A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers
Abstract
:1. Introduction
2. Consensus Process
3. Genetic Testing to Inform PARP Inhibitor Maintenance Strategies
3.1. Consensus Statements
- (a)
- All patients with high-grade EOC should have BRCA1/2 mutation testing to:
- i.
- Inform hereditary cancer predisposition and the need for cascade testing of family members;
- ii.
- Guide first-line PARP inhibitor maintenance in advanced stage cases.
- (b)
- Tumour HRD status is a predictive biomarker of treatment benefit from PARP inhibitors, and testing should be publicly funded.
- (c)
- Assessment of mutations in HRR genes other than BRCA1/2 should not be used as a substitute for HRD testing.
3.2. Summary of Evidence
3.3. Interpretation and Canadian Perspective
4. Selection of PARP Inhibitors as First-Line Maintenance Therapy in Advanced EOC
4.1. Consensus Statements
- (d)
- All BRCA1/2-mutated patients with advanced EOC should receive maintenance therapy with a PARP inhibitor following a response to platinum-based chemotherapy. The choice of PARP inhibitor is influenced by several factors, including the expected toxicity profile of each agent.
- (e)
- Patients with advanced EOC who are BRCA1/2 wild-type and have responded to platinum-based chemotherapy should be considered for maintenance treatment with niraparib.
- (f)
- There is evidence to support the combination of olaparib with bevacizumab as a maintenance regimen in patients with advanced, high-grade, HRD-positive EOC who respond to first-line treatment with platinum chemotherapy and bevacizumab.
4.2. Summary of Evidence
4.3. Interpretation and Canadian Perspective
5. Dosing and Duration of PARP Inhibitor Maintenance Therapy
5.1. Consensus Statements
- (g)
- Olaparib should be given orally at a starting dose of 300 mg, twice-daily for up to two years in patients with a response to first-line platinum-based chemotherapy. Treatment beyond 2 years should only be considered in patients who have evidence of disease at the 2-year time point for whom ongoing treatment is felt to be beneficial.
- (h)
- Niraparib should be given orally at a starting dose of 200 mg, once-daily for patients weighing less than 77 kg or with a platelet count of less than 150,000/µL, or at a starting dose of 300 mg, once-daily for patients weighing greater than or equal to 77 kg and with a platelet count of greater than or equal to 150,000/µL. Treatment beyond 3 years should only be considered in patients who have evidence of disease at the 3-year time point for whom ongoing treatment is felt to be beneficial.
- (i)
- Patients should be informed of the expected treatment duration and data to support completion of treatment at the time of maintenance therapy initiation.
- (j)
- Routine clinical assessments and laboratory monitoring are required, for the duration of therapy, taking into consideration the common adverse events.
- (k)
- Toxicities can be managed through dose interruptions and reductions as described in the product monograph for each PARP inhibitor, followed by a rechallenge upon resolution of toxicity.
- (l)
- Switching between approved PARP inhibitors in the first-line maintenance setting for unmanageable toxicity is considered a reasonable option to allow for the continuation of PARP inhibitor therapy.
5.2. Summary of Evidence
5.3. Interpretation and Canadian Perspective
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Brenner, D.R.; Weir, H.K.; Demers, A.A.; Ellison, L.F.; Louzado, C.; Shaw, A.; Turner, D.; Woods, R.R.; Smith, L.M.; Canadian Cancer Statistics Advisory Committee. Projected estimates of cancer in Canada in 2020. Can. Med. Assoc. J. 2020, 192, E199–E205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA A Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Cancer Society. Survival Rates for Ovarian Cancer. Available online: https://www.cancer.org/cancer/ovarian-cancer/detection-diagnosis-staging/survival-rates.html (accessed on 4 February 2022).
