Diagnostic and Therapeutic Challenges in a Patient with Ureteral Metastases from a Triple Negative Breast Cancer
Abstract
1. Introduction
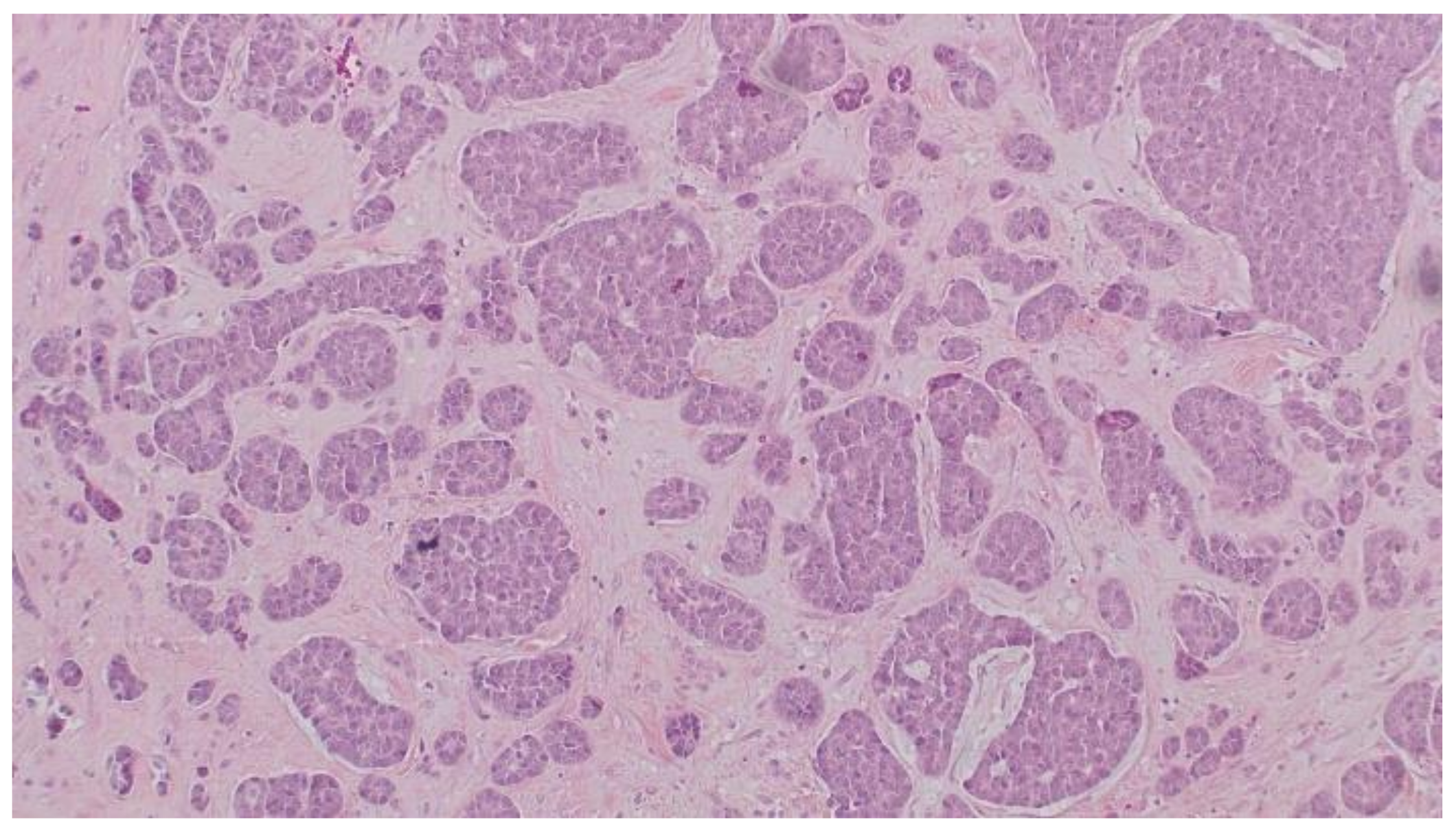
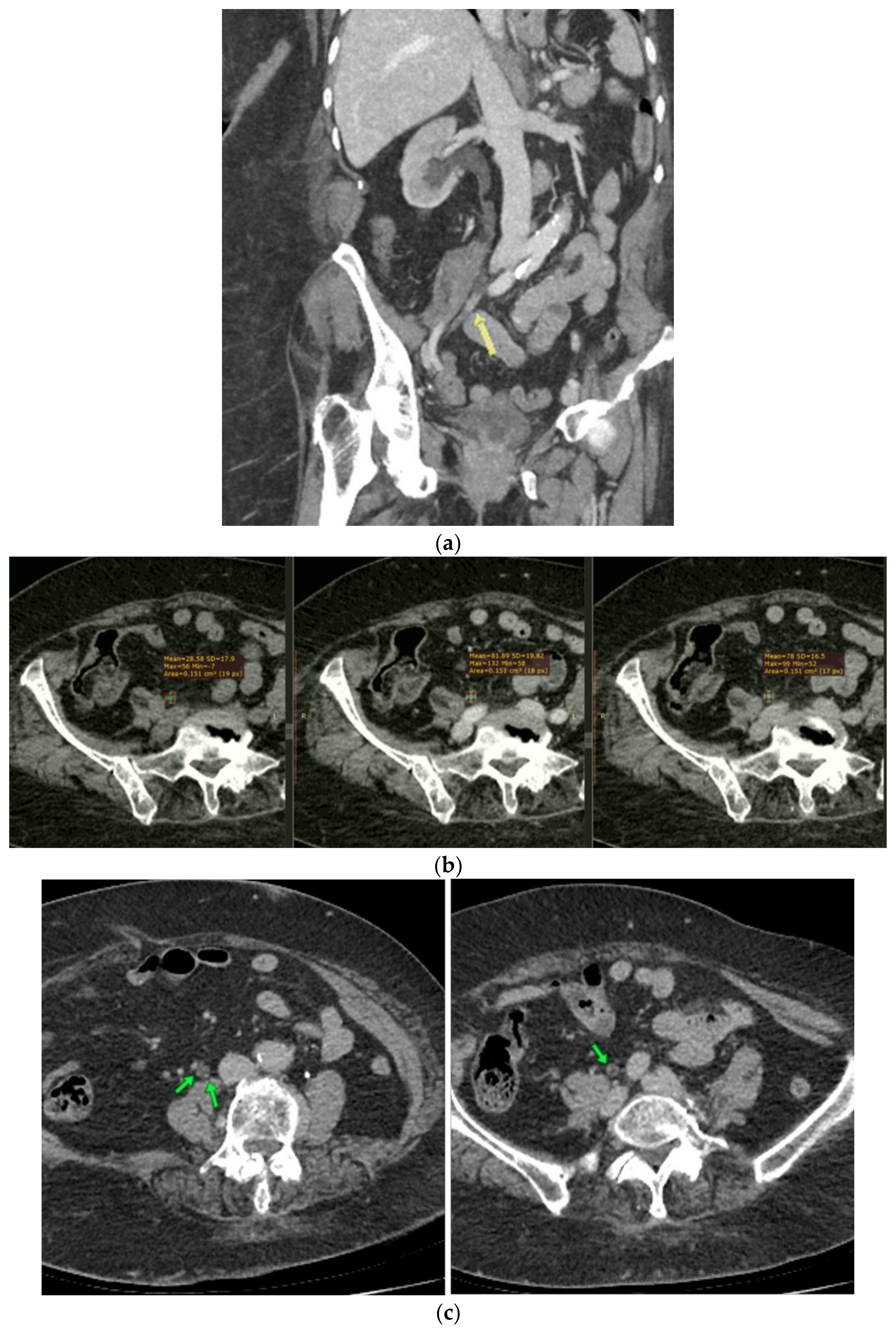
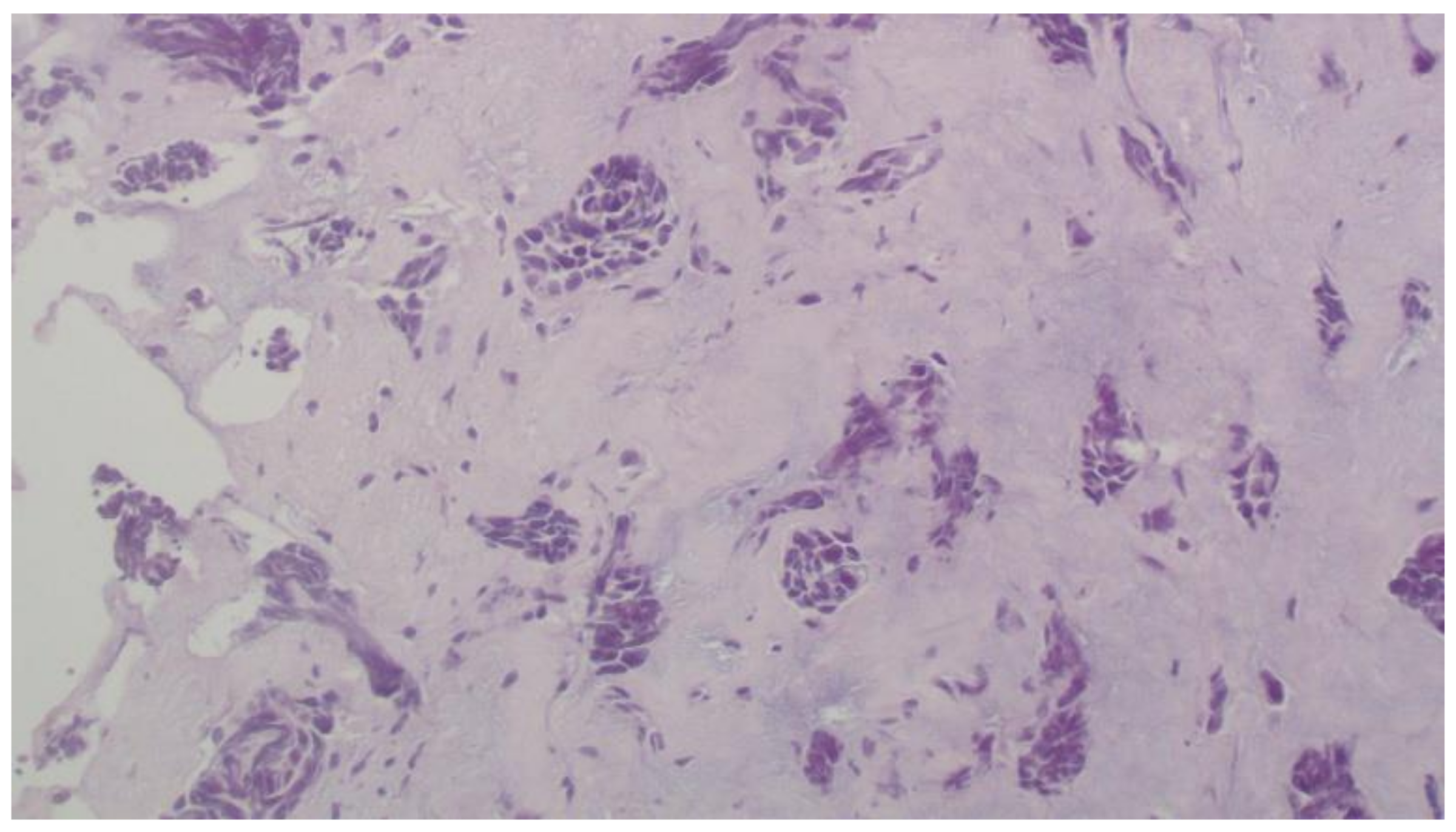
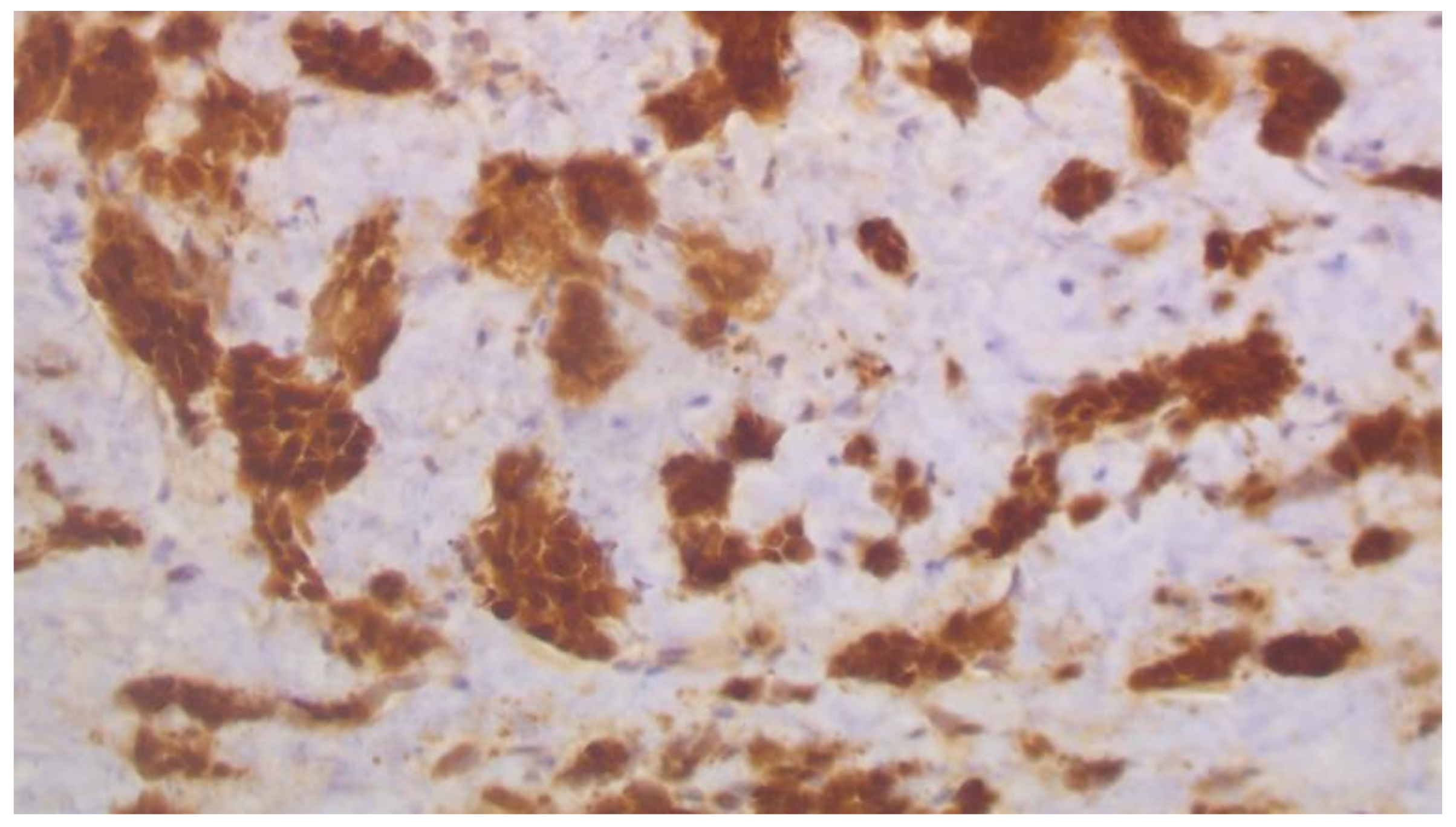
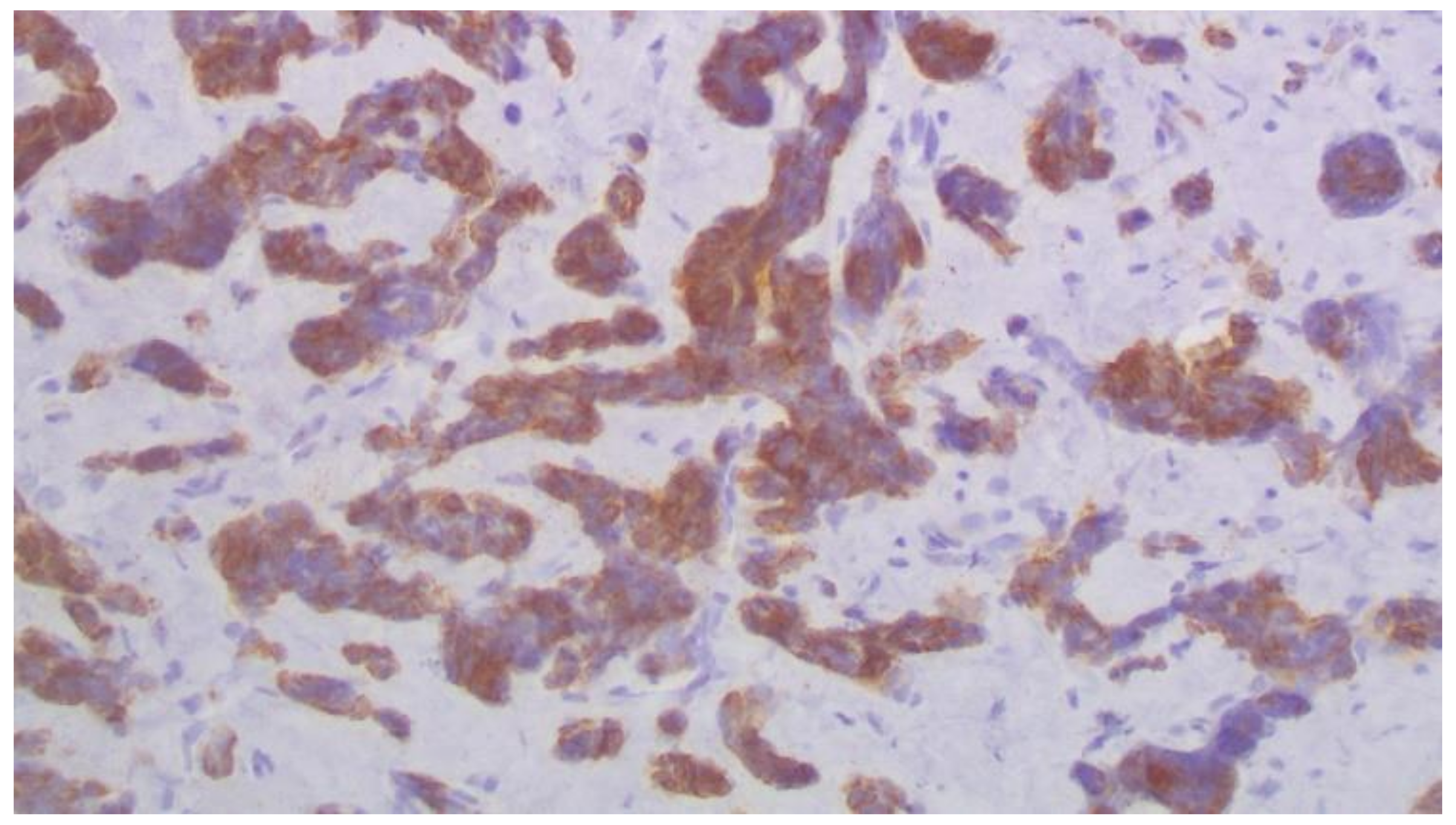
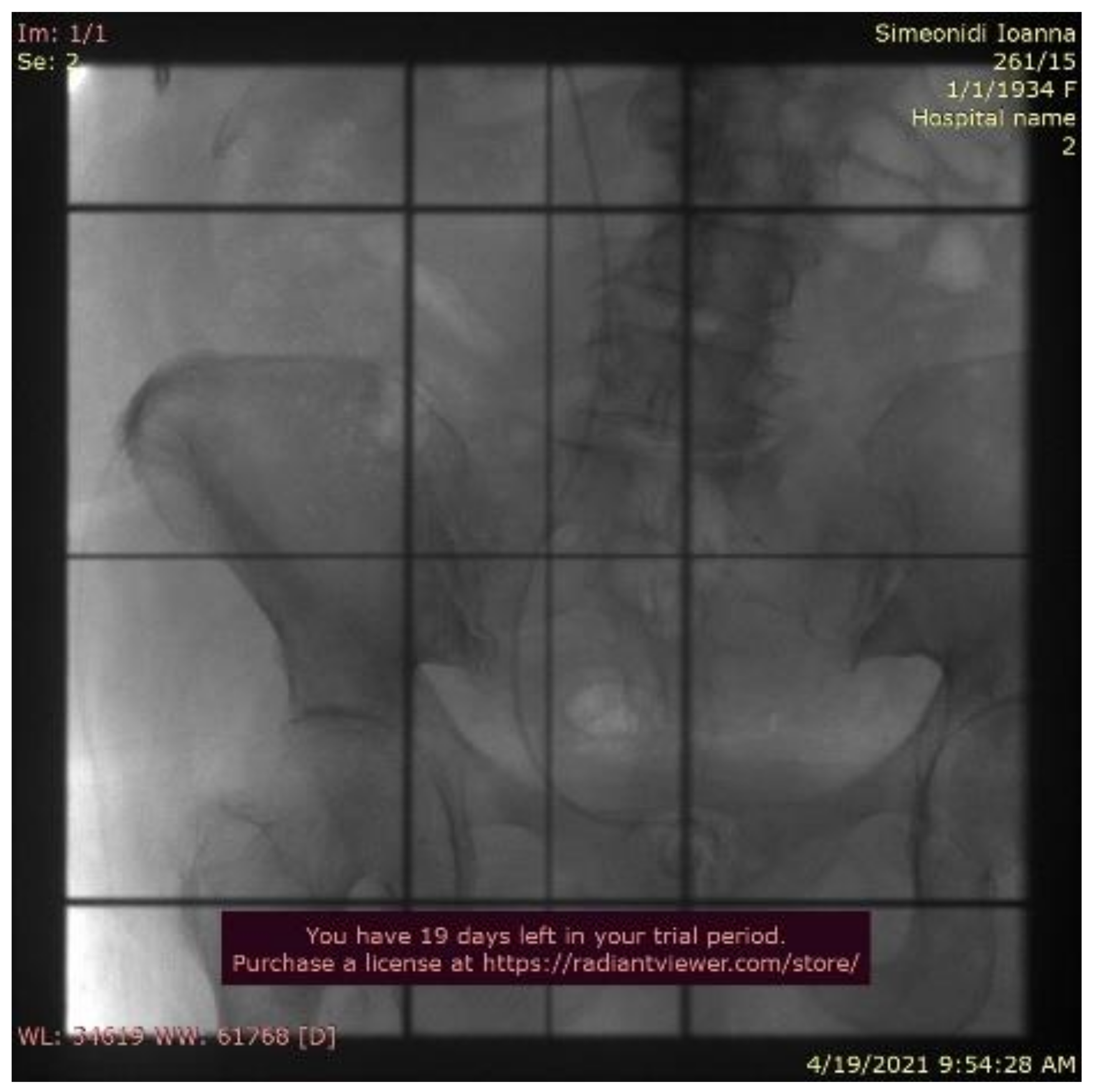
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lei, S.; Zheng, R.; Zhang, S.; Wang, S.; Chen, R.; Sun, K.; Zeng, H.; Zhou, J.; Wei, W. Global Patterns of Breast Cancer Incidence and Mortality: A Population-Based Cancer Registry Data Analysis from 2000 to 2020. Cancer Commun. 2021, 41, 1183–1194. [Google Scholar] [CrossRef] [PubMed]
- Yamamura, J.; Kamigaki, S.; Fujita, J.; Osato, H.; Manabe, H.; Tanaka, Y.; Shinzaki, W.; Hashimoto, Y.; Komoike, Y. New Insights into Patterns of First Metastatic Sites Influencing Survival of Patients with Hormone Receptor-Positive, HER2-Negative Breast Cancer: A Multicenter Study of 271 Patients. BMC Cancer 2021, 21, 476. [Google Scholar] [CrossRef] [PubMed]
