Current and New Novel Combination Treatments for Metastatic Triple-Negative Breast Cancer
Abstract
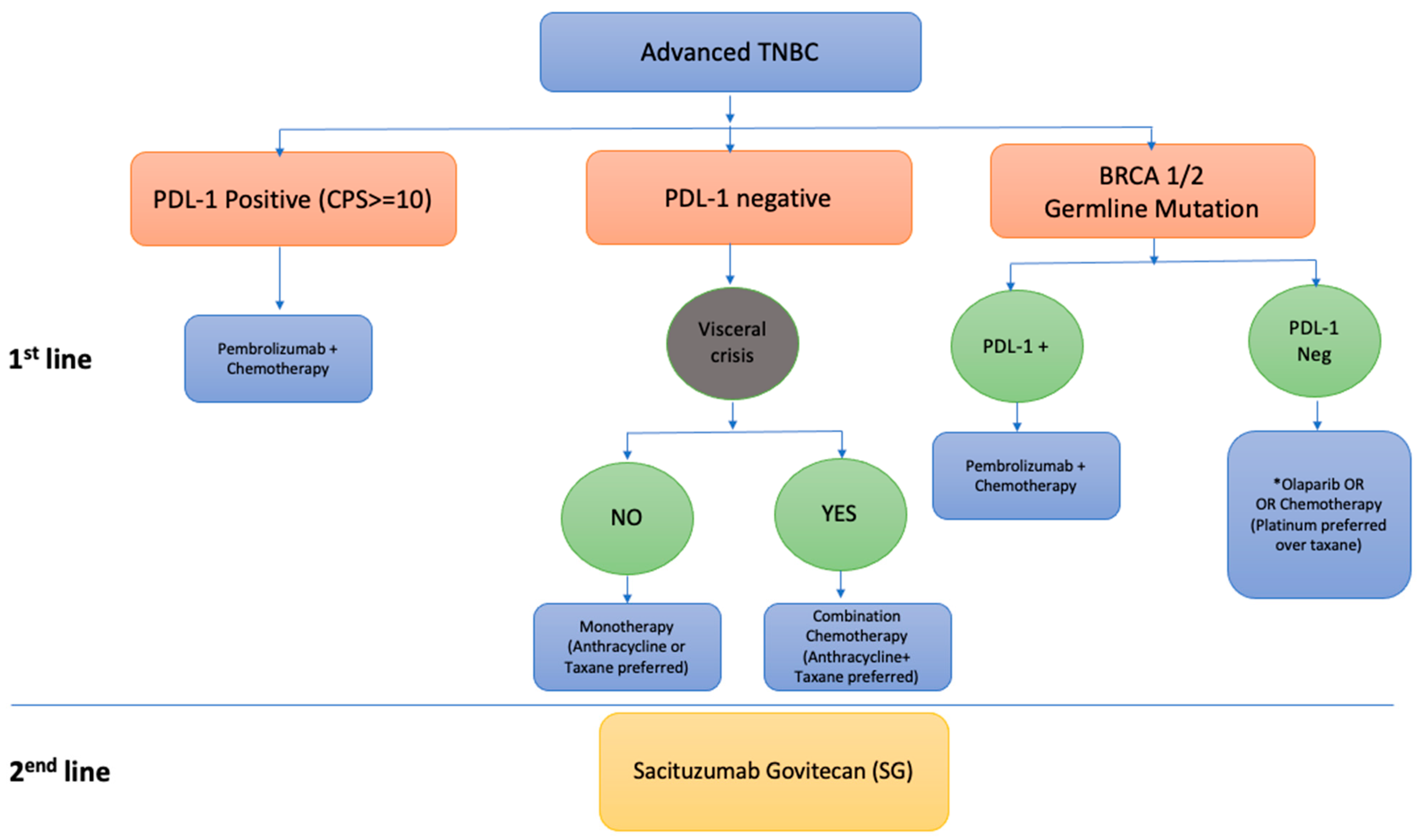
1. Background
1.1. Current Management of Metastatic Breast Cancer
1.2. Immunotherapy
1.3. PARP Inhibitors
1.4. Antibody-Drug Conjugates
2. Emerging Drugs and Novel Combinations for TNBC
2.1. Exploring Combinations with Antibody-Drug Conjugates (ADCs)
2.2. ADCs and Chemotherapy
2.3. ADCs and Immunotherapy
2.4. ADCs and PARP Inhibitors
2.5. ADCs and PI3K Inhibitors
2.6. PI3K/AKT/mTOR Targeted Agents
2.7. Androgen-Targeted Therapy
2.8. Combined Androgen and PI3K/AKT/mTOR-Pathway-Targeted Agents
2.9. Combined Androgen Therapy and Cell Cycle Inhibition
3. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gelmon, K.; LeVasseur, N. Triple negative breast cancer–Understanding the clinical implications of heterogeneity. Med. Res. Arch. 2020, 8. [Google Scholar] [CrossRef][Green Version]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-Negative Breast Cancer: Clinical Features and Patterns of Recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Bauer, J.A.; Chen, X.; Sanders, M.E.; Chakravarthy, A.B.; Shyr, Y.; Pietenpol, J.A. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Investig. 2011, 121, 2750–2767. [Google Scholar] [CrossRef] [PubMed]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Canc. Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Cortes, J.; Cescon, D.W.; Rugo, H.S.; Nowecki, Z.; Im, S.-A.; Yusof, M.M.; Gallardo, C.; Lipatov, O.; Barrios, C.H.; Holgado, E.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): A randomised, placebo-controlled, double-blind, phase 3 clinical trial. Lancet 2020, 396, 1817–1828. [Google Scholar] [CrossRef]
- Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer|NEJM. Available online: https://www.nejm.org/doi/10.1056/NEJMoa1809615?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub%20%200pubmed (accessed on 30 January 2022).
