An Oncology Urgent Care Clinic for the Management of Immune-Related Adverse Events: A Descriptive Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Encounters and Demographics
2.2. irAE Definition and Data Collection
2.3. Statistical Analysis
3. Results
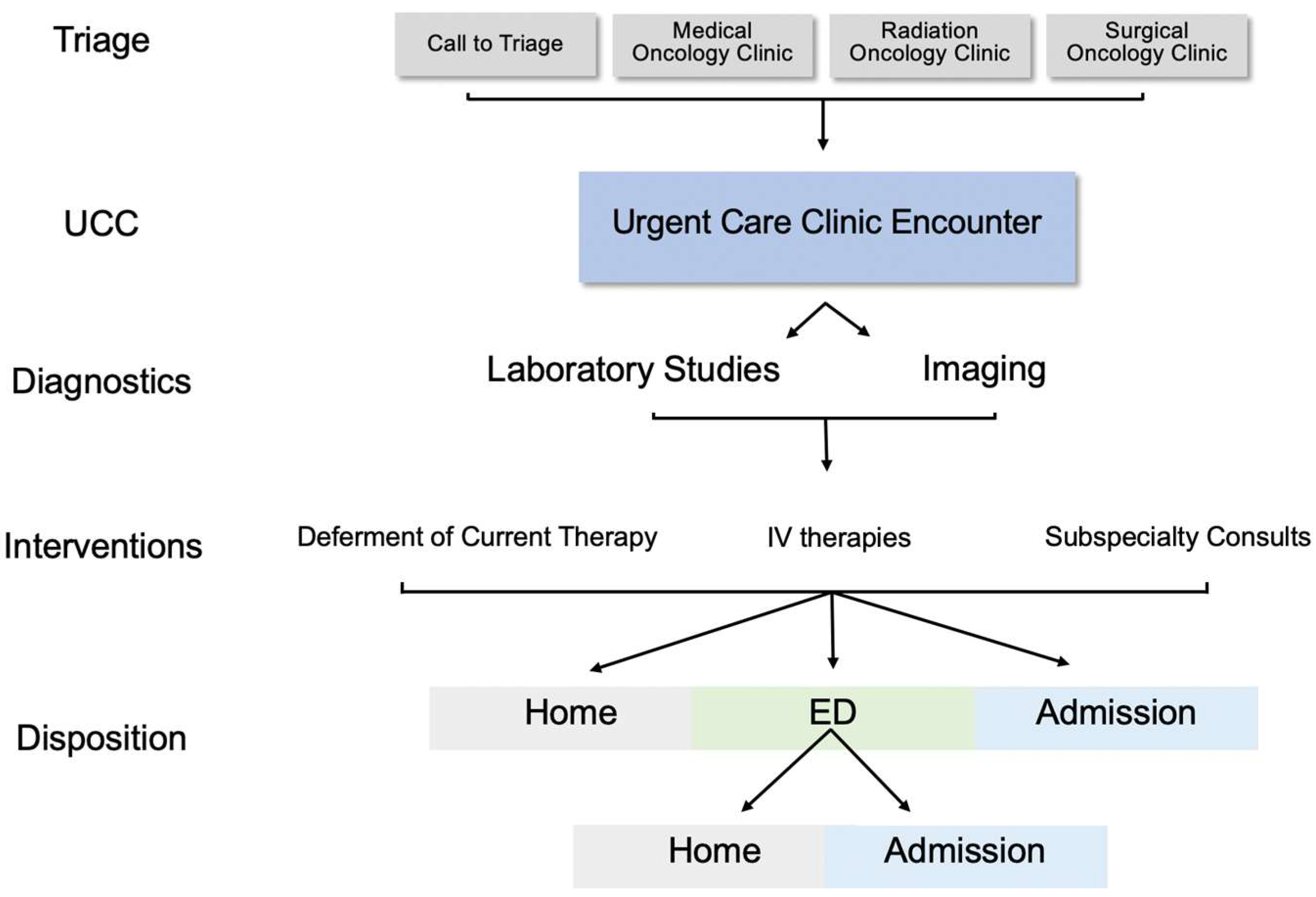
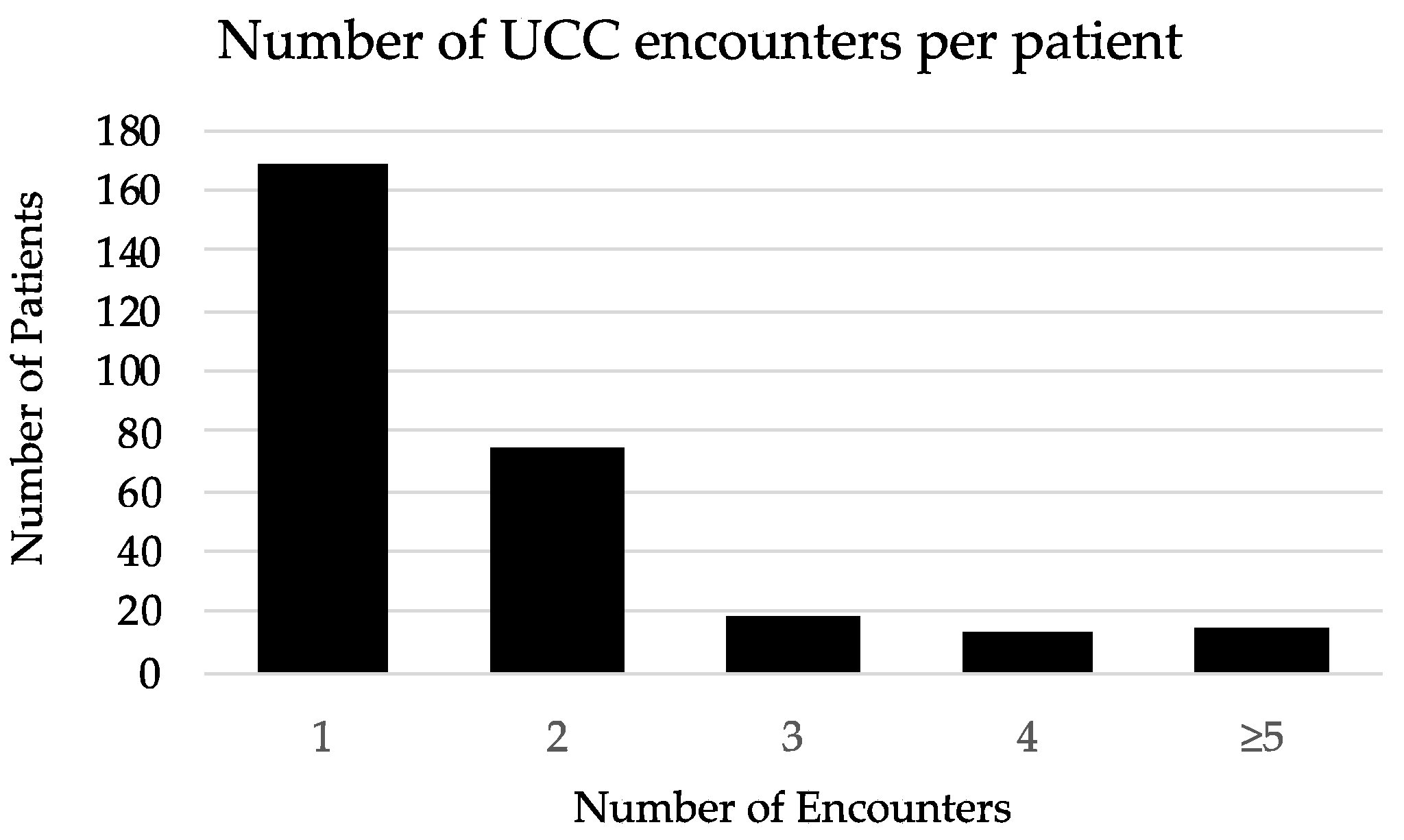
3.1. UCC Encounter Demographics
3.2. Evaluation of Patients with a History of ICI
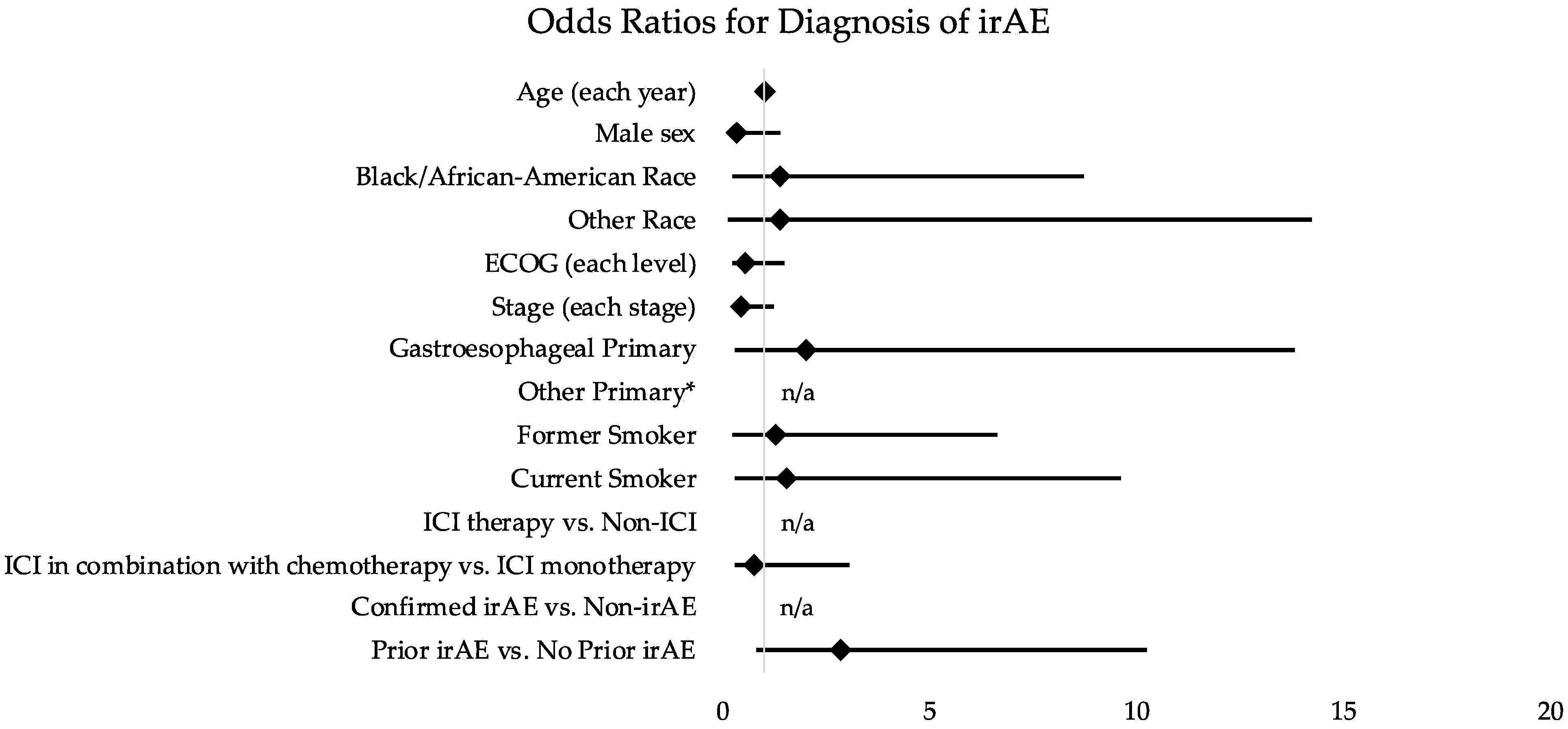
3.3. Distribution of irAEs
3.4. Management, and Outcomes for irAEs by Disposition
3.4.1. Outpatient Management
3.4.2. Inpatient Management
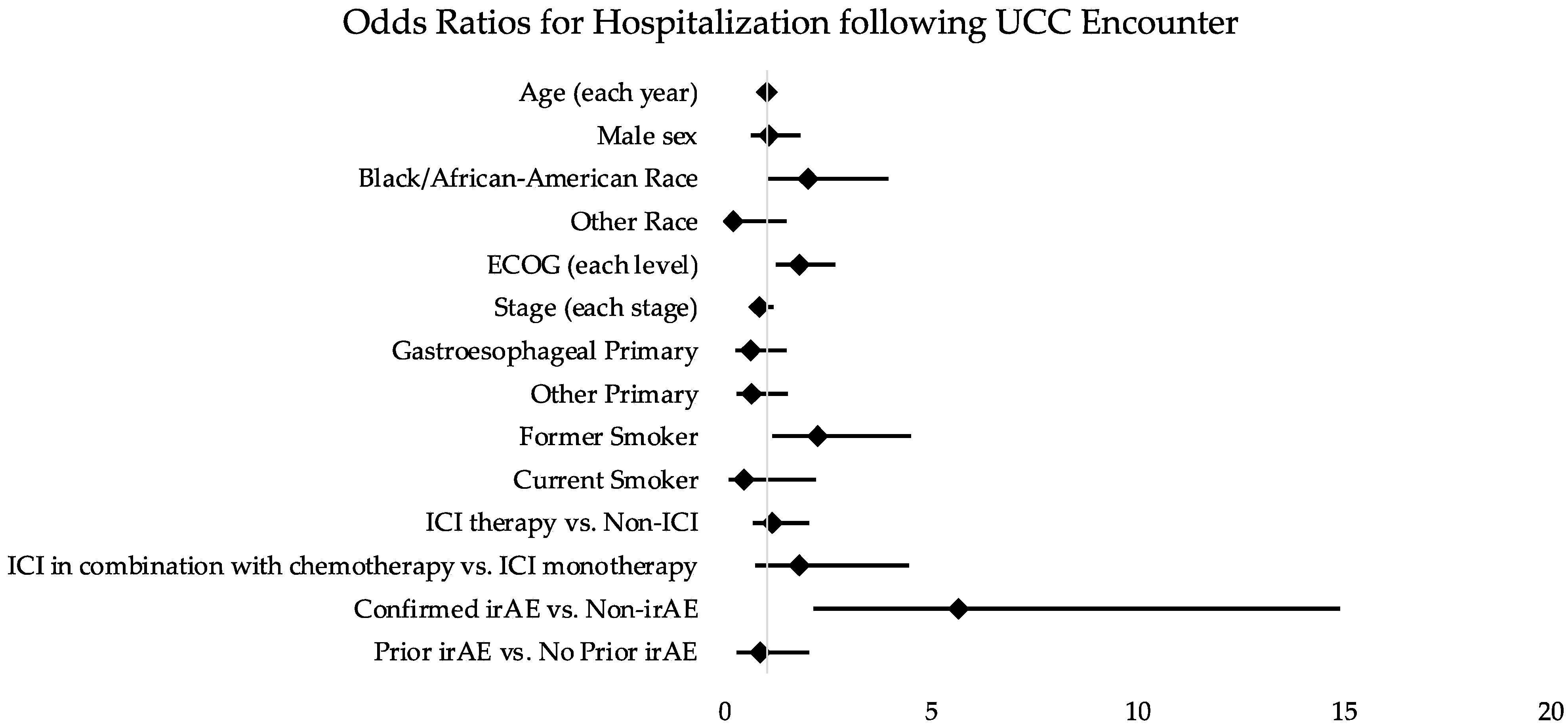
3.5. Associations between Encounter Features and UCC Visit Disposition
4. Discussion
4.1. Main Findings
4.2. Comparison with Other Studies
4.3. Limitations
4.4. Implications and Future Directions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hong, A.S.; Froehlich, T.; Clayton Hobbs, S.; Lee, S.J.C.; Halm, E.A. Impact of a Cancer Urgent Care Clinic on Regional Emergency Department Visits. J. Oncol. Pract. 2019, 15, e501–e509. [Google Scholar] [CrossRef] [PubMed]
- Gould Rothberg, B.E.; Canavan, M.E.; Mun, S.; Sedghi, T.; Carafeno, T.; Raucci, M.; Dest, V.; Sinanis, N.; Gross, C.P.; Adelson, K.B. Impact of a Dedicated Cancer Urgent Care Center on Acute Care Utilization. JCO Oncol. Pract. 2022, 18, e129–e136. [Google Scholar] [CrossRef] [PubMed]