- Nathanson, S.D.; Detmar, M.; Padera, T.P.; Yates, L.R.; Welch, D.R.; Beadnell, T.C.; Scheid, A.D.; Wrenn, E.D.; Cheung, K. Mechanisms of Breast Cancer Metastasis. Clin. Exp. Metastasis 2021, 29, 117–137. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Deng, J.; Guo, J.; Fu, B. Ureteral Involvement by Metastatic Malignant Disease. Clin. Exp. Metastasis 2019, 36, 499–509. [Google Scholar] [CrossRef]
- Haddad, F.S. Metastases to the Ureter. Review of the World Literature, and Three New Case Reports. J. Med. Liban. 1999, 47, 265–271. [Google Scholar]
- López-Martínez, R.A.; Stock, J.A.; Gump, F.E.; Rosen, J.S. Carcinoma of the Breast Metastatic to the Ureter Presenting with Flank Pain and Recurrent Urinary Tract Infection. Am. Surg. 1996, 62, 748–752. [Google Scholar]
- Grabstald, H.; Kaufman, R. Hydronephrosis Secondary to Ureteral Obstruction by Metastatic Breast Cancer. J. Urol. 1969, 102, 569–576. [Google Scholar] [CrossRef]
- Talreja, D.; Opfell, R.W. Ureteral Metastasis in Carcinoma of the Breast. West. J. Med. 1980, 133, 252–254. [Google Scholar]
