Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I–III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database
Abstract
:1. Background
2. Methods and Materials
2.1. Data Sources
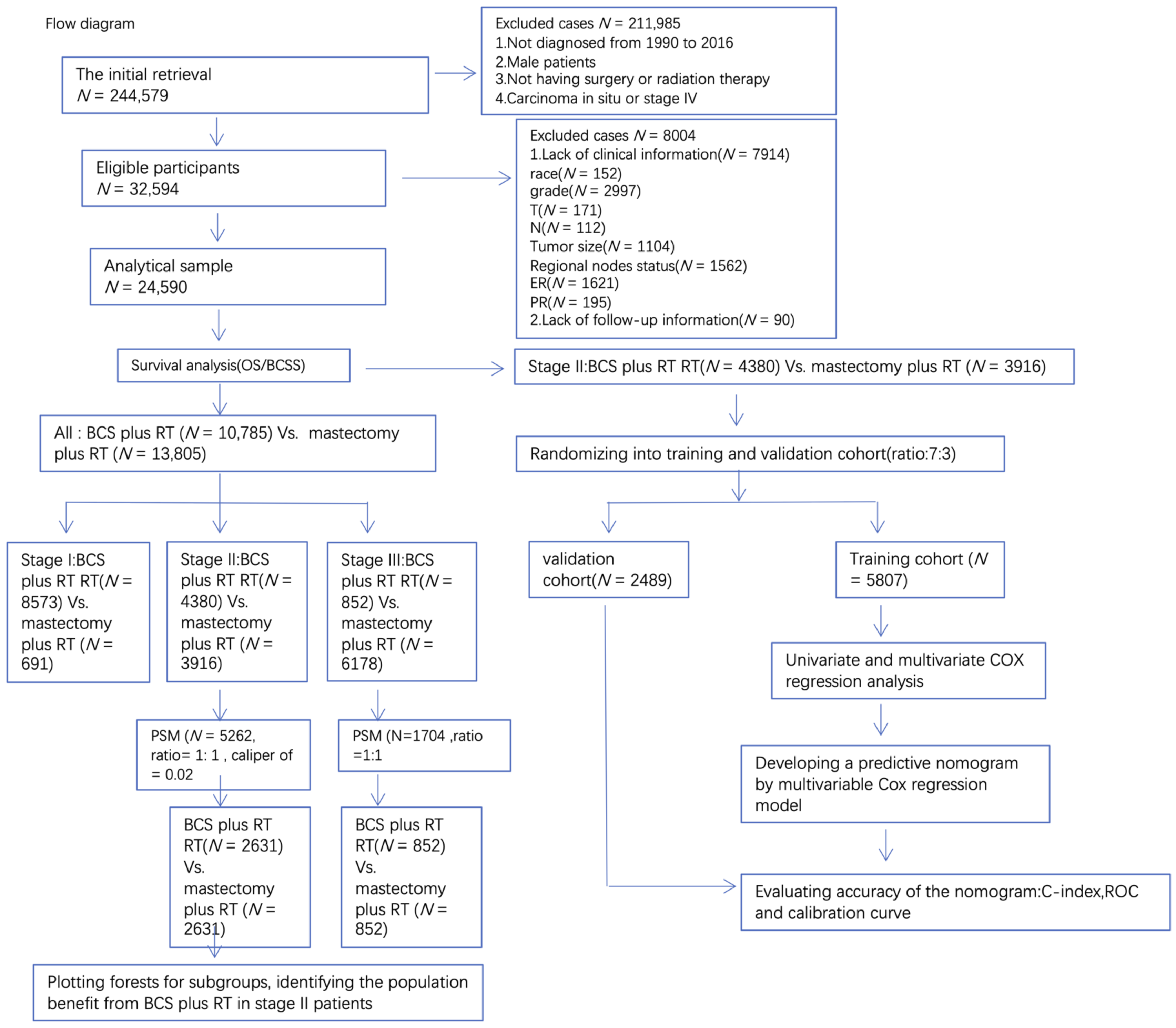
2.2. Cohort Selection
2.3. Variables
2.4. Propensity Score Matching
2.5. Statistical Methods
3. Results
3.1. Patient Characteristics and Determining Independent Prognostic Factors
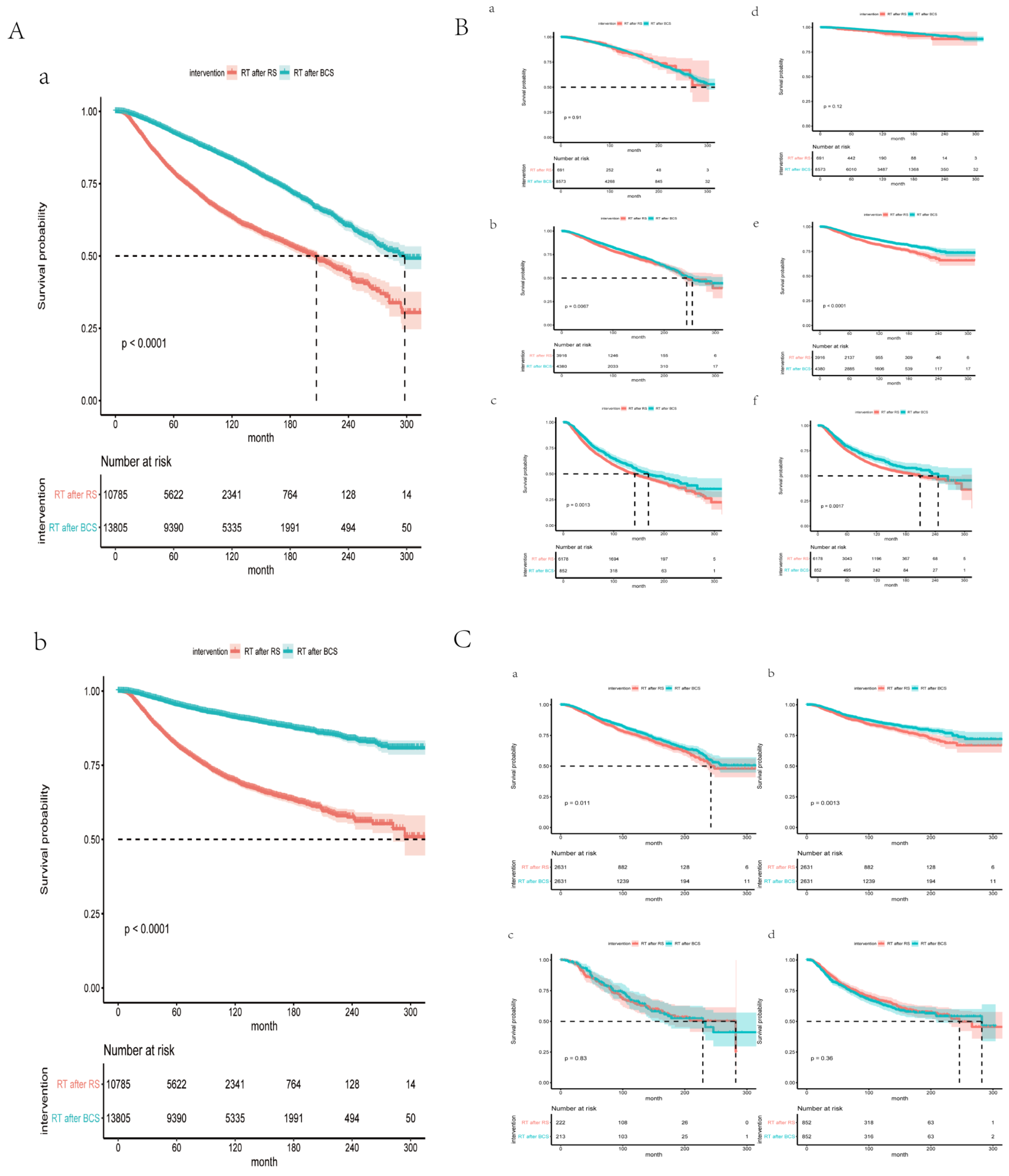
3.2. BCS Plus RT Improved Survival of Stage II Patients before and after PSM
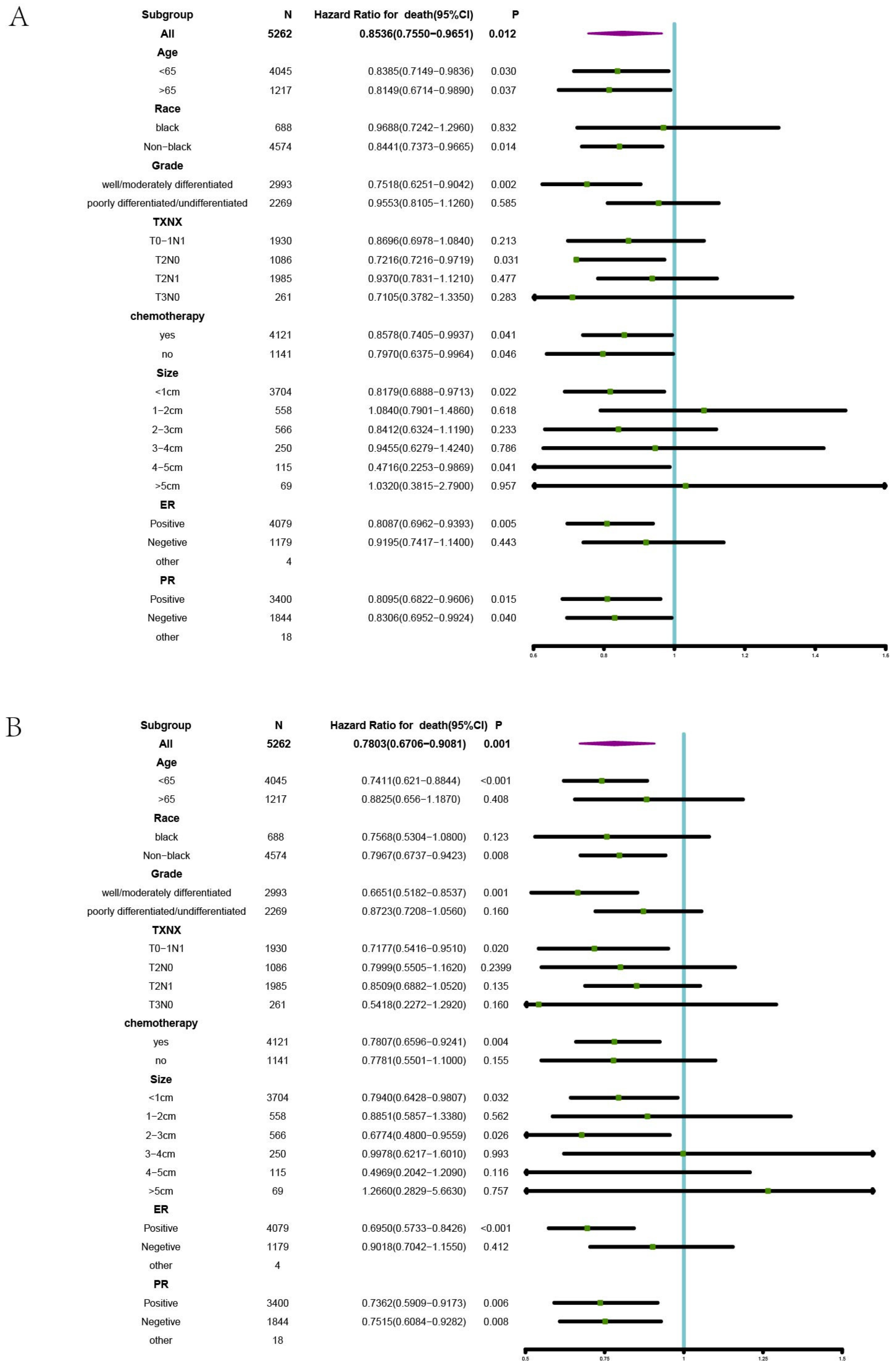
3.3. Subgroups Benefiting from BCS plus RT in Stage II
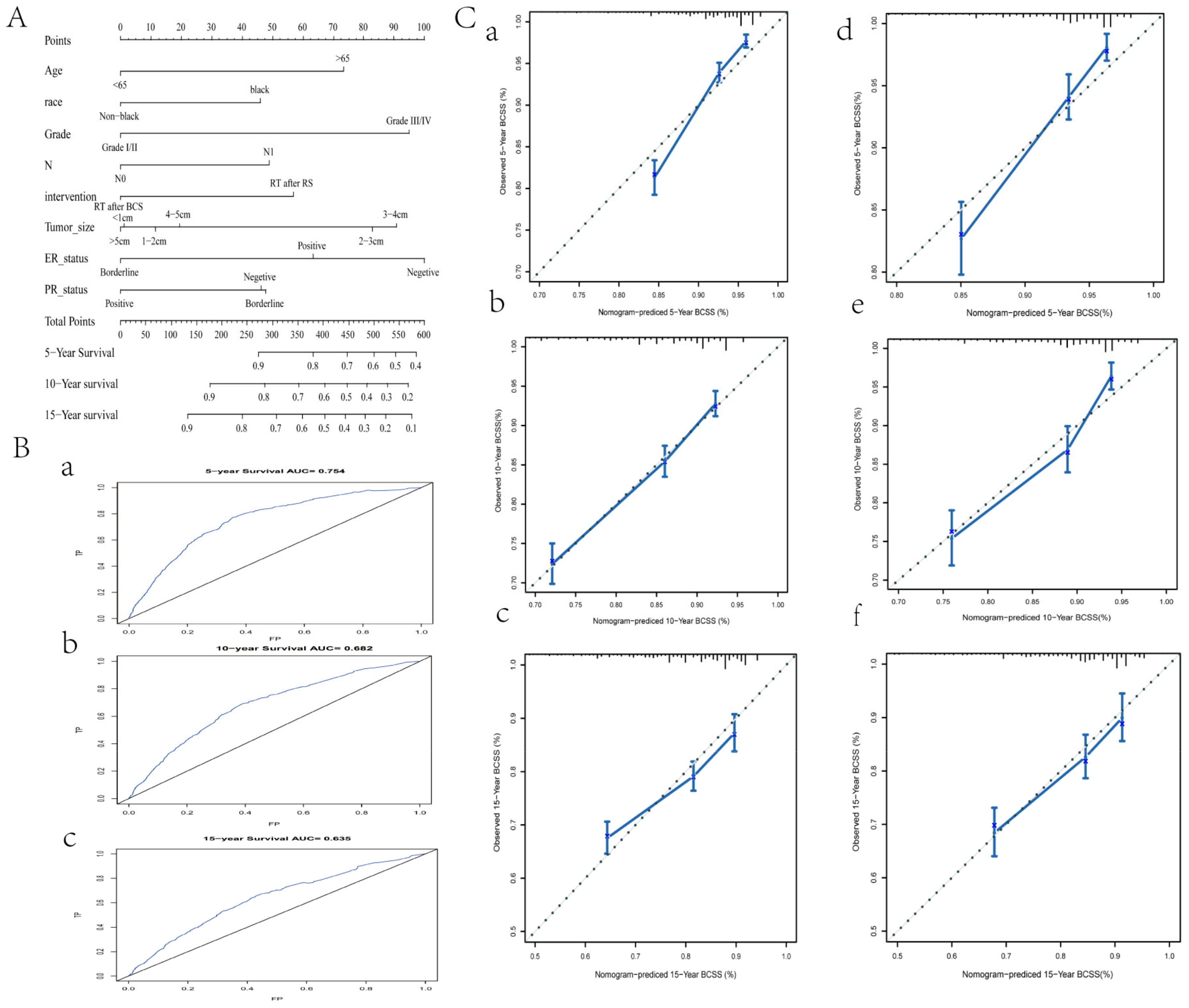
3.4. A Predictive Nomogram for BCSS Based on Stage II Data
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BCSS | Breast cancer-specific survival |
| BCS | Breast-conserving surgery |
| RT | Radiotherapy |
| OS | Overall survival |
| PSM | Propensity score matching |
| ER | Estrogen receptor |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
References
- Veronesi, U.; Cascinelli, N.; Mariani, L.; Greco, M.; Saccozzi, R.; Luini, A.; Aguilar, M.; Marubini, E. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N. Engl. J. Med. 2002, 347, 1227–1232. [Google Scholar] [CrossRef]
- Fisher, B.; Anderson, S.; Bryant, J.; Margolese, R.G.; Deutsch, M.; Fisher, E.R.; Jeong, J.H.; Wolmark, N. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N. Engl. J. Med. 2002, 347, 1233–1241. [Google Scholar] [CrossRef]
- Litière, S.; Werutsky, G.; Fentiman, I.S.; Rutgers, E.; Christiaens, M.R.; Van Limbergen, E.; Baaijens, M.H.; Bogaerts, J.; Bartelink, H. Breast conserving therapy versus mastectomy for stage I-II breast cancer: 20 year follow-up of the EORTC 10801 phase 3 randomised trial. Lancet Oncol. 2012, 13, 412–419. [Google Scholar] [CrossRef]
- Arriagada, R.; Lê, M.G.; Rochard, F.; Contesso, G. Conservative treatment versus mastectomy in early breast cancer: Patterns of failure with 15 years of follow-up data. Institut Gustave-Roussy Breast Cancer Group. J. Clin. Oncol. 1996, 14, 1558–1564. [Google Scholar] [CrossRef]
- Hwang, E.S.; Lichtensztajn, D.Y.; Gomez, S.L.; Fowble, B.; Clarke, C.A. Survival after lumpectomy and mastectomy for early stage invasive breast cancer: The effect of age and hormone receptor status. Cancer Am. Cancer Soc. 2013, 119, 1402–1411. [Google Scholar] [CrossRef]