- Bevins, J.S.; Fullington, H.; Froehlich, T.W.; Hobbs, S.; Halm, E.; Lee, S.C.; Hong, A. Safety and outcomes of a cancer patient urgent care clinic. J. Clin. Oncol. 2019, 37, 6542. [Google Scholar] [CrossRef]
- Parikh, A.; Berizzi, D.; Tsao, C.-K.; Smith, C.B. Characterization of sick visits at an enhanced oncology urgent care center. J. Clin. Oncol. 2018, 36, 285. [Google Scholar] [CrossRef]
- Holstead, R.; Kartolo, A.; Baetz, T. Emergency Department Utilization for Patients Receiving Immune Checkpoint Inhibitors: A Retrospective Analysis of Identification and Outcomes for Those Presenting for Immune-Related Adverse Events. Curr. Oncol. 2020, 28, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Cooksley, T.; Gupta, A.; Al-Sayed, T.; Lorigan, P. Emergency presentations in patients treated with immune checkpoint inhibitors. Eur. J. Cancer 2020, 130, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Puzanov, I.; Diab, A.; Abdallah, K.; Bingham, C.O., 3rd; Brogdon, C.; Dadu, R.; Hamad, L.; Kim, S.; Lacouture, M.E.; LeBoeuf, N.R.; et al. Managing toxicities associated with immune checkpoint inhibitors: Consensus recommendations from the Society for Immunotherapy of Cancer (SITC) Toxicity Management Working Group. J. Immunother. Cancer 2017, 5, 95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, J.; Suresh, K. A Multidisciplinary Approach for Patients with Preexisting Lung Diseases and Immune Checkpoint Inhibitor Toxicities. Oncologist 2020, 25, e1589–e1592. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B.; Reynolds, K.L.; Sullivan, R.J.; Balko, J.M.; Patrinely, J.R.; Cappelli, L.C.; Naidoo, J.; Moslehi, J.J. Immune checkpoint inhibitor toxicities: Systems-based approaches to improve patient care and research. Lancet Oncol. 2020, 21, e398–e404. [Google Scholar] [CrossRef]
