Evolving Role of Risk Tailored Therapy in Early Stage HER2-Positive Breast Cancer: A Canadian Perspective
Abstract
:1. Introduction
2. Systemic Management of HER2-Positive EBC
2.1. Adjuvant HER2 Targeted Therapy
2.2. Adjuvant Chemotherapy in HER2-Positive Disease
2.3. Neoadjuvant Therapy in HER2-Positive Disease
2.4. Neoadjuvant HER2 Targeted Therapy
2.5. Neoadjuvant Chemotherapy for HER2-Positive Disease
2.6. Post-Neoadjuvant HER2 Targeted Therapy
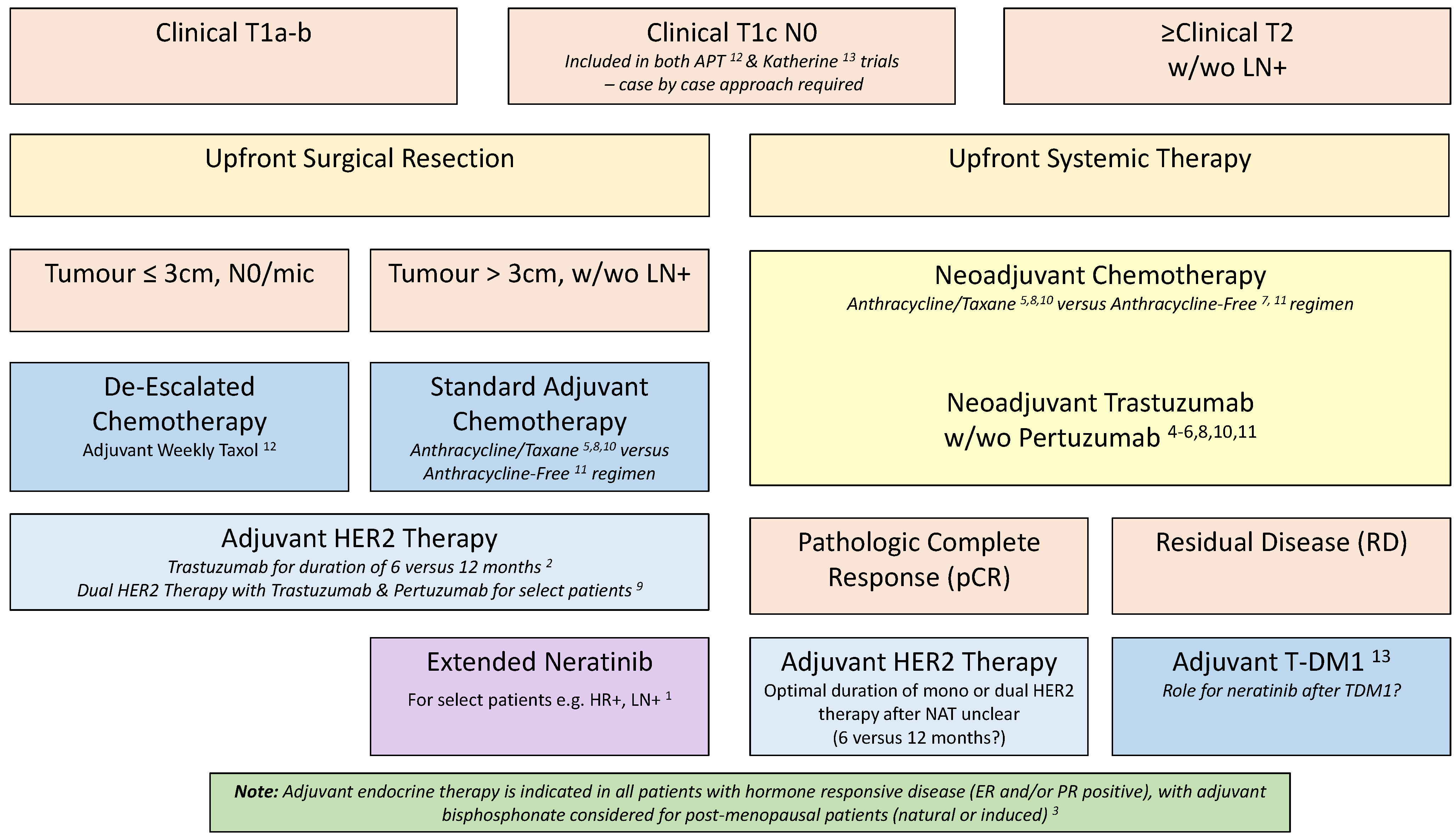
3. Ongoing Trials of Risk Tailored Therapy in HER2-Positive EBC
3.1. Ongoing Trials Leveraging pCR as a Selection Approach for Adjuvant Therapy
3.2. Ongoing De-Escalation Studies
4. Canadian-Led Pragmatic Trials to Optimize Standard of Care
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Piccart-Gebhart, M.J.; Procter, M.; Leyland-Jones, B.; Goldhirsch, A.; Untch, M.; Smith, I.; Gianni, L.; Baselga, J.; Bell, R.H.; Jackisch, C.; et al. Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1659–1672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romond, E.H.; Perez, E.A.; Bryant, J.; Suman, V.J.; Geyer, C.E., Jr.; Davidson, N.E.; Tan-Chiu, E.; Martino, S.; Paik, S.; Kaufman, P.A.; et al. Trastuzumab plus Adjuvant Chemotherapy for Operable HER2-Positive Breast Cancer. N. Engl. J. Med. 2005, 353, 1673–1684. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Kellokumpu-Lehtinen, P.-L.; Bono, P.; Alanko, T.; Kataja, V.; Asola, R.; Utriainen, T.; Kokko, R.; Hemminki, A.; Tarkkanen, M.; et al. Adjuvant Docetaxel or Vinorelbine with or without Trastuzumab for Breast Cancer. N. Engl. J. Med. 2006, 354, 809–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slamon, D.; Eiermann, W.; Robert, N.; Pienkowski, T.; Martin, M.; Press, M.; Mackey, J.; Glaspy, J.; Chan, A.; Pawlicki, M.; et al. Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 2011, 365, 1273–1283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moja, L.; Tagliabue, L.; Balduzzi, S.; Parmelli, E.; Pistotti, V.; Guarneri, V.; D’Amico, R. Trastuzumab containing regimens for early breast cancer. Cochrane Database Syst. Rev. 2012, 2021, CD006243. [Google Scholar] [CrossRef]
