Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.1.1. Participants
2.1.2. Data
2.2. Extensive Acetabular Bone Loss Management
2.2.1. Treatment Strategy
2.2.2. Surgical Procedure
2.3. Study Objectives
2.4. Statistics and Ethics
3. Results
3.1. Population Description
3.2. Parker and MSTS Evaluation
3.3. Reoperation-Free Survival
3.4. Patient Overall Survival
4. Discussion
4.1. Functional Assessment
4.2. Reoperation-Free Survival and Complications
4.3. Overall Survival and Katagiri Score
4.4. Limitations and Strengths
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reflecting on 20 Years of Progress. Nat. Rev. Cancer 2021, 21, 605. [CrossRef] [PubMed]
- Hernandez, R.K.; Wade, S.W.; Reich, A.; Pirolli, M.; Liede, A.; Lyman, G.H. Incidence of Bone Metastases in Patients with Solid Tumors: Analysis of Oncology Electronic Medical Records in the United States. BMC Cancer 2018, 18, 44. [Google Scholar] [CrossRef] [PubMed]
- Kimura, T. Multidisciplinary Approach for Bone Metastasis: A Review. Cancers 2018, 10, 156. [Google Scholar] [CrossRef] [PubMed]
- D’Oronzo, S.; Coleman, R.; Brown, J.; Silvestris, F. Metastatic Bone Disease: Pathogenesis and Therapeutic Options: Up-Date on Bone Metastasis Management. J. Bone Oncol. 2019, 15, 100205. [Google Scholar] [CrossRef]
- Fragnaud, H.; Mattei, J.-C.; Le Nail, L.-R.; Nguyễn, M.-V.; Schubert, T.; Griffin, A.; Wunder, J.; Biau, D.; Gouin, F.; Bonnevialle, P.; et al. Mid and Long-Term Overall Survival after Carcinologic Resections of Thyroid Cancer Bone Metastases. Front. Surg. 2022, 9, 965951. [Google Scholar] [CrossRef]
- Crenn, V.; Carlier, C.; Gouin, F.; Sailhan, F.; Bonnevialle, P. High Rate of Fracture in Long-Bone Metastasis: Proposal for an Improved Mirels Predictive Score. Orthop. Traumatol. Surg. Res. 2020, 106, 1005–1011. [Google Scholar] [CrossRef]
- Cirstoiu, C.; Cretu, B.; Iordache, S.; Popa, M.; Serban, B.; Cursaru, A. Surgical Management Options for Long-Bone Metastasis. EFORT Open Rev. 2022, 7, 206–213. [Google Scholar] [CrossRef]
- Tsukamoto, S.; Errani, C.; Kido, A.; Mavrogenis, A.F. What’s New in the Management of Metastatic Bone Disease. Eur. J. Orthop. Surg. Traumatol. Orthop. Traumatol. 2021, 31, 1547–1555. [Google Scholar] [CrossRef]
- Müller, D.A.; Capanna, R. The Surgical Treatment of Pelvic Bone Metastases. Adv. Orthop. 2015, 2015, e525363. [Google Scholar] [CrossRef]
- Park, J.W.; Lim, H.; Kang, H.G.; Kim, J.H.; Kim, H.-S. Percutaneous Cementoplasty for the Pelvis in Bone Metastasis: 12-Year Experience. Ann. Surg. Oncol. 2022, 29, 1413–1422. [Google Scholar] [CrossRef]