- Balaji, A.; Zhang, J.; Wills, B.; Marrone, K.A.; Elmariah, H.; Yarchoan, M.; Zimmerman, J.W.; Hajjir, K.; Venkatraman, D.; Armstrong, D.K.; et al. Immune-Related Adverse Events Requiring Hospitalization: Spectrum of Toxicity, Treatment, and Outcomes. J. Oncol. Pract. 2019, 15, e825–e834. [Google Scholar] [CrossRef] [PubMed]
- U.S. National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE); Version 5.0; U.S. Department of Health and Human Services: Washington, DC, USA, 2017. [Google Scholar]
- Peyrony, O.; Tieghem, Y.; Franchitti, J.; Ellouze, S.; Morra, I.; Madelaine-Chambrin, I.; Flicoteaux, R.; Baroudjian, B.; Azoulay, E.; Chevret, S.; et al. Immune checkpoint blockade toxicity among patients with cancer presenting to the emergency department. Emerg. Med. J. 2019, 36, 306–309. [Google Scholar] [CrossRef] [PubMed]
- El Majzoub, I.; Qdaisat, A.; Thein, K.Z.; Win, M.A.; Han, M.M.; Jacobson, K.; Chaftari, P.S.; Prejean, M.; Reyes-Gibby, C.; Yeung, S.J. Adverse Effects of Immune Checkpoint Therapy in Cancer Patients Visiting the Emergency Department of a Comprehensive Cancer Center. Ann. Emerg. Med. 2019, 73, 79–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naidoo, J.; Cappelli, L.; Lipson, E.J.; Forde, P.M.; Sharfman, W.H.; Zhang, J.; Mammen, J.; Moseley, K.; Suresh, K.; Mehta, S.; et al. A multidisciplinary toxicity team for cancer immunotherapy-related adverse events. J. Clin. Oncol. 2018, 36, 6538. [Google Scholar] [CrossRef] [Green Version]