- Miles, D.; Gligorov, J.; André, F.; Cameron, D.; Schneeweiss, A.; Barrios, C.; Xu, B.; Wardley, A.; Kaen, D.; Andrade, L.; et al. Primary results from IMpassion131, a double-blind, placebo-controlled, randomised phase III trial of first-line paclitaxel with or without atezolizumab for unresectable locally advanced/metastatic triple-negative breast cancer. Ann. Oncol. 2021, 32, 994–1004. [Google Scholar] [CrossRef]
- ANNONC557_Proof 983..993|Elsevier Enhanced Reader. Available online: https://reader.elsevier.com/reader/sd/pii/S0923753421015556?token=B1A52E1403C06808285E67229C5DC0941D32A2198C4A88BF95F258222B309403A112C0169A2FE537634DE90F30B21EC8&originRegion=us-east-1&originCreation=20220603155637 (accessed on 3 June 2022).
- KEYNOTE-355 Final Analysis Reveals Survival Benefit with Pembrolizumab in Triple-Negative Breast Cancer—The ASCO Post. Available online: https://ascopost.com/issues/december-10-2021/keynote-355-final-analysis-reveals-survival-benefit-with-pembrolizumab-in-triple-negative-breast-cancer/ (accessed on 8 March 2022).
- Winer, E.P.; Lipatov, O.; Im, S.-A.; Goncalves, A.; Muñoz-Couselo, E.; Lee, K.S.; Schmid, P.; Tamura, K.; Testa, L.; Witzel, I.; et al. Pembrolizumab versus investigator-choice chemotherapy for metastatic triple-negative breast cancer (KEYNOTE-119): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 499–511. [Google Scholar] [CrossRef]
- Gonzalez-Angulo, A.M.; Timms, K.M.; Liu, S.; Chen, H.; Litton, J.K.; Potter, J.; Lanchbury, J.S.; Stemke-Hale, K.; Hennessy, B.T.; Arun, B.K.; et al. Incidence and Outcome of BRCA Mutations in Unselected Patients with Triple Receptor-Negative Breast Cancer. Clin. Cancer Res. 2011, 17, 1082–1089. [Google Scholar] [CrossRef]
- D’Andrea, A.D. Mechanisms of PARP inhibitor sensitivity and resistance. DNA Repair 2018, 71, 172–176. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.-A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef]
- Litton, J.K.; Rugo, H.S.; Ettl, J.; Hurvitz, S.A.; Gonçalves, A.; Lee, K.-H.; Fehrenbacher, L.; Yerushalmi, R.; Mina, L.A.; Martin, M.; et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018, 379, 753–763. [Google Scholar] [CrossRef] [PubMed]
- Diéras, V.; Han, H.S.; Kaufman, B.; Wildiers, H.; Friedlander, M.; Ayoub, J.-P.; Puhalla, S.L.; Bondarenko, I.; Campone, M.; Jakobsen, E.H.; et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 1269–1282. [Google Scholar] [CrossRef]
- Robson, M.E.; Tung, N.; Conte, P.; Im, S.-A.; Senkus, E.; Xu, B.; Masuda, N.; Delaloge, S.; Li, W.; Armstrong, A.; et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann. Oncol. 2019, 30, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Litton, J.K.; Hurvitz, S.A.; Mina, L.A.; Rugo, H.S.; Lee, K.-H.; Gonçalves, A.; Diab, S.; Woodward, N.; Goodwin, A.; Yerushalmi, R.; et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall survival results from the EMBRACA trial. Ann. Oncol. 2020, 31, 1526–1535. [Google Scholar] [CrossRef] [PubMed]
- Bardia, A.; Mayer, I.A.; Vahdat, L.T.; Tolaney, S.M.; Isakoff, S.J.; Diamond, J.R.; O’Shaughnessy, J.; Moroose, R.L.; Santin, A.D.; Abramson, V.G.; et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2019, 380, 741–751. [Google Scholar] [CrossRef]
- Bardia, A.; Hurvitz, S.A.; Tolaney, S.M.; Loirat, D.; Punie, K.; Oliveira, M.; Brufsky, A.; Sardesai, S.D.; Kalinsky, K.; Zelnak, A.B.; et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. N. Engl. J. Med. 2021, 384, 1529–1541. [Google Scholar] [CrossRef] [PubMed]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J., Jr.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-Human Trial of a Novel Anti-Trop-2 Antibody-SN-38 Conjugate, Sacituzumab Govitecan, for the Treatment of Diverse Metastatic Solid Tumors. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef]