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef] [Green Version]
- Cass, I.; Baldwin, R.L.; Varkey, T.; Moslehi, R.; Narod, S.A.; Karlan, B.Y. Improved survival in women with BRCA-associated ovarian carcinoma. Cancer 2003, 97, 2187–2195. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.P.; Rothermundt, C.; Thomas, K.; Bancroft, E.; Eeles, R.; Shanley, S.; Ardern-Jones, A.; Norman, A.; Kaye, S.B.; Gore, M.E. “BRCAness” Syndrome in Ovarian Cancer: A Case-Control Study Describing the Clinical Features and Outcome of Patients with Epithelial Ovarian Cancer Associated WithBRCA1 and BRCA2 Mutations. J. Clin. Oncol. 2008, 26, 5530–5536. [Google Scholar] [CrossRef]
- Takaya, H.; Nakai, H.; Takamatsu, S.; Mandai, M.; Matsumura, N. Homologous recombination deficiency status-based classification of high-grade serous ovarian carcinoma. Sci. Rep. 2020, 10, 2757. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Moore, K.N.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 1721–1731. [Google Scholar] [CrossRef]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Depont Christensen, R.; Graybill, W.; Mirza, M.R.; McCormick, C.; Lorusso, D.; Hoskins, P.; Freyer, G.; et al. Niraparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2391–2402. [Google Scholar] [CrossRef] [Green Version]
- The Society of Gynecologic Oncology of Canada. Optimizing BRCA Gene Testing for Ovarian Cancer. Available online: https://g-o-c.org/wp-content/uploads/2015/01/18GOCStmt_OptimizingBRCAGeneTestingForOvarianCancer_FINAL_EN_Feb082018.pdf (accessed on 19 January 2022).
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- AstraZeneca. First and only PARP Inhibitor, LYNPARZA® (OLAPARIB) Approved as a First-Line Maintenance Therapy Treatement in BRCA-Mutated Advanced Ovarian Cancer. Available online: https://www.astrazeneca.ca/en/media/press-releases/2019/first-and-only-parp-inhibitor--lynparza---olaparib--approved-as-.html# (accessed on 4 February 2022).
- GlaxoSmithKline Inc. ZEJULA is Approved in Canada for First-Line Maintenance Treatment of Women with Advanced Ovarian Cancer. Available online: https://www.newswire.ca/news-releases/zejula-is-approved-in-canada-for-first-line-maintenance-treatment-of-women-with-advanced-ovarian-cancer-849289412.html (accessed on 4 February 2022).
- Ramus, S.J.; Gayther, S.A. The Contribution of BRCA1 and BRCA2 to Ovarian Cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef] [Green Version]
- Alsop, K.; Fereday, S.; Meldrum, C.; Defazio, A.; Emmanuel, C.; George, J.; Dobrovic, A.; Birrer, M.J.; Webb, P.M.; Stewart, C.; et al. BRCA Mutation Frequency and Patterns of Treatment Response in BRCA Mutation–Positive Women with Ovarian Cancer: A Report from the Australian Ovarian Cancer Study Group. J. Clin. Oncol. 2012, 30, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belanger, M.H.; Dolman, L.; Arcand, S.; Shen, Z.; Chong, G.; Mes-Masson, A.-M.; Provencher, D.; Tonin, P.N. A targeted analysis identifies a high frequency of BRCA1 and BRCA2 mutation carriers in women with ovarian cancer from a founder population. J. Ovarian Res. 2015, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Hanley, G.E.; McAlpine, J.N.; Miller, D.; Huntsman, D.; Schrader, K.A.; Blake Gilks, C.; Mitchell, G. A population-based analysis of germline BRCA1 and BRCA2 testint among ovarian cancer patients in an era of histotype-specific approaches to ovarian cancer prevention. BMC Cancer 2018, 18, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Khan, S.; Sun, Y.; Hess, K.; Shmulevich, I.; Sood, A.K.; Zhang, W. Association of BRCA1 and BRCA2 Mutations with Survival, Chemotherapy Sensitivity, and Gene Mutator Phenotype in Patients with Ovarian Cancer. JAMA 2011, 306, 1557–1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witjes, V.M.; van Bommel, M.H.D.; Ligtenberg, M.J.L.; Vos, J.R.; Mourits, M.J.E.; Ausems, M.G.E.M.; De Hullu, J.A.; Bosse, T.; Hoogerbrugge, N. Probability of detecting germline BRCA1/2 pathogenic variants in histological subtypes of ovarian carcinoma. A meta-analysis. Gynecol. Oncol. 2022, 164, 221–230. [Google Scholar] [CrossRef]