- Shankar, B.; Zhang, J.; Naqash, A.R.; Forde, P.M.; Feliciano, J.L.; Marrone, K.A.; Ettinger, D.S.; Hann, C.L.; Brahmer, J.R.; Ricciuti, B.; et al. Multisystem Immune-Related Adverse Events Associated With Immune Checkpoint Inhibitors for Treatment of Non-Small Cell Lung Cancer. JAMA Oncol. 2020, 6, 1952–1956. [Google Scholar] [CrossRef] [PubMed]
| Demographic Characteristic | Non-ICI Therapy n = 324 (%) | ICI Therapy n = 170 (%) | p | ICI Therapy | p | |
|---|---|---|---|---|---|---|
| Non-irAE n = 141 (%) | irAE n = 29 (%) | |||||
| Age, years, mean ± SD | 63.8 ± 10.8 | 65.5 ± 9.5 | 0.082 | 65.3 ± 9.6 | 66.9 ± 9.0 | 0.408 |
| Sex | ||||||
| Female | 169 (52) | 79 (46) | 0.230 | 62 (44) | 17 (59) | 0.150 |
| Male | 155 (48) | 91 (54) | 79 (56) | 12 (41) | ||
| Race | ||||||
| White/Caucasian | 258 (80) | 127 (75) | 0.121 | 107 (76) | 20 (69) | 0.617 |
| Black/African-American | 48 (15) | 25 (15) | 20 (14) | 5 (17) | ||
| Other | 18 (5) | 18 (11) | 14 (10) | 4 (14) | ||
| ECOG score (prior to UCC encounter) | ||||||
| 0–1 | 157 (48) | 117 (69) | 0.071 | 96 (68) | 21 (72) | 0.926 |
| 2 | 70 (22) | 47 (28) | 40 (28) | 7 (24) | ||
| 3+ | 23 (7) | 6 (4) | 5 (4) | 1 (3) | ||
| Stage (at UCC encounter) | ||||||
| I | 13 (4) | 1 (0) | 0.009 | 1 (0) | 0 (0) | 0.152 |
| II | 24 (7) | 14 (8) | 9 (6) | 5 (17) | ||
