Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation
Abstract
:1. Introduction
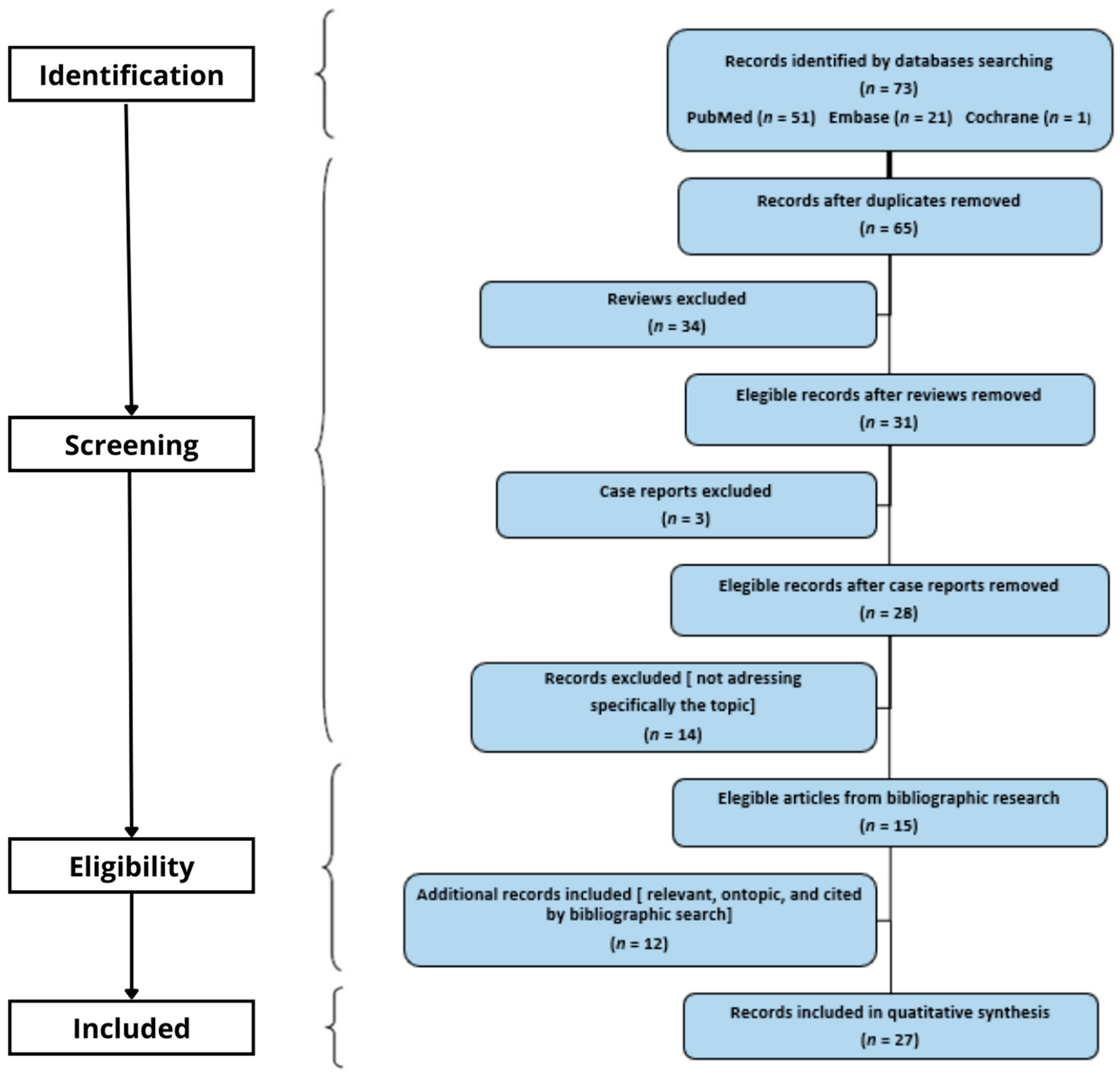
2. Materials and Methods
3. Results
3.1. Tumor Microenvironment
3.2. Tumor Mutational Burden and Neoantigens Expression
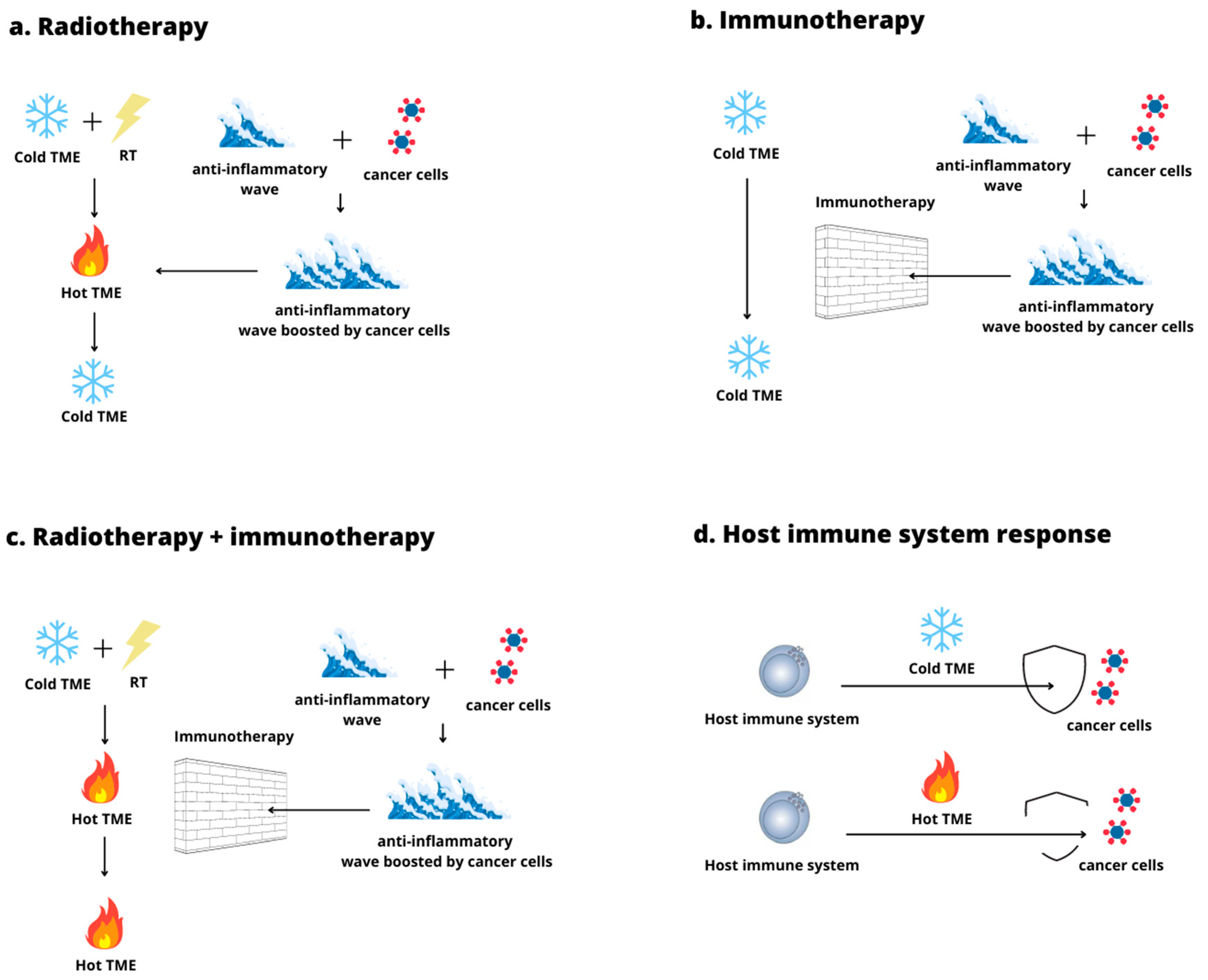
3.3. Hot and Cold Tumor Microenvironment
3.4. Immunosuppression in Cold Tumor Microenvironment
3.5. The Use of Radiation Therapy to Convert a Cold Tumor Microenvironment into a Hot One
3.6. Cold Tumor Microenvironment Radiomodulation: Preclinical Research
3.7. Cold Tumor Microenvironment Radiomodulation: Clinical Research
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shen, C.; Frakes, J.M.; Weiss, J.; Caudell, J.; Hackman, T.; Akulian, J.; El-Haddad, G.E.; Dixon, R.; Hu, Y.; Pearson, A.; et al. NBTXR3 Radiation Enhancing Hafnium Oxide Nanoparticles Activated by Radiotherapy in Combination With Anti-PD-1 Therapy: A Phase I Study. Int. J. Radiat. Oncol. Biol. Phys. 2020, 108, e851. [Google Scholar] [CrossRef]
- Mortezaee, K.; Najafi, M. Immune system in cancer radiotherapy: Resistance mechanisms and therapy perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103180. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Tosolini, M.; Kirilovsky, A.; Waldner, M.; Obenauf, A.C.; Angell, H.; Fredriksen, T.; Lafontaine, L.; Berger, A.; et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity 2013, 39, 782–795. [Google Scholar] [CrossRef] [Green Version]
- Hader, M.; Frey, B.; Fietkau, R.; Hecht, M.; Gaipl, U.S. Immune biological rationales for the design of combined radio- and immunotherapies. Cancer Immunol. Immunother. 2020, 69, 293–306. [Google Scholar] [CrossRef] [Green Version]
- McLaughlin, M.; Patin, E.C.; Pedersen, M.; Wilkins, A.; Dillon, M.T.; Melcher, A.A.; Harrington, K.J. Inflammatory microenvironment remodelling by tumour cells after radiotherapy. Nat. Rev. Cancer 2020, 20, 203–217. [Google Scholar] [CrossRef]
- Ji, D.; Li, Y.; Xia, J.; Wu, Y.; Jia, J.; Cui, X.; Yu, S.; Gu, J. Combination of radiotherapy and suppression of Tregs enhances abscopal antitumor effect and inhibits metastasis in rectal cancer. J. Immunother. Cancer 2020, 8, e000826. [Google Scholar] [CrossRef]