- Agarwal, S.; Pappas, L.; Neumayer, L.; Kokeny, K.; Agarwal, J. Effect of breast conservation therapy vs mastectomy on disease-specific survival for early-stage breast cancer. JAMA Surg. 2014, 149, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.; Gao, H.; Yasui, Y.; Dabbs, K.; Winget, M. Survival in stage I-III breast cancer patients by surgical treatment in a publicly funded health care system. Ann. Oncol. 2015, 26, 1161–1169. [Google Scholar] [CrossRef]
- Hartmann-Johnsen, O.J.; Karesen, R.; Schlichting, E.; Nygard, J.F. Survival is Better After Breast Conserving Therapy than Mastectomy for Early Stage Breast Cancer: A Registry-Based Follow-up Study of Norwegian Women Primary Operated Between 1998 and 2008. Ann. Surg. Oncol. 2015, 22, 3836–3845. [Google Scholar] [CrossRef] [PubMed]
- Hofvind, S.; Holen, Å.; Aas, T.; Roman, M.; Sebuødegård, S.; Akslen, L.A. Women treated with breast conserving surgery do better than those with mastectomy independent of detection mode, prognostic and predictive tumor characteristics. Eur. J. Surg. Oncol. 2015, 41, 1417–1422. [Google Scholar] [CrossRef]
- van Maaren, M.C.; de Munck, L.; de Bock, G.H.; Jobsen, J.J.; van Dalen, T.; Linn, S.C.; Poortmans, P.; Strobbe, L.J.A.; Siesling, S. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: A population-based study. Lancet Oncol. 2016, 17, 1158–1170. [Google Scholar] [CrossRef]
- De Boniface, J.; Frisell, J.; Bergkvist, L.; Andersson, Y. Breast-conserving surgery followed by whole-breast irradiation offers survival benefits over mastectomy without irradiation. J. Br. Surg. 2018, 105, 1607–1614. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology; Breast Cancer, Version 3. 2020. Available online: http://www.nccn.org//patients/guidelines/content/PDF/breast-invasive-patient.pdf (accessed on 10 August 2021).
- Ditsch, N.; Untch, M.; Kolberg-Liedtke, C.; Jackisch, C.; Krug, D.; Friedrich, M.; Janni, W.; Müller, V.; Albert, U.S.; Banys-Paluchowski, M.; et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2020. Breast Care 2020, 15, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Hancock, M.J. Appraisal of Clinical Practice Guideline: Early and locally advanced breast cancer: Diagnosis and management. NICE guideline [NG101]. J. Physiother. 2019, 65, 57. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, C.B.; Xie, F.; Peng, Y.; Wang, S. Clinical practice guidelines for diagnosis and treatment of invasive breast cancer: Chinese Society of Breast Surgery (CSBrS) practice guidelines 2021. Chin. Med. J. 2021, 134, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef] [PubMed]
- Recht, A.; Comen, E.A.; Fine, R.E.; Fleming, G.F.; Hardenbergh, P.H.; Ho, A.Y.; Hudis, C.A.; Hwang, E.S.; Kirshner, J.J.; Morrow, M.; et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. J. Clin. Oncol. 2016, 34, 4431–4442. [Google Scholar] [CrossRef]