- Piccart, M.; Procter, M.; Fumagalli, D.; de Azambuja, E.; Clark, E.; Ewer, M.S.; Restuccia, E.; Jerusalem, G.; Dent, S.; Reaby, L.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer in the APHINITY Trial: 6 Years’ Follow-Up. J. Clin. Oncol. 2021, 39, 1448–1457. [Google Scholar] [CrossRef]
- Conte, P.; Frassoldati, A.; Bisagni, G.; Brandes, A.; Donadio, M.; Garrone, O.; Piacentini, F.; Cavanna, L.; Giotta, F.; Aieta, M.; et al. Nine weeks versus 1 year adjuvant trastuzumab in combination with chemotherapy: Final results of the phase III randomized Short-HER study. Ann. Oncol. 2018, 29, 2328–2333. [Google Scholar] [CrossRef]
- Cameron, D.; Piccart-Gebhart, M.J.; Gelber, R.D.; Procter, M.; Goldhirsch, A.; de Azambuja, E.; Castro, G., Jr.; Untch, M.; Smith, I.; Gianni, L.; et al. 11 years’ follow-up of trastuzumab after adjuvant chemotherapy in HER2-positive early breast cancer: Final analysis of the HERceptin Adjuvant (HERA) trial. Lancet 2017, 389, 1195–1205. [Google Scholar] [CrossRef] [Green Version]
- Joensuu, H.; Fraser, J.; Wildiers, H.; Huovinen, R.; Auvinen, P.; Utriainen, M.; Nyandoto, P.; Villman, K.K.; Halonen, P.; Granstam-Björneklett, H.; et al. Effect of Adjuvant Trastuzumab for a Duration of 9 Weeks vs. 1 Year with Concomitant Chemotherapy for Early Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer. JAMA Oncol. 2018, 4, 1199–1206. [Google Scholar] [CrossRef] [PubMed]
- Mavroudis, D.; Saloustros, E.; Malamos, N.; Kakolyris, S.; Boukovinas, I.; Papakotoulas, P.; Kentepozidis, N.; Ziras, N.; Georgoulias, V. Six versus 12 months of adjuvant trastuzumab in combination with dose-dense chemotherapy for women with HER2-positive breast cancer: A multicenter randomized study by the Hellenic Oncology Research Group (HORG). Ann. Oncol. 2015, 26, 1333–1340. [Google Scholar] [CrossRef]