- Nagayama, A.; Vidula, N.; Ellisen, L.; Bardia, A. Novel antibody–drug conjugates for triple negative breast cancer. Ther. Adv. Med. Oncol. 2020, 12, 1758835920915980. [Google Scholar] [CrossRef]
- Marmé, F. Antibody-Drug Conjugates for Breast Cancer. Oncol. Res. Treat. 2022, 45, 26–36. [Google Scholar] [CrossRef]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef]
- Doi, T.; Shitara, K.; Naito, Y.; Shimomura, A.; Fujiwara, Y.; Yonemori, K.; Shimizu, C.; Shimoi, T.; Kuboki, Y.; Matsubara, N.; et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: A phase 1 dose-escalation study. Lancet Oncol. 2017, 18, 1512–1522. [Google Scholar] [CrossRef]
- Daiichi Sankyo, Inc. A Phase 3, Multicenter, Randomized, Open-Label, Active Controlled Trial of DS-8201a, an Anti-HER2-Antibody Drug Conjugate (ADC), Versus Treatment of Physician’s Choice for HER2-Low, Unresectable and/or Metastatic Breast Cancer Subjects. Report No.: NCT03734029. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03734029 (accessed on 19 May 2022).
- AstraZeneca. A Phase 1b Multicentre, Open-Label, Modular, Dose-Finding and Dose-Expansion Study to Explore the Safety, Tolerability, Pharmacokinetics and Anti-Tumour Activity of Trastuzumab Deruxtecan (T-DXd) in Combination with Other Anti-Cancer Agents in Patients with Metastatic HER2-Low Breast Cancer (DESTINY-Breast08). Report No.: NCT04556773. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04556773 (accessed on 19 May 2022).
- QuantumLeap Healthcare Collaborative. ISPY-P1.01: Evaluating the Safety of Weekly Paclitaxel with Trastuzumab Duocarmazine (SYD985) in Patients with Metastatic Cancer: A Phase I/Ib Trial. Report No.: NCT04602117. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04602117 (accessed on 19 May 2022).
- Cortés, J.; Diab, S.; Basho, R.K.; Oliveira, M.; Pluard, T.; Alemany, C.; Brown-Glaberman, U.; Meisel, J.; Boni, V.; Sinha, R.; et al. 357TiP SGNLVA-002: Single arm, open-label, phase Ib/II study of ladiratuzumab vedotin (LV) in combination with pembrolizumab for first-line treatment of triple-negative breast cancer. Ann. Oncol. 2020, 31, S393. [Google Scholar] [CrossRef]
- Modi, S.; Pusztai, L.; Forero, A.; Mita, M.; Miller, K.; Weise, A.; Krop, I.; Burris, H., III; Kalinsky, K.; Tsai, M.; et al. Abstract PD3-14: Phase 1 study of the antibody-drug conjugate SGN-LIV1A in patients with heavily pretreated triple-negative metastatic breast cancer. Cancer Res. 2018, 78, PD3-14. [Google Scholar] [CrossRef]
- Seagen Inc. Single Arm, Open Label Phase 1b/2 Study of SGN-LIV1A in Combination with Pembrolizumab for First-Line Treatment of Patients with Unresectable Locally-Advanced or Metastatic Triple-Negative Breast Cancer. Report No.: NCT03310957. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03310957 (accessed on 19 May 2022).
- Hoffmann-La Roche. A Phase Ib/II, Open-Label, Multicenter, Randomized Umbrella Study Evaluating the Efficacy and Safety of Multiple Immunotherapy-Based Treatment Combinations in Patients with Metastatic Triple-Negative Breast Cancer (Morpheus-TNBC). Report No.: NCT03424005. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03424005 (accessed on 19 May 2022).
- MacroGenics. A Phase 1/2, First-in-Human, Open-Label, Dose-Escalation Study of MGC018 (Anti-B7-H3 Antibody Drug Conjugate) Alone and in Combination with MGA012 (Anti-PD-1 Antibody) in Patients with Advanced Solid Tumors. Report No.: NCT03729596. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03729596 (accessed on 19 May 2022).
- BioAtla, Inc. A Phase 1/2 Safety and Efficacy Dose Escalation/Dose Expansion Study of a CAB-ROR2-ADC, Alone and in Combination with a PD-1 Inhibitor, in Patients with Advanced Solid Tumors (Ph1) and Melanoma and NSCLC Patients (Ph2). Report No.: NCT03504488. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT03504488 (accessed on 19 May 2022).