- National Institutes of Health Consensus Development Panel. NIH Consensus Development Conference statement on the treatment of early-stage breast cancer. Oncology 1991, 5, 120–124. [Google Scholar]
- Lan, X.W.; Wen, G.; He, Z.; Huang, J.H.; Zou, X.B.; Lin, X.; Tan, Y.T.; Huang, X.B. Comparison of long-term results between radiotherapy after breast-conserving surgery and postmastectomy radiotherapy in stage T1-2N1M0 breast cancer. Cancer Manag. Res. 2019, 11, 6477–6487. [Google Scholar] [CrossRef]
- Sun, G.Y.; Wen, G.; Zhang, Y.J.; Tang, Y.; Jing, H.; Wang, J.Y.; Zhang, J.H.; Yang, Y.; Zhao, X.R.; Chen, S.Y.; et al. Radiotherapy plays an important role in improving the survival outcome in patients with T1-2N1M0 breast cancer—A joint analysis of 4262 real world cases from two institutions. BMC Cancer 2020, 20, 1155. [Google Scholar] [CrossRef]
- de Boniface, J.; Szulkin, R.; Johansson, A.L.V. Survival After Breast Conservation vs Mastectomy Adjusted for Comorbidity and Socioeconomic Status: A Swedish National 6-Year Follow-up of 48 986 Women. JAMA Surg. 2021, 156, 628–637. [Google Scholar] [CrossRef]
- Rosenbaum, P.R.; Rubin, D.B. The Central Role of the Propensity Score in Observational Studies for Causal Effects. Biometrika 1983, 70, 41–55. [Google Scholar] [CrossRef]
- Benedetto, U.; Head, S.J.; Angelini, G.D.; Blackstone, E.H. Statistical primer: Propensity score matching and its alternatives. Eur. J. Cardiothorac. Surg. 2018, 53, 1112–1117. [Google Scholar] [CrossRef]
- Austin, P.C. An Introduction to Propensity Score Methods for Reducing the Effects of Confounding in Observational Studies. Multivar. Behav. Res. 2011, 46, 399–424. [Google Scholar] [CrossRef]
- Duhamel, A.; Labreuche, J.; Gronnier, C.; Mariette, C. Statistical Tools for Propensity Score Matching. Ann. Surg. 2017, 265, E79–E80. [Google Scholar] [CrossRef] [PubMed]
- Onitilo, A.A.; Engel, J.M.; Stankowski, R.V.; Doi, S.A. Survival Comparisons for Breast Conserving Surgery and Mastectomy Revisited: Community Experience and the Role of Radiation Therapy. Clin. Med. Res. 2015, 13, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Tang, Y.; Jing, H.; Sun, G.; Jin, J.; Liu, Y.; Song, Y.; Wang, W.; Fang, H.; et al. Comparison of Treatment Outcomes With Breast-conserving Surgery Plus Radiotherapy Versus Mastectomy for Patients with Stage I Breast Cancer: A Propensity Score-matched Analysis. Clin. Breast Cancer 2018, 18, e975–e984. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Lee, S.B.; Nam, S.J.; Lee, E.S.; Park, B.W.; Park, H.Y.; Lee, H.J.; Kim, J.; Chung, Y.; Kim, H.J.; et al. Survival of Breast-Conserving Surgery Plus Radiotherapy versus Total Mastectomy in Early Breast Cancer. Ann. Surg. Oncol. 2021, 28, 5039–5047. [Google Scholar] [CrossRef]