- Eoh, K.J.; Kim, H.M.; Lee, J.-Y.; Kim, S.; Kim, S.W.; Kim, Y.T.; Nam, E.J. Mutation landscape of germline and somatic BRCA1/2 in patients with high-grade serous ovarian cancer. BMC Cancer 2020, 20, 204. [Google Scholar] [CrossRef] [PubMed]
- Pennington, K.P.; Walsh, T.; Harrell, M.I.; Lee, M.K.; Pennil, C.C.; Rendi, M.H.; Thornton, A.; Norquist, B.M.; Casadei, S.; Nord, A.S.; et al. Germline and Somatic Mutations in Homologous Recombination Genes Predict Platinum Response and Survival in Ovarian, Fallopian Tube, and Peritoneal Carcinomas. Clin. Cancer Res. 2014, 20, 764–775. [Google Scholar] [CrossRef] [Green Version]
- Bolton, K.L. Association Between BRCA1 and BRCA2 Mutations and Survival in Women with Invasive Epithelial Ovarian Cancer. JAMA 2012, 307, 382. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Yang, D.; Sun, Y.; Shmulevich, I.; Xue, F.; Sood, A.K.; Zhang, W. Differing clinical impact of BRCA1 and BRCA2 mutations in serous ovarian cancer. Pharmacogenomics 2012, 13, 1523–1535. [Google Scholar] [CrossRef] [Green Version]
- Chetrit, A.; Hirsh-Yechezkel, G.; Ben-David, Y.; Lubin, F.; Friedman, E.; Sadetzki, S. Effect of BRCA1/2 Mutations on Long-Term Survival of Patients with Invasive Ovarian Cancer: The National Israeli Study of Ovarian Cancer. J. Clin. Oncol. 2008, 26, 20–25. [Google Scholar] [CrossRef]
- Fong, P.C.; Boss, D.S.; Yap, T.A.; Tutt, A.; Wu, P.; Mergui-Roelvink, M.; Mortimer, P.; Swaisland, H.; Lau, A.; O’Connor, M.J.; et al. Inhibition of Poly(ADP-Ribose) Polymerase in Tumors from BRCA Mutation Carriers. N. Engl. J. Med. 2009, 361, 123–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ledermann, J.; Harter, P.; Gourley, C.; Friedlander, M.; Vergote, I.; Rustin, G.; Scott, C.L.; Meier, W.; Shapira-Frommer, R.; Safra, T.; et al. Olaparib maintenance therapy in patients with platinum-sensitive relapsed serous ovarian cancer: A preplanned retrospective analysis of outcomes by BRCA status in a randomised phase 2 trial. Lancet Oncol. 2014, 15, 852–861. [Google Scholar] [CrossRef]
- Mirza, M.R.; Monk, B.J.; Herrstedt, J.; Oza, A.M.; Mahner, S.; Redondo, A.; Fabbro, M.; Ledermann, J.A.; Lorusso, D.; Vergote, I.; et al. Niraparib Maintenance Therapy in Platinum-Sensitive, Recurrent Ovarian Cancer. N. Engl. J. Med. 2016, 375, 2154–2164. [Google Scholar] [CrossRef] [PubMed]
- Pujade-Lauraine, E.; Ledermann, J.A.; Selle, F.; Gebski, V.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Poveda, A.; Pignata, S.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2017, 18, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Coleman, R.L.; Oza, A.M.; Lorusso, D.; Aghajanian, C.; Oaknin, A.; Dean, A.; Colombo, N.; Weberpals, J.I.; Clamp, A.; Scambia, G.; et al. Rucaparib maintenance treatment for recurrent ovarian carcinoma after response to platinum therapy (ARIEL3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1949–1961. [Google Scholar] [CrossRef] [Green Version]
- Mohyuddin, G.R.; Aziz, M.; Britt, A.; Wade, L.; Sun, W.; Baranda, J.; Al-Rajabi, R.; Saeed, A.; Kasi, A. Similar response rates and survival with PARP inhibitors for patients with solid tumors harboring somatic versus Germline BRCA mutations: A Meta-analysis and systematic review. BMC Cancer 2020, 20, 507. [Google Scholar] [CrossRef] [PubMed]