- Kirkinis, M.N.; Lyne, C.J.; Wilson, M.D.; Choong, P.F.M. Metastatic Bone Disease: A Review of Survival, Prognostic Factors and Outcomes Following Surgical Treatment of the Appendicular Skeleton. Eur. J. Surg. Oncol. 2016, 42, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Harrington, K.D. The Management of Acetabular Insufficiency Secondary to Metastatic Malignant Disease. J. Bone Jt. Surg. 1981, 63, 653–664. [Google Scholar] [CrossRef]
- de l’Escalopier, N.; Felden, A.; Anract, P.; Biau, D. Acetabular Reconstruction after Advanced Acetabular Metastasis Resection: Modified Harrington Technique with a Kerboull Reinforcement Device. Orthop. Traumatol. Surg. Res. 2022, 108, 103232. [Google Scholar] [CrossRef] [PubMed]
- Tsagozis, P.; Wedin, R.; Brosjö, O.; Bauer, H. Reconstruction of Metastatic Acetabular Defects Using a Modified Harrington Procedure. Acta Orthop. 2015, 86, 690–694. [Google Scholar] [CrossRef]
- Ho, L.; Ahlmann, E.R.; Menendez, L.R. Modified Harrington Reconstruction for Advanced Periacetabular Metastatic Disease. J. Surg. Oncol. 2010, 101, 170–174. [Google Scholar] [CrossRef]
- Gusho, C.A.; Chapman, R.; Blank, A.T. A Modified Harrington Technique for Periacetabular Reconstruction in Advanced Metastatic Bone Disease and a Discussion of Alternative Treatment Options. Orthop. Rev. 2020, 12, 9011. [Google Scholar] [CrossRef]
- Vielgut, I.; Sadoghi, P.; Gregori, M.; Kovar, F.M.; Pichler, K.; Maurer-Ertl, W.; Leithner, A. The Modified Harrington Procedure for Metastatic Peri-Acetabular Bone Destruction. Int. Orthop. 2013, 37, 1981–1985. [Google Scholar] [CrossRef]
- Parker, M.J.; Palmer, C.R. A New Mobility Score for Predicting Mortality after Hip Fracture. J. Bone Jt. Surg. Br. 1993, 75, 797–798. [Google Scholar] [CrossRef]
- Tunn, P.U.; Pomraenke, D.; Goerling, U.; Hohenberger, P. Functional Outcome after Endoprosthetic Limb-Salvage Therapy of Primary Bone Tumours—A Comparative Analysis Using the MSTS Score, the TESS and the RNL Index. Int. Orthop. 2008, 32, 619–625. [Google Scholar] [CrossRef]
- Katagiri, H.; Takahashi, M.; Wakai, K.; Sugiura, H.; Kataoka, T.; Nakanishi, K. Prognostic Factors and a Scoring System for Patients with Skeletal Metastasis. J. Bone Jt. Surg. Br. 2005, 87, 698–703. [Google Scholar] [CrossRef]
- Katagiri, H.; Okada, R.; Takagi, T.; Takahashi, M.; Murata, H.; Harada, H.; Nishimura, T.; Asakura, H.; Ogawa, H. New Prognostic Factors and Scoring System for Patients with Skeletal Metastasis. Cancer Med. 2014, 3, 1359–1367. [Google Scholar] [CrossRef]