- Garrido-Castro, A.C. Saci-IO TNBC: Randomized Phase II Study of Sacituzumab Govitecan with or without Pembrolizumab in PD-L1-negative Metastatic Triple Negative Breast Cancer (TNBC). Report No.: NCT04468061. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04468061 (accessed on 2 June 2022).
- Bardia, A. Phase 1b/2 Study to Evaluate Antibody-Drug Conjugate Sacituzumab Govitecan in Combination with PARP Inhibitor Talazoparib in Patients with Metastatic Breast Cancer. Report No.: NCT04039230. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT04039230 (accessed on 19 May 2022).
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.-S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 208–217. [Google Scholar] [CrossRef]
- Chang, D.-Y.; Ma, W.-L.; Lu, Y.-S. Role of Alpelisib in the Treatment of PIK3CA-Mutated Breast Cancer: Patient Selection and Clinical Perspectives. Ther. Clin. Risk Manag. 2021, 17, 193–207. [Google Scholar] [CrossRef]
- Sharma, P. Phase I Trial of Alpelisib Plus Sacituzumab Govitecan in Patients with Metastatic or Locally Recurrent HER2-Negative Breast Cancer. Report No.: NCT05143229. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT05143229 (accessed on 19 May 2022).
- Yardley, D.A.; Weaver, R.; Melisko, M.E.; Saleh, M.N.; Arena, F.P.; Forero, A.; Cigler, T.; Stopeck, A.; Citrin, D.; Oliff, I.; et al. EMERGE: A Randomized Phase II Study of the Antibody-Drug Conjugate Glembatumumab Vedotin in Advanced Glycoprotein NMB-Expressing Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Vahdat, L.T.; Schmid, P.; Forero-Torres, A.; Blackwell, K.; Telli, M.L.; Melisko, M.; Möbus, V.; Cortes, J.; Montero, A.J.; Ma, C.; et al. Glembatumumab vedotin for patients with metastatic, gpNMB overexpressing, triple-negative breast cancer (“METRIC”): A randomized multicenter study. Npj Breast Cancer 2021, 7, 57. [Google Scholar] [CrossRef]
- LoRusso, P.M. Inhibition of the PI3K/AKT/mTOR Pathway in Solid Tumors. J. Clin. Oncol. 2016, 34, 3803–3815. [Google Scholar] [CrossRef]
- Carbognin, L.; Miglietta, F.; Paris, I.; Dieci, M.V. Prognostic and Predictive Implications of PTEN in Breast Cancer: Unfulfilled Promises but Intriguing Perspectives. Cancers 2019, 11, 1401. [Google Scholar] [CrossRef]
- Zardavas, D.; te Marvelde, L.; Milne, R.L.; Fumagalli, D.; Fountzilas, G.; Kotoula, V.; Razis, E.; Papaxoinis, G.; Joensuu, H.; Moynahan, M.E.; et al. Tumor PIK3CA Genotype and Prognosis in Early-Stage Breast Cancer: A Pooled Analysis of Individual Patient Data. J. Clin. Oncol. 2018, 36, 981–990. [Google Scholar] [CrossRef]
- Kim, S.-B.; Dent, R.; Im, S.-A.; Espié, M.; Blau, S.; Tan, A.R.; Isakoff, S.J.; Oliveira, M.; Saura, C.; Wongchenko, M.J.; et al. Ipatasertib plus paclitaxel versus placebo plus paclitaxel as first-line therapy for metastatic triple-negative breast cancer (LOTUS): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2017, 18, 1360–1372. [Google Scholar] [CrossRef]
- Overall Survival (OS) Update of the Double-Blind Placebo (PBO)-Controlled Randomized Phase 2 LOTUS Trial of First-Line Ipatasertib (IPAT) + Paclitaxel (PAC) for Locally Advanced/Metastatic Triple-Negative Breast Cancer (mTNBC). Available online: https://oce.ovid.com/article/00005083-201836151-00109/HTML (accessed on 20 May 2022).
- Hoffmann-La Roche. A Double-Blind, Placebo-Controlled, Randomized Phase III Study of Ipatasertib in Combination with Paclitaxel as a Treatment for Patients with PIK3CA/AKT1/PTEN-Altered, Locally Advanced or Metastatic, Triple-Negative Breast Cancer or Hormone Receptor-Positive, HER2-Negative Breast Cancer. Report No.: NCT03337724. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03337724 (accessed on 20 May 2022).