- Poveda, A.; Lheureux, S.; Colombo, N.; Cibula, D.; Lindemann, K.; Weberpals, J.; Bjurberg, M.; Oaknin, A.; Sikorska, M.; González-Martín, A.; et al. Olaparib maintenance monotherapy in platinum-sensitive relapsed ovarian cancer patients without a germline BRCA1/BRCA2 mutation: OPINION primary analysis. Gynecol. Oncol. 2022, 164, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Paluch-Shimon, S.; Cardoso, F.; Sessa, C.; Balmana, J.; Cardoso, M.J.; Gilbert, F.; Senkus, E. Prevention and screening in BRCA mutation carriers and other breast/ovarian hereditary cancer syndromes: ESMO Clinical Practice Guidelines for cancer prevention and screening. Ann. Oncol. 2016, 27, v103–v110. [Google Scholar] [CrossRef]
- Canadian Cancer Statistics Advisory Committee in Collaboration with the Canadian Cancer Society, Statistics Canada and the Public Health Agency of Canada. Canadian Cancer Statistics. 2021. Available online: https://cdn.cancer.ca/-/media/files/research/cancer-statistics/2021-statistics/2021-pdf-en-final.pdf (accessed on 3 March 2022).
- Konstantinopoulos, P.A.; Norquist, B.; Lacchetti, C.; Armstrong, D.; Grisham, R.N.; Goodfellow, P.J.; Kohn, E.C.; Levine, D.A.; Liu, J.F.; Lu, K.H.; et al. Germline and Somatic Tumor Testing in Epithelial Ovarian Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1222–1245. [Google Scholar] [CrossRef]
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-reducing bilateral salpingo-oophorectomy in women with BRCA1 or BRCA2 mutations. Cochrane Database Syst. Rev. 2018, 8, CD012464. [Google Scholar] [CrossRef]
- Colombo, N.; Sessa, C.; du Bois, A.; Ledermann, J.; McCluggage, W.G.; McNeish, I.; Morice, P.; Pignata, S.; Ray-Coquard, I.; Vergote, I.; et al. ESMO-ESGO consensus conference recommendations on ovarian cancer: Pathology and molecular biology, early and advanced stages, borderline tumours and recurrent disease. Ann. Oncol. 2019, 30, 672–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lesueur, F.; Eon-Marchais, S.; Bonnet-Boissinot, S.; Beauvallet, J.; Dondon, M.-G.; Golmard, L.; Rouleau, E.; Garrec, C.; Martinez, M.; Toulas, C.; et al. TUMOSPEC: A Nation-Wide Study of Hereditary Breast and Ovarian Cancer Families with a Predicted Pathogenic Variant Identified through Multigene Panel Testing. Cancers 2021, 13, 3659. [Google Scholar] [CrossRef]
- Norquist, B.M.; Brady, M.F.; Harrell, M.I.; Walsh, T.; Lee, M.K.; Gulsuner, S.; Bernards, S.S.; Casadei, S.; Burger, R.A.; Tewari, K.S.; et al. Mutations in Homologous Recombination Genes and Outcomes in Ovarian Carcinoma Patients in GOG 218: An NRG Oncology/Gynecologic Oncology Group Study. Clin. Cancer Res. 2018, 24, 777–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swisher, E.M.; Lin, K.K.; Oza, A.M.; Scott, C.L.; Giordano, H.; Sun, J.; Konecny, G.E.; Coleman, R.L.; Tinker, A.V.; O’Malley, D.M.; et al. Rucaparib in relapsed, platinum-sensitive high-grade ovarian carcinoma (ARIEL2 Part 1): An international, multicentre, open-label, phase 2 trial. Lancet Oncol. 2017, 18, 75–87. [Google Scholar] [CrossRef] [Green Version]
- Hodgson, D.R.; Dougherty, B.A.; Lai, Z.; Fielding, A.; Grinsted, L.; Spencer, S.; O’Connor, M.J.; Ho, T.W.; Robertson, J.D.; Lanchbury, J.S.; et al. Candidate biomarkers of PARP inhibitor sensitivity in ovarian cancer beyond the BRCA genes. Br. J. Cancer 2018, 119, 1401–1409. [Google Scholar] [CrossRef] [Green Version]
- Miller, R.E.; Leary, A.; Scott, C.L.; Serra, V.; Lord, C.J.; Bowtell, D.; Chang, D.K.; Garsed, D.W.; Jonkers, J.; Ledermann, J.A.; et al. ESMO recommendations on predictive biomarker testing for homologous recombination deficiency and PARP inhibitor benefit in ovarian cancer. Ann. Oncol. 2020, 31, 1606–1622. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 2011, 474, 609–615. [Google Scholar] [CrossRef]
- Ngoi, N.Y.L.; Tan, D.S.P. The role of homologous recombination deficiency testing in ovarian cancer and its clinical implications: Do we need it? Esmo Open 2021, 6, 100144. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Ovarian Cancer, Version 1.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/ovarian.pdf (accessed on 15 March 2022).