- Henderson, E.R.; Groundland, J.S.; Pala, E.; Dennis, J.A.; Wooten, R.; Cheong, D.; Windhager, R.; Kotz, R.I.; Mercuri, M.; Funovics, P.T.; et al. Failure Mode Classification for Tumor Endoprostheses: Retrospective Review of Five Institutions and a Literature Review. J. Bone Jt. Surg. Am. 2011, 93, 418–429. [Google Scholar] [CrossRef] [PubMed]
- Tillman, R.M.; Myers, G.J.C.; Abudu, A.T.; Carter, S.R.; Grimer, R.J. The Three-Pin Modified “Harrington” Procedure for Advanced Metastatic Destruction of the Acetabulum. J. Bone Jt. Surg. Br. 2008, 90, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.; Paziuk, T.; Henry, T.; Krieg, J.; Brown, S. Reinforced Reconstruction: A Technique for the Treatment of Periacetabular Metastases. J. Orthop. 2021, 27, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Mallet, J.; Persigant, M.; Quinette, Y.; Brulefert, K.; Waast, D.; Vaz, G.; Nich, C.; Gouin, F.; Crenn, V. A Novel and Secure Technique of Stemmed Acetabular Cup Implantation in Complex Hip Reconstructions: A Comparative Study and Technical Note. Int. Orthop. 2022, 46, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, J.; Malatray, M.; Al-Qahtani, T.; Pibarot, V.; Confavreux, C.; Freyer, G. Total Hip Arthroplasty for Periacetabular Metastatic Disease. An Original Technique of Reconstruction According to the Harrington Classification. J. Arthroplast. 2018, 33, 2546–2555. [Google Scholar] [CrossRef]
- Felden, A.; Vaz, G.; Kreps, S.; Anract, P.; Hamadouche, M.; Biau, D.J. A Cemented Acetabular Component with a Reinforcement Cross Provides Excellent Medium-Term Fixation in Total Hip Arthroplasty after Pelvic Irradiation. Bone Jt. J. 2015, 97-B, 177–184. [Google Scholar] [CrossRef]
- Lozano-Calderon, S.A.; Kaiser, C.L.; Osler, P.M.; Raskin, K.A. Cemented Total Hip Arthroplasty With Retrograde Ischioacetabular Steinmann Pin Reconstruction for Periacetabular Metastatic Carcinoma. J. Arthroplast. 2016, 31, 1555–1560. [Google Scholar] [CrossRef]
- Kiatisevi, P.; Sukunthanak, B.; Pakpianpairoj, C.; Liupolvanish, P. Functional Outcome and Complications Following Reconstruction for Harrington Class II and III Periacetabular Metastasis. World J. Surg. Oncol. 2015, 13, 4. [Google Scholar] [CrossRef]
- Erol, B.; Aydemir, A.N.; Onay, T.; Topkar, M.O. Reconstruction of Advanced Periacetabular Metastatic Lesions with Modified Harrington Procedure. Acta Orthop. Traumatol. Turc. 2016, 50, 178–185. [Google Scholar] [CrossRef]
- Janssen, S.J.; van Rein, E.A.J.; Paulino Pereira, N.R.; Raskin, K.A.; Ferrone, M.L.; Hornicek, F.J.; Lozano-Calderon, S.A.; Schwab, J.H. The Discrepancy between Patient and Clinician Reported Function in Extremity Bone Metastases. Sarcoma 2016, 2016, 1014248. [Google Scholar] [CrossRef]