- Turner, N.; Dent, R.A.; O’Shaughnessy, J.; Kim, S.-B.; Isakoff, S.J.; Barrios, C.; Saji, S.; Bondarenko, I.; Nowecki, Z.; Lian, Q.; et al. Ipatasertib plus paclitaxel for PIK3CA/AKT1/PTEN-altered hormone receptor-positive HER2-negative advanced breast cancer: Primary results from cohort B of the IPATunity130 randomized phase 3 trial. Breast Cancer Res. Treat. 2022, 191, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Nagayama, A.; Vidula, N.; Bardia, A. Novel Therapies for Metastatic Triple-Negative Breast Cancer: Spotlight on Immunotherapy and Antibody-Drug Conjugates. Oncology 2021, 35, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann-La Roche. A Phase III, Double-Blind, Placebo-Controlled, Randomized Study of Ipatasertib in Combination with Atezolizumab and Paclitaxel as a Treatment for Participants with Locally Advanced Unresectable or Metastatic Triple-Negative Breast Cancer. Report No.: NCT04177108. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04177108 (accessed on 19 May 2022).
- MedSIR. A Multicenter, Open-Label, Non-Comparative, Three-Arm, Phase IIa Trial of Ipatasertib (GDC-0068) in Combination with Non-Taxane Chemotherapy Agents for Taxane-Pretreated Unresectable Locally Advanced or Metastatic Triple-Negative Breast Cancer Patients. Report No.: NCT04464174. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04464174 (accessed on 19 May 2022).
- Capivasertib+Paclitaxel as First Line Treatment for Patients with Locally Advanced or Metastatic TNBC-Full Text View. Available online: https://clinicaltrials.gov/ct2/show/NCT03997123?term=NCT03997123&draw=2&rank=1 (accessed on 20 May 2022).
- Novartis Pharmaceuticals. A Phase III, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy and Safety of Alpelisib (BYL719) in Combination with Nab-Paclitaxel in Patients with Advanced Triple Negative Breast Cancer with Either Phosphoinositide-3-Kinase Catalytic Subunit Alpha (PIK3CA) Mutation or Phosphatase and Tensin Homolog Protein (PTEN) Loss without PIK3CA Mutation. Report No.: NCT04251533. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT04251533 (accessed on 19 May 2022).
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [PubMed]
- Hickey, T.E.; Robinson, J.L.L.; Carroll, J.S.; Tilley, W.D. Minireview: The androgen receptor in breast tissues: Growth inhibitor, tumor suppressor, oncogene? Mol. Endocrinol. 2012, 26, 1252–1267. [Google Scholar] [CrossRef]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- A Phase II Trial of Abiraterone Acetate Plus Prednisone in Patients with Triple-Negative Androgen Receptor Positive Locally Advanced or Metastatic Breast Cancer (UCBG 12-1)-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/27052658/ (accessed on 20 May 2022).
- Lehmann, B.D.; Bauer, J.A.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gómez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. BCR 2014, 16, 406. [Google Scholar] [CrossRef]
- The University of Texas MD Anderson Cancer Center. Phase Ib Study of BYL719 (Alpelisib) in Combination with Androgen Receptor Inhibitor (Enzalutamide) in Patients with Androgen Receptor (AR)-Positive and PTEN Positive Metastatic Breast Cancer. Report No.: NCT03207529. 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT03207529 (accessed on 19 May 2022).
- Lehmann, B.D.; Abramson, V.G.; Sanders, M.E.; Mayer, E.L.; Haddad, T.C.; Nanda, R.; Van Poznak, C.; Storniolo, A.M.; Nangia, J.R.; Gonzalez-Ericsson, P.I.; et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2020, 26, 2111–2123. [Google Scholar] [CrossRef]
- Dent, S.; Cortés, J.; Im, Y.-H.; Diéras, V.; Harbeck, N.; Krop, I.E.; Wilson, T.R.; Cui, N.; Schimmoller, F.; Hsu, J.Y.; et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021, 32, 197–207. [Google Scholar] [CrossRef]
- Memorial Sloan Kettering Cancer Center. Phase I/II Trial of Palbociclib in Combination with Bicalutamide for the Treatment of AR(+) Metastatic Breast Cancer (MBC). Report No.: NCT02605486. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT02605486 (accessed on 19 May 2022).
- Wisinski, K. A Phase I/II, Single Arm, Non-randomized Study of Ribociclib (LEE011), a CDK 4/6 Inhibitor, in Combination with Bicalutamide, an Androgen Receptor (AR) Inhibitor, in Advanced AR+ Triple-Negative Breast Cancer: Big Ten Cancer Research Consortium BRE15-024. Report No.: NCT03090165. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03090165 (accessed on 19 May 2022).
- Providence Health & Services. A Phase II Study of Nivolumab Combined with Bicalutamide and Ipilimumab in Metastatic HER2-Negative Breast Cancer. Report No.: NCT03650894. 2022. Available online: https://clinicaltrials.gov/ct2/show/NCT03650894 (accessed on 19 May 2022).