| III | 107 (33) | 50 (29) | 41 (29) | 9 (31) | ||
| IV | 129 (40) | 101 (59) | 88 (62) | 13 (45) | ||
| Oncologic/Hematologic Primary | ||||||
| Lung | 198 (61) | 148 (87) | <0.001 | 125 (89) | 23 (79) | 0.345 |
| Esophageal | 49 (15) | 21 (12) | 15 (10) | 6 (21) | ||
| Other * | 77 (24) | 1 (0) | 1 (0) | 0 (0) | ||
| Smoking Status | ||||||
| Never | 116 (36) | 29 (17) | <0.001 | 25 (18) | 4 (14) | 0.366 |
| Former | 171 (53) | 124 (73) | 104 (74) | 20 (69) | ||
| Current | 37 (11) | 17 (10) | 12 (9) | 5 (17) | ||
| Prior Therapy | ||||||
| ICI Monotherapy | n/a | 57 (34) | n/a | 48 (34) | 9 (31) | 0.832 |
| ICI in Combination with Chemotherapy | n/a | 113 (66) | 93 (66) | 20 (69) | ||
| ICI Clinical Trial | 0 (0) | 35 (21) | n/a | 27 (19) | 8 (28) | 0.318 |
| ICI SOC | 0 (0) | 135 (79) | 114 (81) | 21 (72) | ||
| Prior irAE | 0 (0) | 37 (22) | <0.001 | 26 (18) | 11 (38) | 0.021 |
| Urgent Care Disposition | ||||||
| Home | 279 (86) | 147 (86) | 0.226 | 128 (91) | 19 (66) | 0.002 |
| ED to Admit | 27 (8) | 12 (7) | 6 (4) | 6 (21) | ||
| ED to Home | 8 (2) | 1 (0) | 1 (0) | 0 (0) | ||
| Direct Admit | 10 (3) | 10 (6) | 6 (4) | 4 (14) | ||
| Clinical Demographic | OR: Outcome Hospitalization (ED to Admit and Direct Admit) | 95% CI |
|---|---|---|
| Age (each year) | 1.02 | 0.99–1.05 |
| Sex | ||
| Female (ref) | - | |
| Male | 1.05 | 0.61–1.82 |
| Race | ||
| White/Caucasian (ref) | - | |
| Black/African American | 2.00 | 1.01–3.96 |
| Other | 0.20 | 0.03–1.47 |