- Chen, I.X.; Chauhan, V.P.; Posada, J.; Ng, M.R.; Wu, M.W.; Adstamongkonkul, P.; Huang, P.; Lindeman, N.; Langer, R.; Jain, R.K. Blocking CXCR4 alleviates desmoplasia, increases T-lymphocyte infiltration, and improves immunotherapy in metastatic breast cancer. Proc. Natl. Acad. Sci. USA 2019, 116, 4558–4566. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, Y.; Tan, Y.; Wei, Q.; Yu, W. Cancer-associated fibroblasts in radiotherapy: Challenges and new opportunities. Cell Commun. Signal. 2019, 17, 47. [Google Scholar] [CrossRef]
- Portella, L.; Scala, S. Ionizing radiation effects on the tumor microenvironment. Semin. Oncol. 2019, 46, 254–260. [Google Scholar] [CrossRef]
- Darragh, L.B.; Oweida, A.J.; Karam, S.D. Overcoming Resistance to Combination Radiation-Immunotherapy: A Focus on Contributing Pathways Within the Tumor Microenvironment. Front. Immunol. 2018, 9, 3154. [Google Scholar] [CrossRef] [PubMed]
- Krisnawan, V.E.; Stanley, J.A.; Schwarz, J.K.; Denardo, D.G. Tumor Microenvironment as a Regulator of Radiation Therapy: New Insights into Stromal-Mediated Radioresistance. Cancers 2020, 12, 2916. [Google Scholar] [CrossRef] [PubMed]
- Goodman, C.R.; Seagle, B.L.; Friedl, T.W.P.; Rack, B.; Lato, K.; Fink, V.; Cristofanilli, M.; Donnelly, E.D.; Janni, W.; Shahabi, S.; et al. Association of Circulating Tumor Cell Status with Benefit of Radiotherapy and Survival in Early-Stage Breast Cancer. JAMA Oncol. 2018, 4, e180163. [Google Scholar] [CrossRef] [PubMed]
| Overall | Mastectomy Plus RT | BCS Plus RT | p | Test | |
|---|---|---|---|---|---|
| n | 24,590 | 10,785 | 13,805 | ||
| Median follow-up time | 79.00 [37.00, 135.00] | 63.00 [29.00, 110.00] | 95.00 [47.00, 149.00] | <0.001 | nonnorm |
| Age_diagnosis | |||||
| (median [IQR]) | 57.00 [48.00, 66.75] | 53.00 [45.00, 63.00] | 59.00 [51.00, 68.00] | <0.001 | nonnorm |
| Age (%) | <0.001 | ||||
| <65 | 17,589 (71.5) | 8476 (78.6) | 9113 (66.0) | ||
| >65 | 7001 (28.5) | 2309 (21.4) | 4692 (34.0) | ||
| Race (%) | <0.001 | ||||
| Black | 2885 (11.7) | 1390 (12.9) | 1495 (10.8) | ||
| White | 19,766 (80.4) | 8373 (77.6) | 11,393 (82.5) | ||
| Other | 1939 (7.9) | 1022 (9.5) | 917 (6.6) | ||
| Histologic_Type (%) | <0.001 | ||||
| Duct carcinoma | 18,092 (73.6) | 7454 (69.1) | 10,638 (77.1) | ||