- City of Hope Medical Center. A Phase 2 Clinical Trial of the Combination of Pembrolizumab and Selective Androgen Receptor Modulator (SARM) GTX-024 in Patients with Metastatic Androgen Receptor (AR) Positive Triple Negative Breast Cancer (TNBC) Report No.: NCT02971761. 2021. Available online: https://clinicaltrials.gov/ct2/show/NCT02971761 (accessed on 19 May 2022).
| Trial Identifier | Therapeutic Agent | Class of Agent | Line of Therapy | Phase | Intervention | Key Efficacy Results | Primary Toxicity |
|---|---|---|---|---|---|---|---|
| Keynote-119 (NCT02555657) | Pembrolizumab | PD-1 inhibitor | ≥2nd-line metastatic treatment | III | Pembrolizumab vs. single-agent chemotherapy of physicians’ choice in mTNBC ≥2nd-line metastatic treatment | PDL-1 CPS ≥10 -mOS 12.7 m vs. 11.6 months (HR 0.78, 95% CI 0.57–1.06) PDL-1 CPS ≥ 1 -mOS 10.7 months vs. 10.2 months (HR 0.86, 95% CI 0.69–1.06) Overall Population -mOS 9.9 vs. 10.8 (HR 0.97, 95% CI 0.82–1.15) | Fatigue, gastrointestinal toxicity, myelosuppression, alopecia, hypothyroidism, hyperthyroidism, pneumonitis, skin reactions, adrenal insufficiency |
| Keynote-355 (NCT02819518) | Pembrolizumab | PD-1 inhibitor | 1st-line treatment | III | Pembrolizumab + chemotherapy (paclitaxel (P) or nab paclitaxel (NP) or carbo/gemcitabine (CG) vs. placebo + chemotherapy (P or NP or CG) in mTNBC as 1st-line treatment | -mPFS 9.7 months vs. 5.6 months (HR 0.65, 95% CI 0.49–0.86) -mOS 23 months vs. 16 months in PDL- positive disease with CPS ≥ 10 (HR 0.73, 95% CI 0.55–0.95) | Fatigue, gastrointestinal toxicity, myelosuppression alopecia, hypothyroidism, hyperthyroidism, pneumonitis, skin reactions, adrenal insufficiency |
| Impassion-130 (NCT02425891) | Atezolizumab | PD-L1 inhibitor | 1st-line treatment | III | Atezolizumab/nab-paclitaxel vs. nab-paclitaxel in mTNBC as 1st-line treatment | ITT -mPFS 7.2 months vs. 5.5 (HR 0.80, 95% CI 0.69–0.92) -mOS 21.3 months vs. 17.6 months (HR 0.84, 95% CI 0.69–1.02) PDL-1 > 1% -mPFS 7.5 months vs. 5.0 months (HR 0.62, 95% CI 0.49–0.78) -mOS 25 months vs. 15.5 months (HR 0.62, 95% CI 0.45–0.86) | Alopecia, nausea, cough, peripheral neuropathy, neutropenia, pyrexia, hypothyroidism |
| OlympiAD (NCT02000622) | Olaparib | PARP inhibitor | 1st-3rd-line treatment (No more than 2 prior lines of treatment) | III | Olaparib vs. physician’s choice of chemotherapy (capecitabinr, vinorelbine or eribulin) in metastatic germline BRCA 1/2 mutated breast cancer that are HER-2-negative | -mPFS 7 vs. 4.2 months (HR 0.58, 95% CI 0.43–0.80) -mOS 19.3 vs. 17.1 months (HR 0.91, 95% CI 0.66–1.23) | Anaemia, thrombocytopenia, gastrointestinal toxicity |
| BROCADE 3 (NCT02163694) | Veliparib in combination with carboplatin and paclitaxel | PARP inhibitor | 1st-3rd-line treatment (no more than 2 prior lines of treatment) | III | Veliparib in combination with a platinum doublet vs. placebo in combination with platinum in metastatic germline BRCA 1/2 mutated breast cancer that are HER-2-negative | -mPFS 14.5 months vs. 12.6 months (HR 0.71, 95% CI 0.57–0.88) | Myelosuppression |
| EMBRACA (NCT01945775) | Talazoparib | PARP inhibitor | 1st-4th-line treatment (no more than 3 prior lines of treatment) | III | Talazoparib vs. physician choice of chemotherapy (gemcitabine, capecitabine, eribulin or vinorelbine) in metastatic germline BRCA 1/2 mutated breast cancer that are HER-2-negative | -mPFS 8.6 months vs. 5.6 months (HR 0.54, 95% CI 0.41–0.71) -mOS 19.3 months vs. 19.5 months (HR 0.84, 95% CI 0.67–1.07) | Anaemia, thrombocytopenia, gastrointestinal toxicity |
| Ascent (NCT02574455) | Sacituzumab govitecan | Antibody-drug conjugate targeting trop-2 | Post two or more lines of treatment | III | Sacituzumab govitecan vs. choice of chemotherapy (capecitabinr, vinorelbine or eribulin or gemcitabine) in metastatic TNBC | -mPFS 5.6 months vs. 1.7 months (HR 0.41, 95% CI 0.32–0.52) -mOS 12.1 months vs. 6.7 months (HR 0.48, 95% CI 0.38–0.59) | Myelosuppression, gastrointestinal toxicity, fatigue, electrolyte abnormalities, skin changes, infection |