- Richie, J.P.; Withers, G.; Ehrlich, R.M. Ureteral Obstruction Secondary to Metastatic Tumors. Surg. Gynecol. Obstet. 1979, 148, 355–357. [Google Scholar]
- Karaosmanoglu, A.D.; Onur, M.R.; Karcaaltincaba, M.; Akata, D.; Ozmen, M.N. Secondary Tumors of the Urinary System: An Imaging Conundrum. Korean J. Radiol. 2018, 19, 742–751. [Google Scholar] [CrossRef]
- Fröber, R. Surgical Anatomy of the Ureter. BJU Int. 2007, 100, 949–965. [Google Scholar] [CrossRef]
- Gabsi, A.; Yahiaoui, Y.; Zenhani, A.; Herbegue, K.; Meddeb, K.; Mokrani, A.; Letaief, F.; Ayadi, M.; Rais, H.; Chraiet, N.; et al. Ureteral Metastasis in Carcinoma of the Breast. Urology case reports. November 2018, 21, 38–40. [Google Scholar] [CrossRef]
- Jani, K. Ureteric Obstruction Secondary to Metastatic Breast Carcinoma. Pakistan J. Med. Sci. 2006, 22, 197–199. [Google Scholar]
- Logothetis, C.; Assikis, V.; Sarriera, J. Algorithm for the Management of Urinary Obstruction. In Holland-Frei Cancer Medicine, 6th ed.; Kufe, D.W., Pollock, R.E., Weichselbaum, R.R., Best, R.C., Jr., Gansler, T.S., Holland, J.F., Frei, E., Eds.; BC Decker: Hamilton, ON, Canada, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK13333/ (accessed on 10 April 2022).