- Ruscetti, M.; Morris, J.P., 4th; Mezzadra, R.; Russell, J.; Leibold, J.; Romesser, P.B.; Simon, J.; Kulick, A.; Ho, Y.J.; Fennell, M.; et al. Senescence-Induced Vascular Remodeling Creates Therapeutic Vulnerabilities in Pancreas Cancer. Cell 2020, 181, 424–441.e421, Erratum in: Cell 2021, 184, 4838–4839. [Google Scholar] [CrossRef]
- Massaccesi, M.; Boldrini, L.; Romano, A.; Rossi, E.; Schinzari, G.; Lepre, E.; Gambacorta, M.A.; Valentini, V. Unconventional radiotherapy to enhance immunotherapy efficacy in bulky tumors: A case report. Immunotherapy 2021, 13, 1457–1463. [Google Scholar] [CrossRef]
- Liu, Y.; Dong, Y.; Kong, L.; Shi, F.; Zhu, H.; Yu, J. Abscopal effect of radiotherapy combined with immune checkpoint inhibitors. J. Hematol. Oncol. 2018, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef]
- Hinshaw, D.C.; Shevde, L.A. The Tumor Microenvironment Innately Modulates Cancer Progression. Cancer Res. 2019, 79, 4557–4566. [Google Scholar] [CrossRef] [Green Version]
- Kather, J.N.; Suarez-Carmona, M.; Charoentong, P.; Weis, C.A.; Hirsch, D.; Bankhead, P.; Horning, M.; Ferber, D.; Kel, I.; Herpel, E.; et al. Topography of cancer-associated immune cells in human solid tumors. eLife 2018, 7, e36967. [Google Scholar] [CrossRef]
- Thomas, A.; Routh, E.D.; Pullikuth, A.; Jin, G.; Su, J.; Chou, J.W.; Hoadley, K.A.; Print, C.; Knowlton, N.; Black, M.A.; et al. Tumor mutational burden is a determinant of immune-mediated survival in breast cancer. Oncoimmunology 2018, 7, e1490854. [Google Scholar] [CrossRef]
- Lee, J.C.; Mehdizadeh, S.; Smith, J.; Young, A.; Mufazalov, I.A.; Mowery, C.T.; Daud, A.; Bluestone, J.A. Regulatory T cell control of systemic immunity and IT response in liver metastasis. Sci Immunol. 2020, 5, eaba0759. [Google Scholar] [CrossRef]
- Snyder, A.; Makarov, V.; Merghoub, T.; Yuan, J.; Zaretsky, J.M.; Desrichard, A.; Walsh, L.A.; Postow, M.A.; Wong, P.; Ho, T.S.; et al. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N. Engl. J. Med. 2014, 371, 2189–2199, Erratum in: N. Engl. J. Med. 2018, 379, 2185. [Google Scholar] [CrossRef] [Green Version]
- Jarosz-Biej, M.; Smolarczyk, R.; Cichoń, T.; Kułach, N. Tumor Microenvironment as A “Game Changer” in Cancer Radiotherapy. Int. J. Mol. Sci. 2019, 20, 3212. [Google Scholar] [CrossRef] [Green Version]