| Lobular carcinoma | 2120 (8.6) | 1262 (11.7) | 858 (6.2) | ||
| Other | 4378 (17.8) | 2069 (19.2) | 2309 (16.7) | ||
| Grade (%) | <0.001 | ||||
| Grade I | 4388 (17.8) | 988 (9.2) | 3400 (24.6) | ||
| Grade II | 10,743 (43.7) | 4581 (42.5) | 6162 (44.6) | ||
| Grade III | 9188 (37.4) | 5070 (47.0) | 4118 (29.8) | ||
| Grade IV | 271 (1.1) | 146 (1.4) | 125 (0.9) | ||
| Laterality (%) | 0.015 | ||||
| Left | 12,397 (50.4) | 5520 (51.2) | 6877 (49.8) | ||
| right | 12,187 (49.6) | 5260 (48.8) | 6927 (50.2) | ||
| others | 6 (0.0) | 5 (0.0) | 1 (0.0) | ||
| Stage (%) | <0.001 | ||||
| I | 9264 (37.7) | 691 (6.4) | 8573 (62.1) | ||
| II | 8296 (33.7) | 3916 (36.3) | 4380 (31.7) | ||
| III | 7030 (28.6) | 6178 (57.3) | 852 (6.2) | ||
| T (%) | <0.001 | ||||
| T0 | 34 (0.1) | 26 (0.2) | 8 (0.1) | ||
| T1 | 12,813 (52.1) | 2475 (22.9) | 10,338 (74.9) | ||
| T2 | 7711 (31.4) | 4586 (42.5) | 3125 (22.6) | ||
| T3 | 2680 (10.9) | 2416 (22.4) | 264 (1.9) | ||
| T4 | 1352 (5.5) | 1282 (11.9) | 70 (0.5) | ||
| N (%) | <0.001 | ||||
| N0 | 12,041 (49.0) | 1729 (16.0) | 10,312 (74.7) | ||
| N1 | 7180 (29.2) | 4406 (40.9) | 2774 (20.1) | ||
| N2 | 3381 (13.7) | 2885 (26.8) | 496 (3.6) | ||
| N3 | 1988 (8.1) | 1765 (16.4) | 223 (1.6) | ||
| Intervention (%) | <0.001 | ||||
| Mastectomy plus RT | 10,785 (43.9) | 10,785 (100.0) | 0 (0.0) | ||
| BCS plus RT | 13,805 (56.1) | 0 (0.0) | 13,805 (100.0) | ||
| Chemotherapy (%) | <0.001 | ||||
| Yes | 14,689 (59.7) | 9198 (85.3) | 5491 (39.8) | ||
| No and unknown | 9901 (40.3) | 1587 (14.7) | 8314 (60.2) | ||
| Tumor_size (%) | <0.001 | ||||
| <1cm | 17,843 (72.6) | 7389 (68.5) | 10,454 (75.7) | ||
| 1–2 cm | 3176 (12.9) | 974 (9.0) | 2202 (16.0) | ||
| 2–3 cm | 1561 (6.3) | 770 (7.1) | 791 (5.7) | ||
| 3–4cm | 665 (2.7) | 467 (4.3) | 198 (1.4) | ||
| 4–5 cm | 427 (1.7) | 347 (3.2) | 80 (0.6) | ||
| >5 cm | 918 (3.7) | 838 (7.8) | 80 (0.6) | ||
| Regional_nodes_positive | |||||
| (mean (SD)) | 2.47 (4.60) | 4.70 (5.71) | 0.72 (2.29) | <0.001 | |
| ER_status (%) | <0.001 | ||||
| Positive | 19,769 (80.4) | 8312 (77.1) | 11,457 (83.0) | ||
| Negative | 4780 (19.4) | 2455 (22.8) | 2325 (16.8) | ||
| Borderline | 41 (0.2) | 18 (0.2) | 23 (0.2) | ||
| PR_status (%) | <0.001 | ||||
| Positive | 17,237 (70.1) | 7110 (65.9) | 10,127 (73.4) | ||
| Negative | 7253 (29.5) | 3632 (33.7) | 3621 (26.2) | ||
| Borderline | 100 (0.4) | 43 (0.4) | 57 (0.4) | ||
| Status | <0.001 | ||||
| Alive | 19,268 (78.4) | 7791 (72.2) | 11,477 (83.1) | ||
| Dead | 5322 (21.6) | 2994 (27.8) | 2328 (16.9) |