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- The Society of Gynecologic Oncology of Canada. Why Is Tumour Testing in Ovarian Cancer Needed in Canada? Available online: https://g-o-c.org/wp-content/uploads/2020/02/20BRCACollaborative_TumourTestinginCanada_FINAL_Jan30.pdf (accessed on 19 January 2022).
- McCuaig, J.M.; Stockley, T.L.; Shaw, P.; Fung-Kee-Fung, M.; Altman, A.D.; Bentley, J.; Bernardini, M.Q.; Cormier, B.; Hirte, H.; Kieser, K.; et al. Evolution of genetic assessment for BRCA-associated gynaecologic malignancies: A Canadian multisociety roadmap. J. Med. Genet. 2018, 55, 571–577. [Google Scholar] [CrossRef] [PubMed]
- Pan-Canadian Oncology Drug Review. pCODR Expert Review Committee (pERC): Final Recommendation (Niraparib). Available online: https://www.cadth.ca/sites/default/files/pcodr/Reviews2021/10224NiraparibOC_fnRec_pERC%20Chair%20Approved_Post29Apr2021_final.pdf (accessed on 2 February 2022).
- Coleman, R.L.; Fleming, G.F.; Brady, M.F.; Swisher, E.M.; Steffensen, K.D.; Friedlander, M.; Okamoto, A.; Moore, K.N.; Efrat Ben-Baruch, N.; Werner, T.L.; et al. Veliparib with First-Line Chemotherapy and as Maintenance Therapy in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2403–2415. [Google Scholar] [CrossRef] [PubMed]
- Bradley, W.; Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Abstract 39: Maintenance olaparib for patients with newly diagnosed, advanced ovarian cancer and a BRCA mutation: 5-year follow-up from SOLO-1. In Proceedings of the Society of Gynecologic Oncology (SGO) 2021 Annual Meeting on Women’s Cancer, Virtual Meeting, 19–25 March 2021. [Google Scholar]
- Colombo, N.; Moore, K.; Scambia, G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; Gourley, C.; et al. Tolerability of maintenance olaparib in newly diagnosed patients with advanced ovarian cancer and a BRCA mutation in the randomized phase III SOLO1 trial. Gynecol. Oncol. 2021, 163, 41–49. [Google Scholar] [CrossRef] [PubMed]
- O’Cearbhaill, R.; Pérez-Fidalgo, J.A.; Monk, B.; Tusquets, I.; McCormick, C.; Fuentes, J.; Moore, R.; Vulsteke, C.; Shahin, M.; Forget, F.; et al. Efficacy of niraparib by timing of surgery and residual disease: A post-hoc analysis of patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol. Oncol. 2021, 162, S65. [Google Scholar] [CrossRef]
- Monk, B.J.; Coleman, R.L.; Fujiwara, K.; Wilson, M.K.; Oza, A.M.; Oaknin, A.; O’Malley, D.M.; Lorusso, D.; Westin, S.N.; Safra, T.; et al. ATHENA (GOG-3020/ENGOT-ov45): A randomized, phase III trial to evaluate rucaparib as monotherapy (ATHENA–MONO) and rucaparib in combination with nivolumab (ATHENA–COMBO) as maintenance treatment following frontline platinum-based chemotherapy in ovarian cancer. Int. J. Gynecol. Cancer 2021, 31, 1589–1594. [Google Scholar]
- Poveda, A.; Floquet, A.; Ledermann, J.A.; Asher, R.; Penson, R.T.; Oza, A.M.; Korach, J.; Huzarski, T.; Pignata, S.; Friedlander, M.; et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): A final analysis of a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 2021, 22, 620–631. [Google Scholar] [CrossRef]
- Lafargue, C.J.; Dal Molin, G.Z.; Sood, A.K.; Coleman, R.L. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019, 20, e15–e28. [Google Scholar] [CrossRef]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef]
- AstraZeneca Canada Inc. Product Monograph: Pr LYNPARZA® Olaparib Tablets. Available online: https://www.astrazeneca.ca/content/dam/az-ca/downloads/productinformation/lynparza-tablets-product-monograph-en.pdf (accessed on 4 February 2022).