- Halaby, M.J.; Hezaveh, K.; Lamorte, S.; Ciudad, M.T.; Kloetgen, A.; MacLeod, B.L.; Guo, M.; Chakravarthy, A.; Medina, T.D.S.; Ugel, S.; et al. GCN2 drives macrophage and MDSC function and immunosuppression in the tumor microenvironment. Sci. Immunol. 2019, 4, eaax8189. [Google Scholar] [CrossRef]
- Newman, J.H.; Chesson, C.B.; Herzog, N.L.; Bommareddy, P.K.; Aspromonte, S.M.; Pepe, R.; Estupinian, R.; Aboelatta, M.M.; Buddhadev, S.; Tarabichi, S.; et al. Intratumoral injection of the seasonal flu shot converts immunologically cold tumors to hot and serves as an IT for cancer. Proc. Natl. Acad. Sci. USA 2020, 117, 1119–1128. [Google Scholar] [CrossRef] [Green Version]
- Marabelle, A.; Kohrt, H.; Caux, C.; Levy, R. Intratumoral immunization: A new paradigm for cancer therapy. Clin. Cancer Res. 2014, 20, 1747–1756. [Google Scholar] [CrossRef] [Green Version]
- Urs, S.; Draper, D.; Franklin, M.R. Targeted radiation in combination with IT agents in murine breast cancer models. Cancer Res. 2020, 80 (Suppl. 16), 6650. [Google Scholar] [CrossRef]
- Ren, J.; Xu, M.; Chen, J.; Ding, J.; Wang, P.; Huo, L.; Li, F.; Liu, Z. PET imaging facilitates antibody screening for synergistic radioIT with a (177)Lu-labeled αPD-L1 antibody. Theranostics 2021, 11, 304–315. [Google Scholar] [CrossRef]
- Vanpouille-Box, C.; Alard, A.; Aryankalayil, M.J.; Sarfraz, Y.; Diamond, J.M.; Schneider, R.J.; Inghirami, G.; Coleman, C.N.; Formenti, S.C.; Demaria, S. DNA exonuclease Trex1 regulates radiotherapy-induced tumour immunogenicity. Nat. Commun. 2017, 8, 15618. [Google Scholar] [CrossRef]
- Ochoa de Olza, M.; Navarro Rodrigo, B.; Zimmermann, S.; Coukos, G. Turning up the heat on non-immunoreactive tumours: Opportunities for clinical development. Lancet Oncol. 2020, 21, e419–e430. [Google Scholar] [CrossRef]
- Gerard, C.L.; Delyon, J.; Wicky, A.; Homicsko, K.; Cuendet, M.A.; Michielin, O. Turning tumors from cold to inflamed to improve immunotherapy response. Cancer Treat. Rev. 2021, 101, 102227. [Google Scholar] [CrossRef]