| PSM before | PSM after | |||||
|---|---|---|---|---|---|---|
| Mastectomy Plus RT | BCS Plus RT | p | Mastectomy Plus RT | BCS Plus RT | p | |
| n | 3916 | 4380 | 2631 | 2631 | ||
| Age (%) | <0.001 | 0.433 | ||||
| <65 | 3184 (81.3) | 3008 (68.7) | 2035(77.3) | 2010(76.4) | ||
| >65 | 732 (18.7) | 1372 (31.3) | 596 (22.7) | 621 (23.6) | ||
| Race (%) | 0.558 | 0.54 | ||||
| Black | 498 (12.7) | 577 (13.2) | 352(13.4) | 336(12.8) | ||
| Non-black | 3418 (87.3) | 3803 (86.8) | 2279 (86.6) | 2295 (87.2) | ||
| Grade (%) | 0.111 | 0.824 | ||||
| well/moderately | 2185 (55.8) | 2521 (57.6) | 1492(56.7) | 1501(57.1) | ||
| poorly differentiated | 1731 (44.2) | 1859 (42.4) | 1139 (43.3) | 1130 (42.9) | ||
| T (%) | <0.001 | 0.477 | ||||
| T0 | 8 (0.2) | 4 (0.1) | 2 (0.1) | 4 (0.2) | ||
| T1 | 978 (25.0) | 1472 (33.6) | 939 (35.7) | 985 (37.4) | ||
| T2 | 2538 (64.8) | 2771 (63.3) | 1559 (59.3) | 1512 (57.5) | ||
| T3 | 392 (10.0) | 133 (3.0) | 131 (5.0) | 130 4.9) | ||
| N (%) | <0.001 | 0.165 | ||||
| N0 | 980 (25.0) | 1850 (42.2) | 2509(85.6) | 2520(86.9) | ||
| N1 | 2936 (75.0) | 2530 (57.8) | 122 (14.4) | 111 (13.1) | ||
| Chemotherapy (%) | <0.001 | 0.894 | ||||
| Yes | 3269 (83.5) | 2849 (65.0) | 2063(78.4) | 2058(78.2) | ||
| No and unknown | 647 (16.5) | 1531 (35.0) | 568 (21.6) | 573 (21.8) | ||
| Tumor_size (%) | <0.001 | 0.215 | ||||
| <1 cm | 2814 (71.9) | 2935 (67.0) | 1863 (70.8) | 1841 (70.0) | ||
| 1–2 cm | 270 (6.9) | 483 (11.0) | 253 (9.6) | 305 (11.6) | ||
| 2–3 cm | 343 (8.8) | 703 (16.1) | 298 (11.3) | 268 (10.2) | ||
| 3–4 cm | 210 (5.4) | 165 (3.8) | 122 (4.6) | 128 (4.9) | ||
| 4–5 cm | 166 (4.2) | 61 (1.4) | 58 (2.2) | 57 (2.2) | ||
| >5 cm | 113 (2.9) | 33 (0.8) | 37 (1.4) | 32 (1.2) | ||
| ER_status (%) | 0.212 | 0.064 | ||||
| Positive | 3129 (79.9) | 3442 (78.6) | 2075 (78.9) | 2004 (76.2) | ||
| Negative | 782 (20.0) | 935 (21.3) | 554 (21.1) | 625 (23.8) | ||
| Borderline | 5 (0.1) | 3 (0.1) | 2 (0.1) | 2 (0.1) | ||
| PR_status (%) | 0.689 | <0.001 | ||||
| Positive | 2705 (69.1) | 2999 (68.5) | 1804 (68.6) | 1596 (60.7) | ||
| Negative | 1200 (30.6) | 1365 (31.2) | 820 (31.2) | 1024 (38.9) | ||
| Borderline | 11 (0.3) | 16 (0.4) | 7 (0.3) | 11 (0.4) | ||
| PSM before | PSM after | |||||
|---|---|---|---|---|---|---|
| Mastectomy Plus RT | BCS Plus RT | p | Mastectomy Plus RT | BCS Plus RT | p | |
| n | 6178 | 852 | 852 | 852 | ||
| Age (%) | 0.93 | 1 | ||||
| <65 | 4737 (76.7) | 655 (76.9) | 654 (76.8) | 655 (76.9) | ||
| >65 | 1441 (23.3) | 197 (23.1) | 198 (23.2) | 197 (23.1) | ||
| Race (%) | 0.002 | 0.395 | ||||
