5.274 | 1.967 | 1.048–3.693 | 2.605 | 1.728–3.927 | 1.379 | 0.797–2.386 | ||||
| Other | 3.329 | 1.643–6.743 | 1.288 | 0.508–3.264 | 1.525 | 0.727–3.199 | 0.633 | 0.275–1.455 | ||||
| Adjuvant chemotherapy | 0.042 | <0.001 | 0.001 | 0.008 | ||||||||
| Yes | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||
| No | 1.519 | 1.014–2.275 | 3.616 | 1.872–6.987 | 2.155 | 1.386–3.350 | 2.803 | 1.306–6.017 | ||||
| TG/HDL-C | 0.001 | 0.017 | <0.001 | 0.007 | ||||||||
| Low | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | 1.000 | Reference | ||||
| High | 1.974 | 1.317–2.961 | 1.674 | 1.094–2.559 | 2.236 | 1.499–3.336 | 1.770 | 1.173–2.672 | ||||
| Characteristics | Combined Cohort | |||||
|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | |||||
| HR | 95%CI | p Value | HR | 95%CI | p Value | |
| Age (years) | 0.097 | |||||
| ≤60 | 1.000 | Reference | ||||
| >60 | 1.267 | 0.958–1.675 | ||||
| Gender | 0.337 | |||||
| Male | 1.000 | Reference | ||||
| Female | 0.862 | 0.636–1.167 | ||||
| Smoking history | 0.416 | |||||
| Yes | 1.000 | Reference | ||||
| No | 0.885 | 0.660–1.187 | ||||
| Stage | <0.001 | <0.001 | ||||
| I-II | 1.000 | Reference | 1.000 | Reference | ||
| IIIA | 3.789 | 2.851–5.035 | 2.480 | 1.518–4.050 | ||
| Pathological tumor classification(pT) | 0.002 | 0.619 | ||||
| pT1-2 | 1.000 | Reference | 1.000 | Reference | ||
| pT3-4 | 1.756 | 1.233–2.502 | 1.117 | 0.723–1.724 | ||
| Pathological lymph node stage(pN) | <0.001 | <0.001 | ||||
| pN0 | 1.000 | Reference | 1.000 | Reference | ||
| pN1-2 | 3.877 | 2.872–5.235 | 4.224 | 2.558–6.976 | ||
| Histological type | 0.097 | |||||
| Squamous cell carcinoma | 1.000 | Reference | ||||
| Adenocarcinoma | 0.946 | 0.693–1.292 | ||||
| Large cell carcinoma | 1.578 | 0.958–2.598 | ||||
| Surgery type | <0.001 | 0.046 | ||||
| Lobectomy | 1.000 | Reference | 1.000 | Reference | ||
| Pneumonectomy | 2.848 | 2.106–3.851 | 1.545 | 0.987–2.418 | ||
| Other | 2.107 | 1.266–3.509 | 0.864 | 0.477–1.563 | ||
| Adjuvant chemotherapy | <0.001 | <0.001 | ||||
| Yes | 1.000 | Reference | 1.000 | Reference | ||
| No | 1.767 | 1.317–2.370 | 3.486 | 2.113–5.754 | ||
| TG/HDL-C | <0.001 | <0.001 | ||||
| Low | 1.000 | Reference | 1.000 | Reference | ||
| High | 2.108 | 1.586–2.801 | 1.715 | 1.279–2.301 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Ma, C.; Yuan, X.; Wang, X.; Li, N.; Yu, R.; Liao, H. Preoperative Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio Can Predict Prognosis in Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study. Curr. Oncol. 2022, 29, 6125-6136. https://doi.org/10.3390/curroncol29090481
Li J, Ma C, Yuan X, Wang X, Li N, Yu R, Liao H. Preoperative Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio Can Predict Prognosis in Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study. Current Oncology. 2022; 29(9):6125-6136. https://doi.org/10.3390/curroncol29090481
Chicago/Turabian StyleLi, Junhong, Cong Ma, Xuhui Yuan, Xiaoyan Wang, Na Li, Ronghui Yu, and Hui Liao. 2022. "Preoperative Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio Can Predict Prognosis in Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study" Current Oncology 29, no. 9: 6125-6136. https://doi.org/10.3390/curroncol29090481
APA StyleLi, J., Ma, C., Yuan, X., Wang, X., Li, N., Yu, R., & Liao, H. (2022). Preoperative Serum Triglyceride to High-Density Lipoprotein Cholesterol Ratio Can Predict Prognosis in Non-Small Cell Lung Cancer: A Multicenter Retrospective Cohort Study. Current Oncology, 29(9), 6125-6136. https://doi.org/10.3390/curroncol29090481