- Merchan, J.; Jhaveri, K. Chemotherapy Nephrotoxicity and dose Modification in Patients with Kidney Impairment: Conventional Cytotoxic Agents-UpToDate. Available online: https://www.uptodate.com/contents/chemotherapy-nephrotoxicity-and-dose-modification-in-patients-with-kidney-impairment-conventional-cytotoxic-agents#H1991902678 (accessed on 12 March 2022).
- Numakura, K.; Tsuchiya, N.; Obara, T.; Tsuruta, H.; Saito, M.; Narita, S.; Inoue, T.; Horikawa, Y.; Satoh, S.; Habuchi, T. A Case of Ureteral Malignant Lymphoma Diagnosed by Laparoscopic Needle Biopsy. Jpn. J. Clin. Oncol. 2011, 41, 440–442. [Google Scholar] [CrossRef][Green Version]
- Presman, D.; Ehrich, L. Metastatic Tumors of the Ureter. J. Urol. 1948, 59, 312–325. [Google Scholar] [CrossRef]
- Chahin, M.; Chhatrala, H.; Krishnan, N.; Brow, D.; Zuberi, L. Triple-Negative Lobular Breast Cancer Causing Hydronephrosis. J. Investig. Med. High Impact Case Rep. 2020, 8, 2324709620905954. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Hu, T.; Wang, J.; Su, F. Male Breast Cancer with Ureteral Metastasis: A Case Report. Annals of palliative medicine. China July 2021, 8346–8351. [Google Scholar] [CrossRef]