| ECOG (prior to UCC encounter) (each level) | 1.81 | 1.23–2.67 |
| Stage (at UCC visit) (each stage) | 0.82 | 0.58–1.17 |
| Oncologic/Hematologic Primary | ||
| Lung (ref) | - | |
| Esophageal 1 | 0.61 | 0.25–1.49 |
| Other 2 | 0.64 | 0.27–1.52 |
| Smoking Status | ||
| Never Smoker (ref) | - | |
| Former Smoker 3 | 2.25 | 1.13–4.49 |
| Current Smoker 4 | 0.47 | 0.10–2.19 |
| ICI therapy vs. Non -ICI | 1.15 | 0.66–2.03 |
| ICI in combination with chemotherapy vs. ICI monotherapy | 1.79 | 0.72–4.44 |
| Confirmed irAE vs. Non-irAE | 5.66 | 2.15–14.89 |
| Prior irAE vs. No Prior irAE | 0.88 | 0.28–2.71 |
| Clinical Demographic | OR: Outcome Confirmed irAE | 95% CI |
|---|---|---|
| Age (each year) | 1.02 | 0.95–1.09 |
| Sex | ||
| Female (ref) | - | |
| Male | 0.31 | 0.07–1.39 |
| Race | ||
| White (ref) | - | |
| Black/African American | 1.39 | 0.22–8.72 |
| Other | 1.37 | 0.13–14.25 |
| ECOG (prior to UCC encounter) (each level) | 0.55 | 0.20–1.48 |
| Stage (at UCC visit) (each stage) | 0.44 | 0.16–1.21 |
| Oncologic/Hematologic Primary | ||
| Lung (ref) | - | |
| Esophageal 1 | 2.01 | 0.29–13.79 |
| Other 2 | n/a | n/a |
| Smoking Status | ||
| Never Smoker (ref) | - | |
| Former Smoker 3 | 1.27 | 0.24–6.62 |
| Current Smoker 4 | 1.56 | 0.25–9.60 |
| ICI therapy vs. Non-ICI | n/a | n/a |
| ICI in combination with chemotherapy vs. ICI monotherapy | 0.73 | 0.27–3.08 |
| Confirmed irAE vs. Non-irAE | n/a | n/a |