- Enright, P.L. The Six-Minute Walk Test. Respir. Care 2003, 48, 783–785. [Google Scholar] [PubMed]
- Nayar, S.K.; Kostakos, T.A.; Savvidou, O.; Vlasis, K.; Papagelopoulos, P.J. Outcomes of Hip Reconstruction for Metastatic Acetabular Lesions: A Scoping Review of the Literature. Curr. Oncol. 2022, 29, 307. [Google Scholar] [CrossRef] [PubMed]
- Kircher, J.; Dürr, H.-R.; Jansson, V. Intrapelvic Pin Migration after Periacetabular Reconstruction and Arthroplasty of the Hip in Metastatic Pelvic Disease—A Case Report. Acta Orthop. 2005, 76, 941–943. [Google Scholar] [CrossRef] [PubMed]
- Bagsby, D.T.; Wurtz, L.D. Effectiveness of Constrained Liner Use During Harrington Hip Reconstruction in Oncology Patient. J. Arthroplasty 2017, 32, 1250–1254. [Google Scholar] [CrossRef] [PubMed]
- Kask, G.; Nieminen, J.; van Iterson, V.; Naboistsikov, M.; Pakarinen, T.-K.; Laitinen, M.K. Modified Harrington’s Procedure for Periacetabular Metastases in 89 Cases: A Reliable Method for Cancer Patients with Good Functional Outcome, Especially with Long Expected Survival. Acta Orthop. 2020, 91, 341–346. [Google Scholar] [CrossRef]
- Clayer, M. The Survivorship of Protrusio Cages for Metastatic Disease Involving the Acetabulum. Clin. Orthop. 2010, 468, 2980–2984. [Google Scholar] [CrossRef]
- Crenn, V.; Briand, S.; Rosset, P.; Mattei, J.-C.; Fouasson-Chailloux, A.; Le Nail, L.-R.; Waast, D.; Ropars, M.; Gouin, F. Clinical and Dynamometric Results of Hip Abductor System Repair by Trochanteric Hydroxyapatite Plate with Modular Implant after Resection of Proximal Femoral Tumors. Orthop. Traumatol. Surg. Res. 2019, 105, 1319–1325. [Google Scholar] [CrossRef]
- Philippeau, J.-M.; Durand, J.-M.; Carret, J.-P.; Leclercq, S.; Waast, D.; Gouin, F. Dual Mobility Design Socket Use in Preventing Total Hip Replacement Dislocation Following Tumor Resection. Orthop. Traumatol. Surg. Res. 2010, 96, 2–8. [Google Scholar] [CrossRef]
- Chimutengwende-Gordon, M.; Coomber, R.; Peat, F.; Tarazi, N.; Chou, D.; Carrothers, A. The Harrington plus Reconstruction for Pelvic and Acetabular Metastases. J. Bone Oncol. 2022, 33, 100414. [Google Scholar] [CrossRef]
- Gazendam, A.; Axelrod, D.; Wilson, D.; Ghert, M. Emerging Concepts in the Surgical Management of Peri-Acetabular Metastatic Bone Disease. Curr. Oncol. 2021, 28, 238. [Google Scholar] [CrossRef] [PubMed]