- Ennishi, D.; Takata, K.; Béguelin, W.; Duns, G.; Mottok, A.; Farinha, P.; Bashashati, A.; Saberi, S.; Boyle, M.; Meissner, B.; et al. Molecular and Genetic Characterization of MHC Deficiency Identifies EZH2 as Therapeutic Target for Enhancing Immune Recognition. Cancer Discov. 2019, 9, 546–563. [Google Scholar] [CrossRef]
- Nie, L.; Wei, Y.; Zhang, F.; Hsu, Y.-H.; Chan, L.-C.; Xia, W.; Ke, B.; Zhu, C.; Deng, R.; Tang, J.; et al. DK2-Mediated Site-Specific Phosphorylation of EZH2 Drives and Maintains Triple-Negative Breast Cancer. Nat. Commun. 2019, 10, 5114. [Google Scholar] [CrossRef]
- Tanaka, H.; Yoshida, S.; Komai, Y.; Sakai, Y.; Urakami, S.; Yuasa, T.; Yamamoto, S.; Masuda, H.; Koizumi, M.; Kohno, A.; et al. Clinical Value of 18F-Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography in Upper Tract Urothelial Carcinoma: Impact on Detection of Metastases and Patient Management. Urol. Int. 2016, 96, 65–72. [Google Scholar] [CrossRef]