| Prior irAE vs. No Prior irAE | 2.87 | 0.80–10.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, K.-l.; Tackett, S.; Myers, S.; Brahmer, J.R.; Browner, I.S.; Ettinger, D.S.; Forde, P.M.; Hales, R.K.; Hann, C.L.; Lam, V.K.; et al. An Oncology Urgent Care Clinic for the Management of Immune-Related Adverse Events: A Descriptive Analysis. Curr. Oncol. 2022, 29, 4342-4353. https://doi.org/10.3390/curroncol29060347
Liang K-l, Tackett S, Myers S, Brahmer JR, Browner IS, Ettinger DS, Forde PM, Hales RK, Hann CL, Lam VK, et al. An Oncology Urgent Care Clinic for the Management of Immune-Related Adverse Events: A Descriptive Analysis. Current Oncology. 2022; 29(6):4342-4353. https://doi.org/10.3390/curroncol29060347
Chicago/Turabian StyleLiang, Kai-li, Sean Tackett, Samantha Myers, Julie R. Brahmer, Ilene S. Browner, David S. Ettinger, Patrick M. Forde, Russell K. Hales, Christine L. Hann, Vincent K. Lam, and et al. 2022. "An Oncology Urgent Care Clinic for the Management of Immune-Related Adverse Events: A Descriptive Analysis" Current Oncology 29, no. 6: 4342-4353. https://doi.org/10.3390/curroncol29060347
APA StyleLiang, K.-l., Tackett, S., Myers, S., Brahmer, J. R., Browner, I. S., Ettinger, D. S., Forde, P. M., Hales, R. K., Hann, C. L., Lam, V. K., Marrone, K. A., Patel, T., Peterson, V., Sagorsky, S., Turner, M., Voong, K. R., Naidoo, J., & Feliciano, J. L. (2022). An Oncology Urgent Care Clinic for the Management of Immune-Related Adverse Events: A Descriptive Analysis. Current Oncology, 29(6), 4342-4353. https://doi.org/10.3390/curroncol29060347