| Trial Identifier (Clinical Trial.gov) | Class of Agent | Intervention | Phase | Patient Population | Primary (1′) and Key Secondary (2′) Endpoints | Status |
|---|---|---|---|---|---|---|
| PI3K/AKT/mTOR targeted drug combinations: | ||||||
| NCT03337724 | AKT inhibitor + chemotherapy | Ipatasertib in combination with paclitaxel vs. paclitaxel | III | Patients with PIK3CA/AKT1/PTEN-altered, locally advanced or metastatic, triple-negative breast cancer or hormone receptor-positive, HER2-negative breast cancer | 1′: PFS 2′: ORR, DOR, CBR, OS | Active, not recruiting, Start date: 6 January 2018 Estimate date of completion: 22 December 2022 |
| NCT04177108 | AKT inhibitor + anti-PDL1 | Ipatasertib in combination with atezolizumab and paclitaxel | III | Locally advanced or metastatic triple-negative breast cancer | 1′: PFS, OS 2′: AEs, ORR, DOR, CBR | Active, not recruiting, Start date: 25 November 2019 Estimate date of completion: 10 October 2025 |
| NCT04464174 (PATHFINDER) | AKT inhibitor + chemotherapy | Ipatasertib plus chemotherapy (capecitabine or eribulin or carboplatin plus gemcitabine) | III | Taxane-pretreated, unresectable, locally advanced or metastatic triple-negative breast cancer patients | 1′: Safety and tolerability 2′: PFS, TTR, ORR, DOR, CBR, OS | Recruiting Start date: 8 October 2020 Estimated end date: 31 March 2022 |
| NCT03997123 (CAPItello-290) | AKT inhibitor + chemotherapy | Capivasertib + paclitaxel | III | First-line treatment for patients with locally advanced (inoperable) or metastatic TNBC | 1′: OS 2′: PFS, ORR, AEs, DOR, CBR | Recruiting Estimated start date: 25 June 2019 Estimated end date: 24 March 2023 |
| NCT04251533 | PI3K inhibitor + chemotherapy | Alpelisib in combination with nab-paclitaxel vs. nab-paclitaxel | III | Patients with advanced TNBC with either PIK3CA or PTEN loss without PIK3CA mutation | 1′: PFS, ORR 2′: OS, CBR, TTR, DOR | Recruiting Estimated start date: 8 June 2020 Estimated end date: 9 January 2026 |
| Novel antibody-drug conjugate (ADC) combinations: | ||||||
| NCT03310957 (SGNLVA-002) | ADC + PD-1 inhibitor | SGN-LIV1A (iadiratuzumab vedotin) plus pembrolizumab | IB/II | First-line treatment of patients with unresectable locally advanced or metastatic triple-negative breast cancer | 1′: ORR, AEs, lab abnormalities 2′: DOR, PFS, OS | Recruiting Estimated start date: 27 February 2018 Estimated End date: 30 April 2023 |
| NCT03424005 (Morpheus-TNBC) | ADC + PD-1 inhibitor | Multiple immunotherapy-based treatment combinations including ADC combinations (atezolizumab + sacituzumab govitecan and atezolizumab + SGN-LIV1A) | I/IIB | Metastatic or inoperable locally advanced TNBC | 1′: ORR, AEs 2′: PFS, DCR, OS, DOR | Recruiting Estimated start date: 2 April 2018 Estimated End date: 30 March 2023 |
| NCT05143229 (ASSET) | PI3K inhibitor + ADC | Alpelisib plus sacituzumab govitecan | I | Metastatic or locally recurrent HER2-negative breast cancer including TNBC | 1′: RP2D 2′: ORR, pharmacokinetics | Recruiting Estimated start date: 28 March 2022 Estimated end date: June 2024 |
| NCT04602117 (ISPY-P1.01) | ADC + chemotherapy | Vic-trastuzumab duocarmazine (SYD985) + weekly paclitaxel | I | Evaluating the safety of ADC+ chemotherapy in patients with metastatic cancer including TNBC | 1′: AEs, CBR, ORR 2′: PFS, DOR | Recruiting Estimated start date: 28 July 2021 Estimated end date: 1 December 2022 |
| NCT04556773 (DESTINY-Breast08) | ADC + other anti-cancer agents (chemotherapy, immunotherapy + chemotherapy, AKT inhibitor, aromatase inhibitor, or oestrogen receptor antagonist) | Trastuzumab deruxtecan (T-DXd) in combination with other anti-cancer agents (capecitabine, durvalumab and paclitaxel, capivasertib, anastrozole or fulvestrant) | IB | Metastatic HER2-low breast cancer (including TNBC) | 1′: AEs, SAEs 2′: ORR, PFS, DOR, OS | Recruiting Estimated start date: 17 December 2020 Estimated end date: 28 August 2023 |