- Jing, W.; McAllister, D.; Vonderhaar, E.P.; Palen, K.; Riese, M.J.; Gershan, J.; Johnson, B.D.; Dwinell, M.B. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J. Immunother. Cancer. 2019, 7, 115. [Google Scholar] [CrossRef]
- Morris, Z.S.; Guy, E.I.; Francis, D.M.; Gressett, M.M.; Werner, L.R.; Carmichael, L.L.; Yang, R.K.; Armstrong, E.A.; Huang, S.; Navid, F.; et al. In Situ Tumor Vaccination by Combining Local Radiation and Tumor-Specific Antibody or Immunocytokine Treatments. Cancer Res. 2016, 76, 3929–3941. [Google Scholar] [CrossRef] [Green Version]
- Voeller, J.; Erbe, A.K.; Slowinski, J.; Rasmussen, K.; Carlson, P.M.; Hoefges, A.; VandenHeuvel, S.; Stuckwisch, A.; Wang, X.; Gillies, S.D.; et al. Combined innate and adaptive IT overcomes resistance of immunologically cold syngeneic murine neuroblastoma to checkpoint inhibition. J. Immunother. Cancer 2019, 7, 344. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Palese, P.; Goff, P.H. Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma. EBioMedicine 2019, 49, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Bates, A.M.; O’Leary, K.A.; Emma, S.; Nystuen, E.; Sumiec, E.G.; Schuler, L.A.; Morris, Z.S. Enhancing immunogenicity in immunologically cold ER+ breast cancer using estrogen receptor blockade and radiation therapy. Cancer Res. 2020, 80 (Suppl. 16), 2255. [Google Scholar] [CrossRef]
- Knitz, M.W.; Bickett, T.E.; Darragh, L.B.; Oweida, A.J.; Bhatia, S.; Van Court, B.; Bhuvane, S.; Piper, M.; Gadwa, J.; Mueller, A.C.; et al. Targeting resistance to radiation-IT in cold HNSCCs by modulating the Treg-dendritic cell axis. J. Immunother. Cancer 2021, 9, e001955. [Google Scholar] [CrossRef]
- Chang, W.I.; Han, M.G.; Kang, M.H.; Park, J.M.; Kim, E.E.; Bae, J.; Ahn, S.; Kim, I.A. PI3Kαδ Inhibitor Combined with Radiation Enhances the Antitumor Immune Effect of Anti-PD1 in a Syngeneic Murine Triple-Negative Breast Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 845–858. [Google Scholar] [CrossRef]
- Wilkins, A.; Fontana, E.; Nyamundanda, G.; Ragulan, C.; Patil, Y.; Mansfield, D.; Kingston, J.; Errington-Mais, F.; Bottomley, D.; von Loga, K.; et al. Differential and longitudinal immune gene patterns associated with reprogrammed microenvironment and viral mimicry in response to neoadjuvant radiotherapy in rectal cancer. J. Immunother. Cancer 2021, 9, e001717. [Google Scholar] [CrossRef]