- GlaxoSmithKline Inc. Product Monograph: Pr ZEJULA Niraparib Capsules. Available online: https://ca.gsk.com/media/6229/zejula_pm_en.pdf (accessed on 4 February 2022).
- Berek, J.S.; Matulonis, U.A.; Peen, U.; Ghatage, P.; Mahner, S.; Redondo, A.; Lesoin, A.; Colombo, N.; Vergote, I.; Rosengarten, O.; et al. Safety and dose modification for patients receiving niraparib. Ann. Oncol. 2018, 29, 1784–1792. [Google Scholar] [CrossRef]
- Mirza, M.R.; Martin, A.G.; Graybill, W.; O’Malley, D.M.; Gaba, L.; Yap, O.W.S.; Guerra, E.M.; Rose, P.G.; Baurain, J.-F.; Ghamande, S.A.; et al. Evaluation of an individualized starting-dose of niraparib in the PRIMA/ENGOT-OV26/GOG-3012 study. J. Clin. Oncol. 2020, 38, 6050. [Google Scholar] [CrossRef]
- Gallagher, J.R.; Heap, K.J.; Carroll, S.; Travers, K.; Harrow, B.; Westin, S.N. Real-world adverse events with niraparib 200 mg/day maintenance therapy in ovarian cancer: A retrospective study. Future Oncol. 2019, 15, 4197–4206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madariaga, A.; Bowering, V.; Ahrari, S.; Oza, A.M.; Lheureux, S. Manage wisely: Poly (ADP-ribose) polymerase inhibitor (PARPi) treatment and adverse events. Int. J. Gynecol. Cancer 2020, 30, 903–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matulonis, U.; Herrstedt, J.; Oza, A.; Mahner, S.; Redondo, A.; Berton, D.; Berek, J.; Lund, B.; Marmé, F.; González-Martín, A.; et al. Long-term safety and secondary efficacy endpoints in the ENGOT-OV16/NOVA phase III trial of niraparib in recurrent ovarian cancer. Gynecol. Oncol. 2021, 162, S24–S25. [Google Scholar] [CrossRef]
- Canadian Agency for Drug and Technologies in Health. CADTH pCODR Final Clinical Guidance Report: Niraparib (Zejula). Available online: https://cadth.ca/sites/default/files/pcodr/Reviews2021/10224NiraparibOC_fnCGR_NOREDACT_Post29Apr2021_final.pdf (accessed on 3 February 2022).