| Black | 824 (13.3) | 147 (17.3) | 133 (15.6) | 147 (17.3) | ||
| Non-black | 5354 (86.7) | 705 (82.7) | 719 (84.4) | 705 (82.7) | ||
| Grade (%) | 0.099 | 1 | ||||
| Well/moderately | 2931 (47.4) | 378 (44.4) | 377 (44.2) | 378 (44.4) | ||
| Differentiated | ||||||
| Poorly differentiated/ | 3247 (52.6) | 474 (55.6) | 475 (55.8) | 474 (55.6) | ||
| Undifferentiated | ||||||
| T(%) | <0.001 | 0.964 | ||||
| T0 | 17 (0.3) | 3 (0.4) | 3 (0.4) | 3 (0.4) | ||
| T1 | 807 (13.1) | 294 (34.5) | 305 (35.8) | 294 (34.5) | ||
| T2 | 2048 (33.1) | 354 (41.5) | 344 (40.4) | 354 (41.5) | ||
| T3 | 2024 (32.8) | 131 (15.4) | 135 (15.8) | 131 (15.4) | ||
| T4 | 1282 (20.8) | 70 (8.2) | 65 (7.6) | 70 (8.2) | ||
| N (%) | <0.001 | 0.958 | ||||
| N0 | 140 (2.3) | 22 (2.6) | 19 (2.2) | 22 (2.6) | ||
| N1 | 1388 (22.5) | 111 (13.0) | 114 (13.4) | 111 (13.0) | ||
| N2 | 2885 (46.7) | 496 (58.2) | 500 (58.7) | 496 (58.2) | ||
| N3 | 1765 (28.6) | 223 (26.2) | 219 (25.7) | 223 (26.2) | ||
| chemotherapy (%) | 0.082 | 0.361 | ||||
| Yes | 5548 (89.8) | 748 (87.8) | 761 (89.3) | 748 (87.8) | ||
| No and unknown | 630 (10.2) | 104 (12.2) | 91 (10.7) | 104 (12.2) | ||
| Tumor_size (%) | <0.001 | 0.875 | ||||
| <1 cm | 4014 (65.0) | 542 (63.6) | 541 (63.5) | 542 (63.6) | ||
| 1–2 cm | 577 (9.3) | 128 (15.0) | 136 (16.0) | 128 (15.0) | ||
| 2–3 cm | 427 (6.9) | 85 (10.0) | 72 (8.5) | 85 (10.0) | ||
| 3–4 cm | 257 (4.2) | 31 (3.6) | 30 (3.5) | 31 (3.6) | ||
| 4–5 cm | 181 (2.9) | 19 (2.2) | 23 (2.7) | 19 (2.2) | ||
| >5 cm | 722 (11.7) | 47 (5.5) | 50 (5.9) | 47 (5.5) | ||
| ER_status (%) | 0.625 | 0.555 | ||||
| Positive | 4613 (74.7) | 624 (73.2) | 617 (72.4) | 624 (73.2) | ||
| Negative | 1555 (25.2) | 227 (26.6) | 235 (27.6) | 227 (26.6) | ||
| Borderline | 10 (0.2) | 1 (0.1) | 0 (0.0) | 1 (0.1) | ||
| PR_status (%) | 0.76 | 0.13 | ||||
| Positive | 3909 (63.3) | 528 (62.0) | 536 (62.9) | 528 (62.0) | ||
| Negative | 2240 (36.3) | 320 (37.6) | 316 (37.1) | 320 (37.6) | ||
| Borderline | 29 (0.5) | 4 (0.5) | 0 (0.0) | 4 (0.5) | ||
| Variables | Univariable Cox | Multivariable Cox | ||
|---|---|---|---|---|
| HR | p | HR | p | |
| Intervention | ||||
| Mastectomy plus RT | control group | control group | ||
| BCS plus RT | 0.6893(0.5957–0.7977) | <0.001 | 0.6730(0.5754–0.787) | <0.001 |
| Age | ||||
| <65 | control group | control group | ||
| >65 | 1.333(1.132–1.57) | <0.001 | 1.6659(1.4093–1.969) | <0.001 |
| Race | ||||
| Black | control group | control group | ||
| Non-black | 0.5986(0.4939–0.7255) | <0.001 | 0.7257(0.5965–0.883) | 0.0014 |
| Grade | ||||