- Keam, P.; Halse, H.; Nguyen, T.; Wang, M.; Van Kooten Losio, N.; Mitchell, C.; Caramia, F.; Byrne, D.J.; Haupt, S.; Ryland, G.; et al. High dose-rate brachytherapy of localized prostate cancer converts tumors from cold to hot. J. Immunother. Cancer 2020, 8, e000792. [Google Scholar] [CrossRef]
- Huang, S.; Zhou, N.; Zhao, L.; Gimple, R.C.; Ahn, Y.H.; Zhang, P.; Wang, W.; Shao, B.; Yang, J.; Zhang, Q.; et al. Pharmacological Activation of Estrogen Receptor Beta Overcomes Tumor Resistance to Immune Checkpoint Blockade Therapy. iScience 2020, 23, 101458. [Google Scholar] [CrossRef]
- Bardoscia, L.; Pasinetti, N.; Triggiani, L.; Cozzi, S.; Sardaro, A. Biological Bases of Immune-Related Adverse Events and Potential Crosslinks with Immunogenic Effects of Radiation. Front. Pharmacol. 2021, 12, 746853. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iori, F.; Bruni, A.; Cozzi, S.; Ciammella, P.; Di Pressa, F.; Boldrini, L.; Greco, C.; Nardone, V.; Salvestrini, V.; Desideri, I.; et al. Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation. Curr. Oncol. 2022, 29, 4612-4624. https://doi.org/10.3390/curroncol29070366
Iori F, Bruni A, Cozzi S, Ciammella P, Di Pressa F, Boldrini L, Greco C, Nardone V, Salvestrini V, Desideri I, et al. Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation. Current Oncology. 2022; 29(7):4612-4624. https://doi.org/10.3390/curroncol29070366
Chicago/Turabian StyleIori, Federico, Alessio Bruni, Salvatore Cozzi, Patrizia Ciammella, Francesca Di Pressa, Luca Boldrini, Carlo Greco, Valerio Nardone, Viola Salvestrini, Isacco Desideri, and et al. 2022. "Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation" Current Oncology 29, no. 7: 4612-4624. https://doi.org/10.3390/curroncol29070366
APA StyleIori, F., Bruni, A., Cozzi, S., Ciammella, P., Di Pressa, F., Boldrini, L., Greco, C., Nardone, V., Salvestrini, V., Desideri, I., De Felice, F., & Iotti, C. (2022). Can Radiotherapy Empower the Host Immune System to Counterattack Neoplastic Cells? A Systematic Review on Tumor Microenvironment Radiomodulation. Current Oncology, 29(7), 4612-4624. https://doi.org/10.3390/curroncol29070366