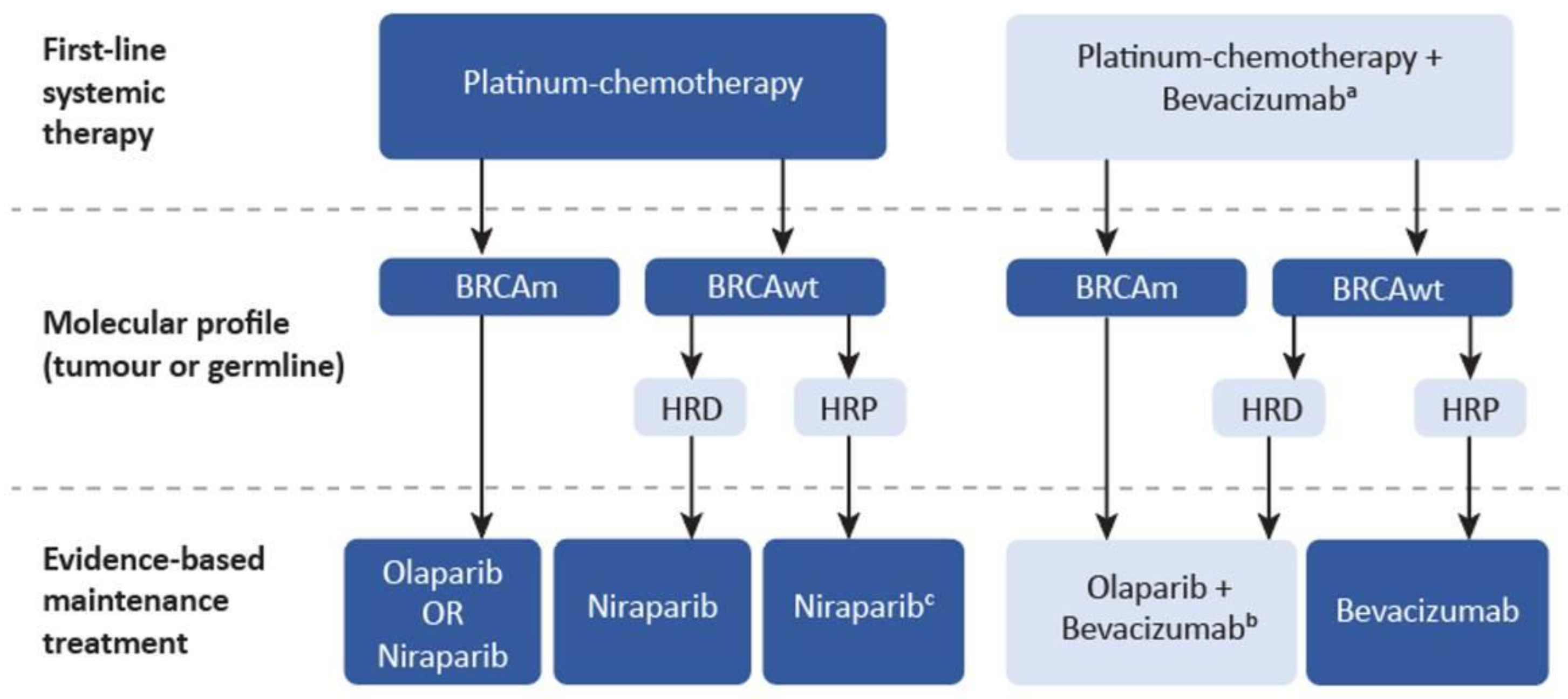
| Genetic Testing to Inform PARP Inhibitor Maintenance Strategies |
|---|
|
| Selection of PARP inhibitors as first-line maintenance therapy in advanced EOC |
|
| Dosing and duration of PARPi maintenance therapy |
|
| Trial Name, Study Phase | Treatment Arms | Study Population | HRD Testing Method | PFS Results (PARP Inhibitor vs. Control) | ||
|---|---|---|---|---|---|---|
| Population | Median (Months) | HR (95% CI) | ||||
| SOLO-1 [8] (NCT01844986) Phase III | olaparib vs. placebo | Stage III/IV BRCAm EOC following PR/CR to CT | N/A | BRACm(ITT) | 56.0 vs. 13.8 | 0.33 (0.25–0.43) |
| PRIMA [9] (NCT02655016) Phase III | niraparib vs. placebo | Stage III/IV high-risk EOC with visible residual disease following PR/CR to CT | Myriad myChoice CDx (HRD = GIS ≥ 42 or BRCA1/2 mutation) | ITT | 13.8 vs. 8.2 | 0.62 (0.50–0.76) |
| HRD | 21.9 vs. 10.4 | 0.43 (0.31–0.59) | ||||
| BRCAm | 22.1 vs. 10.9 | 0.40 (0.27–0.62) | ||||
| HRD/BRCAwt | 19.6 vs. 8.2 | 0.50 (0.31–0.83) | ||||
| HRP | 8.1 vs. 5.4 | 0.68 (0.49–0.94) | ||||
| PAOLA1 * [44] (NCT02477644) Phase III | olaparib + bevacizumab vs. placebo + bevacizumab | Stage III/IV EOC following PR/CR to CT + bevacizumab | Myriad myChoice CDx (HRD = GIS ≥ 42 or BRCA1/2 mutation) | ITT | 22.1 vs. 16.6 | 0.59 (0.49–0.72) |
| HRD | 37.2 vs.17.7 | 0.33 (0.25–0.45) | ||||
| BRCAm | 37.2 vs. 21.7 | 0.31 (0.20–0.47) | ||||
| HRD/BRCAwt | 28.1 vs. 16.6 | 0.43 (0.28–0.66) | ||||
| HRP/HRnd | 16.9 vs. 16.0 | 0.92 (0.72–1.17) | ||||
| VELIA *,†,‡ [50] (NCT02470585) Phase III | CT + veliparib → veliparib vs. CT + 66veliparib → placebo vs. CT + placebo → placebo | Stage III/IV high-grade serous ovarian carcinoma | Myriad myChoice CDx (HRD = GIS ≥ 33 or BRCA1/2 mutation) | ITT | 23.5 vs. 17.3 | 0.68 (0.56–0.83) |
| HRD | 31.9 vs. 20.5 | 0.57 (0.43–0.76) | ||||
| BRCAm | 34.7 vs. 22.0 | 0.44 (0.28–0.68) | ||||
| BRCAwt | 18.2 vs. 15.1 | 0.80 (0.64–1.00) | ||||
| HRP | 15.0 vs. 11.5 | 0.81 (0.60–1.09) | ||||
| Study Attribute | SOLO-1 (N = 391) | PRIMA (N = 733) |
|---|---|---|