- Wan, X.; Hou, Y.; Yu, Z. Helical CT Diagnosis of the Primary Ureteral Carcinoma and Ureteral Metastatic Carcinoma. J. Clin. Radiol. 2010, 29, 985–987. [Google Scholar]
- Huang, Y.; He, H.; Wei, W.; Li, Q.; Long, X.; Li, Y.; Chen, R.; Yi, X. 18F-FDG PET/CT Features of Ureteral Metastases from Breast Cancer: A Case Report. J. Int. Med. Res. 2021, 49, 3000605211014090. [Google Scholar] [CrossRef]
- Kitajima, K.; Yamamoto, S.; Fukushima, K.; Minamimoto, R.; Kamai, T.; Jadvar, H. Update on Advances in Molecular PET in Urological Oncology. Jpn. J. Radiol. 2016, 34, 470–485. [Google Scholar] [CrossRef]
- Peart, O. Metastatic Breast Cancer. Radiol. Technol. 2017, 88, 519M–539M. [Google Scholar]
- Lin, N.U.; Thomssen, C.; Cardoso, F.; Cameron, D.; Cufer, T.; Fallowfield, L.; Francis, P.A.; Kyriakides, S.; Pagani, O.; Senkus, E.; et al. International Guidelines for Management of Metastatic Breast Cancer (MBC) from the European School of Oncology (ESO)-MBC Task Force: Surveillance, Staging, and Evaluation of Patients with Early-Stage and Metastatic Breast Cancer. Breast 2013, 22, 203–210. [Google Scholar] [CrossRef]