- Anderson, A.B.; Wedin, R.; Fabbri, N.; Boland, P.; Healey, J.; Forsberg, J.A. External Validation of PATHFx Version 3.0 in Patients Treated Surgically and Nonsurgically for Symptomatic Skeletal Metastases. Clin. Orthop. 2020, 478, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Ben Gal, O.; Soh, T.C.F.; Vaughan, S.; Jayasanker, V.; Mahendra, A.; Gupta, S. The Prediction of Survival after Surgical Management of Bone Metastases of the Extremities—A Comparison of Prognostic Models. Curr. Oncol. 2022, 29, 373. [Google Scholar] [CrossRef] [PubMed]
- Meares, C.; Badran, A.; Dewar, D. Prediction of Survival after Surgical Management of Femoral Metastatic Bone Disease—A Comparison of Prognostic Models. J. Bone Oncol. 2019, 15, 100225. [Google Scholar] [CrossRef]
- Kubota, H.; Soejima, T.; Sulaiman, N.S.; Sekii, S.; Matsumoto, Y.; Ota, Y.; Tsujino, K.; Fujita, I.; Fujimoto, T.; Morishita, M.; et al. Predicting the Survival of Patients with Bone Metastases Treated with Radiation Therapy: A Validation Study of the Katagiri Scoring System. Radiat. Oncol. 2019, 14, 13. [Google Scholar] [CrossRef]
- Tanaka, A.; Katagiri, H.; Murata, H.; Wasa, J.; Miyagi, M.; Honda, Y.; Takahashi, M. Surgery for Femoral Metastases. Bone Jt. J. 2020, 102-B, 285–292. [Google Scholar] [CrossRef]






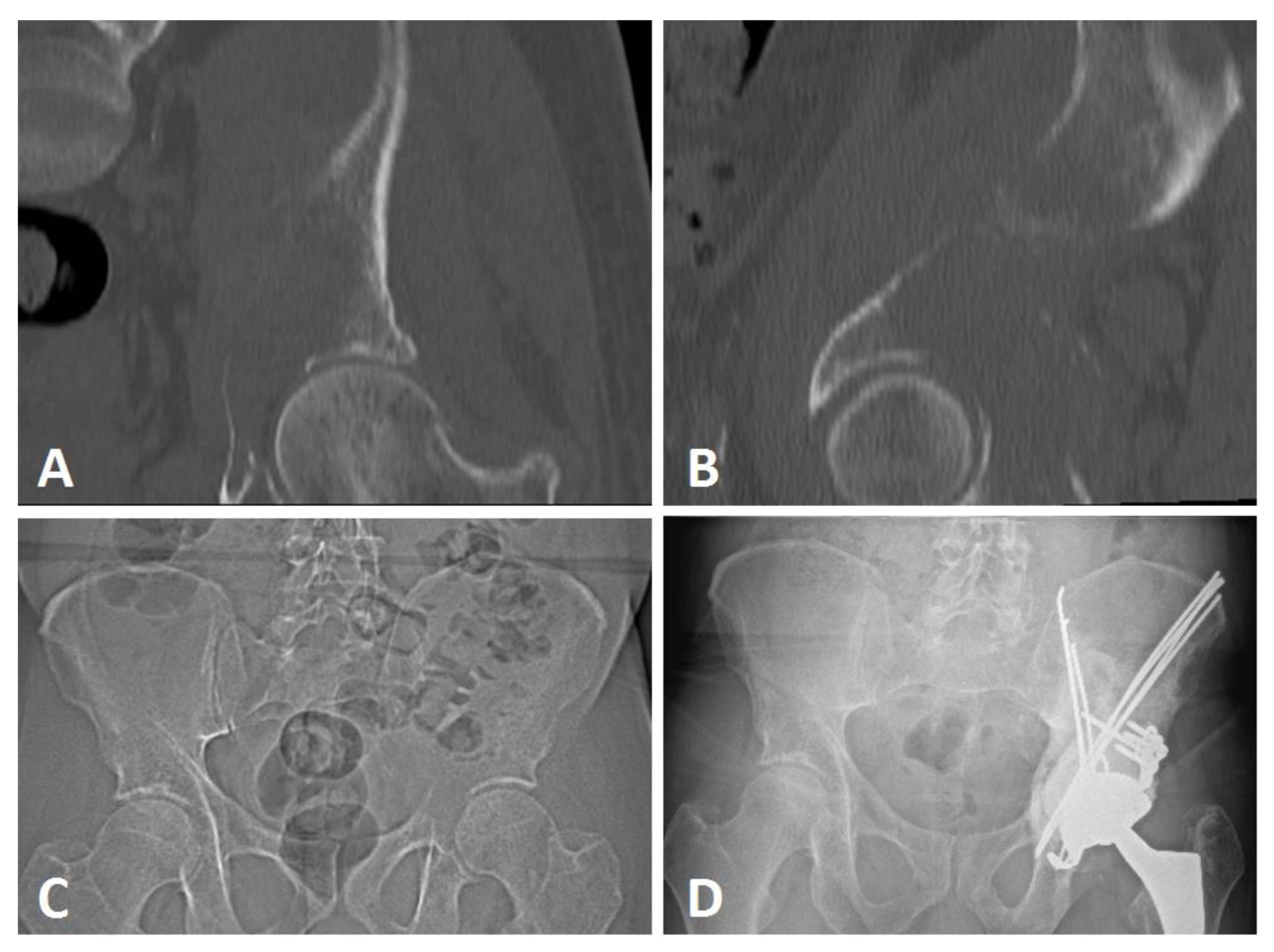

| Total, n = 21 | |||
|---|---|---|---|
| Demographic data | Sex ratio (M/F) | 10/11 | |
| BMI (kg/m2) | 24.4 ± 5.4 | ||
| Age (years) | 60.7 ± 13.0 | ||
| ASA Score | ASA 2 | 10 (47.6%) | |
| ASA 3 | 11 (52.4%) | ||
| Comorbidities | Active smoker | 3 (14.3%) | |
| Obesity | 2 (9.5%) | ||
| COPD or asthma | 2 (9.5%) | ||
| Cardiac condition | 3 (14.3%) | ||
| Diabetes | 3 (14.3%) | ||
| Oncologic data | Pathologic fracture | 3 (14.3%) | |
| Primitive tumor | Breast cancer | 7 (33.3%) | |
| Pulmonary cancer | 7 (33.3%) | ||
| Renal carcinoma | 4 (19.0%) | ||
| Prostatic cancer | 1 (4.8%) | ||
| GIST cancer | 1 (4.8%) | ||
| Harrington Grade | Grade 2 | 8 (32.3%) | |
| Grade 3 | 14 (66.7%) | ||
| Enneking Zones | Zone 1 | 10 (47.6%) | |
| Zone 2 | 21 (100%) | ||
| Zone 3 | 3 (14.3%) | ||
| Associated treatments | Radiation therapy | Preoperative | 8 (38.1%) |
| Postoperative (n = 18) | 14 (77.8%) | ||
| Chemotherapy | Preoperative | 16 (76.2%) | |
| Postoperative | 17 (81.0%) | ||
| Preoperative embolization | 3 (14.3%) | ||
| Surgical Data | Surgery duration (minutes) | 135 ± 29 | |
| Blood loss(mL) (n = 13) | 1433 ± 1177 | ||
| RBCs Transfusion (mL) | 982 ± 726 | ||
| Steinman pins number | 3.0 ± 1.9 | ||
| Associated gesture | Trochanterotomy | 1 (4.8%) | |
| Sacro-iliac joint screwing | 2 (9.5%) | ||
| Acetabular implant | Kerboul cross-plate | 18 (85.7%) | |
| Stemmed acetabular cup | 3 (14.3%) | ||
| Parker Score (%) | MSTS Score (%) | ||
|---|---|---|---|
| Preoperative (n = 21) | Mean ± SD | 3.6 ± 2.0 | 32.2 ± 16.2 |