| I/II | control group | control group | ||
| III/IV | 2.364(2.032–2.75) | <0.001 | 1.9366(1.6396–2.287) | <0.001 |
| N | ||||
| N0 | control group | control group | ||
| N1 | 1.212(1.033–1.421) | 0.0181 | 1.4052(1.1801–1.673) | <0.001 |
| Tumor_size | ||||
| <1 cm | control group | control group | ||
| 1–2 cm | 1.003(0.7865–1.278) | 0.982 | 1.0748(0.8361–1.382) | 0.5737 |
| 2–3 cm | 1.692(1.3974–2.048) | <0.001 | 1.7675(1.4528–2.150) | <0.001 |
| 3–4 cm | 2.089(1.6061–2.717) | <0.001 | 1.8681(1.4330–2.435) | <0.001 |
| 4–5 cm | 1.371(0.9250–2.031) | 0.116 | 1.1356(0.7626–1.691) | 0.5313 |
| >5 cm | 1.016(0.5563–1.856) | 0.959 | 0.9930(0.5339–1.847) | 0.9822 |
| ER_status | ||||
| Positive | control group | control group | ||
| Negetive | 2.1286(1.8272–2.480) | <0.001 | 1.2908(1.0317–1.615) | 0.0255 |
| Borderline | 0.7376(0.1034–5.262) | 0.761 | 0.6434(0.0880–4.705) | 0.664 |
| PR_status | ||||
| Positive | control group | control group | ||
| Negetive | 2.045(1.7672–2.367) | <0.001 | 1.3798(1.1202–1.700) | 0.0025 |
| Borderline | 1.771(0.6613–4.745) | 0.255 | 1.3948(0.5136–3.788) | 0.5139 |
| T | ||||
| T0 | control group | |||
| T1 | 305,014.32 | 0.985 | ||
| T2 | 538,153.28 | 0.984 | ||
| T3 | 457,474.89 | 0.985 | ||
| chemotherapy | ||||
| YES | control group | |||
| NO | 0.9175(0.7711–1.092) | 0.331 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiang, W.; Wu, C.; Wu, H.; Fang, S.; Liu, N.; Yu, H. Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I–III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database. Curr. Oncol. 2022, 29, 5731-5747. https://doi.org/10.3390/curroncol29080452
Xiang W, Wu C, Wu H, Fang S, Liu N, Yu H. Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I–III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database. Current Oncology. 2022; 29(8):5731-5747. https://doi.org/10.3390/curroncol29080452
Chicago/Turabian StyleXiang, Wenbin, Chaoyan Wu, Huachao Wu, Sha Fang, Nuomin Liu, and Haijun Yu. 2022. "Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I–III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database" Current Oncology 29, no. 8: 5731-5747. https://doi.org/10.3390/curroncol29080452
APA StyleXiang, W., Wu, C., Wu, H., Fang, S., Liu, N., & Yu, H. (2022). Survival Comparisons between Breast Conservation Surgery and Mastectomy Followed by Postoperative Radiotherapy in Stage I–III Breast Cancer Patients: Analysis of the Surveillance, Epidemiology, and End Results (Seer) Program Database. Current Oncology, 29(8), 5731-5747. https://doi.org/10.3390/curroncol29080452