- Pagani, O.; Senkus, E.; Wood, W.; Colleoni, M.; Cufer, T.; Kyriakides, S.; Costa, A.; Winer, E.P.; Cardoso, F. International Guidelines for Management of Metastatic Breast Cancer: Can Metastatic Breast Cancer Be Cured? J. Natl. Cancer Inst. 2010, 102, 456–463. [Google Scholar] [CrossRef]
| Author and Year {Ref} | Patient’s Characteristics | Clinical Findings | Laboratory Findings | Treatment |
|---|---|---|---|---|
| Chahin et al., 2020 [18] | 54-year-old female Invasive lobular carcinoma, G I, ER-PR-HER2-negative | Hydronephrosis | CT scan: Hemi-pelvic mass | Ureteral stent |
| Chen et al., 2021 [19] | 60-year-old male Invasive ductal carcinoma, GIII, ER-PR-HER2-negative | Hematuria | CT scan: Ureteral mass and dilated ureter | Middle ureter dissection and anastomosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saranti, G.; Zolota, V.; Kalogeropoulou, C.; Papathanasiou, N.; Katsila, T.; Kitsou, K.; Haliassos, I.; Kardamakis, D. Diagnostic and Therapeutic Challenges in a Patient with Ureteral Metastases from a Triple Negative Breast Cancer. Curr. Oncol. 2022, 29, 4791-4798. https://doi.org/10.3390/curroncol29070380