| Median [Min; Max] | 4.0 [0.0; 9.0] | 31.6 [6.7; 66.6] | |
| 6 months (n = 12) | Mean ± SD | 6.6 ± 3.2 | 67.8 ± 30.6 |
| Median [Min; Max] | 8.0 [0.0; 9.0] | 78.4 [3.3; 100.0] | |
| 12 months (n = 8) | Mean ± SD | 7.6 ± 2.1 | 82.4 ± 24.0 |
| Median [Min; Max] | 9.0 [4.0; 9.0] | 89.9 [30.0; 100.0] | |
| Total, n = 12 | ||
|---|---|---|
| Delay between surgery and complication (months) | Mean ± SD | 12.4 ± 11.9 |
| Median [Q1; Q3] | 5.2 [1.6; 30.7] | |
| Complication type | Pin migration | 6 (53.9%) |
| Infection | 3 (25.0%) | |
| Seroma | 2 (16.7%) | |
| Acetabular loosening | 1 (8.3%) | |
| Henderson Classification | 1 | 2 (16.7%) |
| 3 | 6 (50.0%) | |
| 4 | 3 (25.0%) | |
| 5 | 1 (8.3%) | |
| Score | Total, n = 21 | ||
|---|---|---|---|
| Primary tumor growth characteristics | Slow | 0 | 9 (42.9%) |
| Moderate | 2 | 6 (28.6%) | |
| Rapid | 3 | 6 (28.6%) | |
| Visceral metastases | No | 0 | 14 (66.7%) |
| Nodular | 1 | 0 (0.0%) | |
| Disseminated | 2 | 7 (33.3%) | |
| Laboratory data * (n = 13) | Normal | 0 | 7 (33.3%) |
| Abnormal | 1 | 4 (19.0%) | |
| Critical | 2 | 2 (9.5%) | |
| ECOG PS | 0–2 | 0 | 13 (61.9%) |
| 3–4 | 1 | 8 (38.1%) | |
| Previous chemotherapy | Yes | 1 | 16 (76.2%) |
| No | 0 | 5 (23.8%) | |
| Multiple skeletal metastases | Yes | 1 | 19 (90.5%) |
| No | 0 | 2 (8.5%) | |
| Katagiri Score | Low-risk | (0–3) | 6 (28.6%) |
| Intermediate-risk | (4–6) | 13 (61.9%) | |
| High-risk | (7–10) | 2 (9.5%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plaud, A.; Gaillard, J.; Gouin, F.; Le Thuaut, A.; Ageneau, P.; Berchoud, J.; Fouasson-Chailloux, A.; Crenn, V. Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions. Curr. Oncol. 2022, 29, 5875-5890. https://doi.org/10.3390/curroncol29080464
Plaud A, Gaillard J, Gouin F, Le Thuaut A, Ageneau P, Berchoud J, Fouasson-Chailloux A, Crenn V. Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions. Current Oncology. 2022; 29(8):5875-5890. https://doi.org/10.3390/curroncol29080464
Chicago/Turabian StylePlaud, Andrea, Jean Gaillard, François Gouin, Aurélie Le Thuaut, Peggy Ageneau, Juliane Berchoud, Alban Fouasson-Chailloux, and Vincent Crenn. 2022. "Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions" Current Oncology 29, no. 8: 5875-5890. https://doi.org/10.3390/curroncol29080464
APA StylePlaud, A., Gaillard, J., Gouin, F., Le Thuaut, A., Ageneau, P., Berchoud, J., Fouasson-Chailloux, A., & Crenn, V. (2022). Functional and Survival Outcomes of Patients following the Harrington Procedure for Complex Acetabular Metastatic Lesions. Current Oncology, 29(8), 5875-5890. https://doi.org/10.3390/curroncol29080464