| Design | International, randomized (2:1), double-blind | |
| Treatment arms | Olaparib vs. placebo | Niraparib vs. placebo |
| Dosing | Olaparib 300 mg twice-daily up to 24 months or until progression for patients in PR | Niraparib 300 mg once-daily * up to 36 months (or until progression for patients in PR) |
| Eligibility criteria | BRCA1/2 mutated No prior bevacizumab Stage III/IV CR/PR to platinum-CT | Stage III inoperable/visible residual disease and stage IV † CR/PR to platinum-CT |
| Stage IV | 17% | 35% |
| PDS/NACT-IDS | 63%/35% | 32%/67% |
| NED or CR after platinum-CT | 74% | 69% |
| BRCA1/2 mutated | 100% | 30% |
| HRD testing | None | Myriad myChoice HRD GIS score ≥ 42 or BRCA1/2 mutation |
| Primary endpoint | PFS Investigator-assessed | PFS Blinded Independent central review HRD and ITT (Hierarchical testing) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tinker, A.V.; Altman, A.D.; Bernardini, M.Q.; Ghatage, P.; Gien, L.T.; Provencher, D.; Salvador, S.; Doucette, S.; Oza, A.M. A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers. Curr. Oncol. 2022, 29, 4354-4369. https://doi.org/10.3390/curroncol29060348
Tinker AV, Altman AD, Bernardini MQ, Ghatage P, Gien LT, Provencher D, Salvador S, Doucette S, Oza AM. A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers. Current Oncology. 2022; 29(6):4354-4369. https://doi.org/10.3390/curroncol29060348
Chicago/Turabian StyleTinker, Anna V., Alon D. Altman, Marcus Q. Bernardini, Prafull Ghatage, Lilian T. Gien, Diane Provencher, Shannon Salvador, Sarah Doucette, and Amit M. Oza. 2022. "A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers" Current Oncology 29, no. 6: 4354-4369. https://doi.org/10.3390/curroncol29060348
APA StyleTinker, A. V., Altman, A. D., Bernardini, M. Q., Ghatage, P., Gien, L. T., Provencher, D., Salvador, S., Doucette, S., & Oza, A. M. (2022). A Pan-Canadian Consensus Statement on First-Line PARP Inhibitor Maintenance for Advanced, High-Grade Serous and Endometrioid Tubal, Ovarian, and Primary Peritoneal Cancers. Current Oncology, 29(6), 4354-4369. https://doi.org/10.3390/curroncol29060348