Saranti G, Zolota V, Kalogeropoulou C, Papathanasiou N, Katsila T, Kitsou K, Haliassos I, Kardamakis D. Diagnostic and Therapeutic Challenges in a Patient with Ureteral Metastases from a Triple Negative Breast Cancer. Current Oncology. 2022; 29(7):4791-4798. https://doi.org/10.3390/curroncol29070380
Chicago/Turabian StyleSaranti, Georgia, Vasiliki Zolota, Christina Kalogeropoulou, Nikolaos Papathanasiou, Theodora Katsila, Konstantina Kitsou, Ilias Haliassos, and Dimitrios Kardamakis. 2022. "Diagnostic and Therapeutic Challenges in a Patient with Ureteral Metastases from a Triple Negative Breast Cancer" Current Oncology 29, no. 7: 4791-4798. https://doi.org/10.3390/curroncol29070380
APA StyleSaranti, G., Zolota, V., Kalogeropoulou, C., Papathanasiou, N., Katsila, T., Kitsou, K., Haliassos, I., & Kardamakis, D. (2022). Diagnostic and Therapeutic Challenges in a Patient with Ureteral Metastases from a Triple Negative Breast Cancer. Current Oncology, 29(7), 4791-4798. https://doi.org/10.3390/curroncol29070380