| NCT04039230 | ADC + PARP inhibitor | sacituzumab govitecan plus talazoparib | IB/II | Metastatic TNBC | 1′: Dose-limiting toxicity 2′: DOR, TTR, PFS, OS | Recruiting Estimated start date: 9 October 2019 Estimated end date: 31 October 2024 |
| NCT03729596 | Anti-B7-H3 antibody-drug conjugate alone and + Anti-PD-1 antibody | MGC018 alone and in combination with retifanlimab | I/II | Advanced solid tumours including TNBC | 1′: AEs, MTD 2′: Preliminary anti-tumour activity, patient outcome, radiographic PFS | Recruiting Estimated start date: 21 November 2018 Estimated end date: May 2023 |
| NCT03504488 | ROR2-targeted ADC alone and + PD-1 inhibitor | CAB-ROR2-ADC alone and plus PD-1 inhibitor | I/II | Locally advanced unresectable or metastatic solid tumour including TNBC | 1′: ORR, pharmacokinetics 2′: DOR, OR, DCR, TTR, PFS, OS | Recruiting Estimated start date: 27 June 2018 Estimated end date: 30 June 2023 |
| NCT04468061 | ADC + PD-1 inhibitor | Sacituzumab govitecan with or without pembrolizumab | II | PD-L1-negative metastatic triple negative breast cancer | 1′: PFS 2′: ORR, CBR, DOR, TTP, TTOR, OS | Recruiting Estimated start date: 20 July 2020 Estimated end date: 1 April 2027 |
| Novel androgen receptor (AR) inhibitor combinations: | ||||||
| NCT03207529 | PI3K inhibitor + AR inhibitor | Alpelisib plus enzalutamide | IB | Patients with androgen receptor (AR)- positive and PTEN-positive metastatic breast cancer (including TNBC) | 1′: MTD 2′: AEs, PFS, CBR | Recruiting Estimated start date: 7 June 2019 Estimated end date: 31 December 2020 |
| NCT03090165 (Big Ten Cancer Research Consortium BRE15–024) | CDK 4/6 inhibitor + AR inhibitor | Ribociclib plus bicalutamide | I/II | Metastatic or unresectable AR+ triple-negative breast cancer (TNBC)-AR-positive defined as IHC staining of >0% | 1′: phase I Max tolerated dose, CBR 2′: ORR, DOR, AEs, PFS, OS | Active, not recruiting Estimated start date: 2 March 2017 Estimated end date: September 2024 |
| NCT02605486 | CDK 4/6 inhibitor + AR inhibitor | Palbociclib plus bicalutamide | I/II | AR(+) metastatic breast cancer (MBC) including TNBC in phase I part | 1′: RP2D, PFS 2′: ORR, CBR, PFS after 1 year, AEs | Active, not recruiting Estimated start date: 11 November 2015 Estimated end date: November 2023 |
| NCT03650894 | Immunotherapy + AR inhibitor | Nivolumab combined with ipilimumab plus bicalutamide | II | Metastatic HER2-negative breast cancer—TNBCs were allowed in the study as long they had confirmation of androgen receptor (AR) positivity at screening | 1′: CBR 2′: ORR, PFS, OS | Recruiting Estimated start date: 3 April 2019 Estimated end date: April 2025 |
| NCT02971761 | Immunotherapy + selective androgen receptor modulator (SARM) | Pembrolizumab plus enobosarm | II | Patients with metastatic androgen receptor (AR)-positive triple-negative breast cancer (TNBC) | 1′: AEs, RR, DLT 2′: CBR, EFS, TTF, PFS, OS | Active, not recruiting Estimated start date: 1 June 2017 Estimated end date: 3 November 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauls, M.; Chia, S.; LeVasseur, N. Current and New Novel Combination Treatments for Metastatic Triple-Negative Breast Cancer. Curr. Oncol. 2022, 29, 4748-4767. https://doi.org/10.3390/curroncol29070377
Pauls M, Chia S, LeVasseur N. Current and New Novel Combination Treatments for Metastatic Triple-Negative Breast Cancer. Current Oncology. 2022; 29(7):4748-4767. https://doi.org/10.3390/curroncol29070377
Chicago/Turabian StylePauls, Mehrnoosh, Stephen Chia, and Nathalie LeVasseur. 2022. "Current and New Novel Combination Treatments for Metastatic Triple-Negative Breast Cancer" Current Oncology 29, no. 7: 4748-4767. https://doi.org/10.3390/curroncol29070377
APA StylePauls, M., Chia, S., & LeVasseur, N. (2022). Current and New Novel Combination Treatments for Metastatic Triple-Negative Breast Cancer. Current Oncology, 29(7), 4748-4767. https://doi.org/10.3390/curroncol29070377