- Earl, H.M.; Hiller, L.; Vallier, A.-L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Harnett, A.N.; Ah-See, M.-L.; Simcock, R.; Rea, D.; et al. 6 vs. 12 months of adjuvant trastuzumab for HER2-positive early breast cancer (PERSEPHONE): 4-year disease-free survival results of a randomised phase 3 non-inferiority trial. Lancet 2019, 393, 2599–2612. [Google Scholar] [CrossRef] [Green Version]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab in early breast cancer (PHARE): Final analysis of a multicentre, open-label, phase 3 randomised trial. Lancet 2019, 393, 2591–2598. [Google Scholar] [CrossRef]
- Niraula, S.; Gyawali, B. Optimal duration of adjuvant trastuzumab in treatment of early breast cancer: A meta-analysis of randomized controlled trials. Breast Cancer Res. Treat. 2018, 173, 103–109. [Google Scholar] [CrossRef]
- Goldvaser, H.; Korzets, Y.; Shepshelovich, D.; Yerushalmi, R.; Sarfaty, M.; Ribnikar, D.; Thavendiranathan, P.; Amir, E. Deescalating Adjuvant Trastuzumab in HER2-Positive Early-Stage Breast Cancer: A Systemic Review and Meta-Analysis. JNCI Cancer Spectr. 2019, 3, pkz033. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhou, W.; Hu, X.; Yi, M.; Ye, C.; Yao, G. Short-duration versus 1-year adjuvant trastuzumab in early HER2 positive breast cancer: A meta-analysis of randomized controlled trials. Cancer Treat. Rev. 2019, 75, 12–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inno, A.; Barni, S.; Ghidini, A.; Zaniboni, A.; Petrelli, F. One year versus a shorter duration of adjuvant trastuzumab for HER2-positive early breast cancer: A systematic review and meta-analysis. Breast Cancer Res. Treat. 2018, 173, 247–254. [Google Scholar] [CrossRef]
- Conte, P.; Guarneri, V.; Bisagni, G.; Piacentini, F.; Brandes, A.; Cavanna, L.; Giotta, F.; Aieta, M.; Gebbia, V.; Frassoldati, A.; et al. 9 weeks versus 1 year adjuvant trastuzumab for HER2+ early breast cancer: Subgroup analysis of the ShortHER trial allows to identify patients for whom a shorter trastuzumab administration may have a favourable risk/benefit ratio. Ann. Oncol. 2018, 29, viii705. [Google Scholar] [CrossRef]
- Earl, H.; Hiller, L.; Dunn, J.; Conte, P.; D’Amico, R.; Guarneri, V.; Joensuu, H.; Huttunen, T.; Georgoulias, V.; Abraham, J.; et al. LBA11 Individual patient data meta-analysis of 5 non-inferiority RCTs of reduced duration single agent adjuvant trastuzumab in the treatment of HER2 positive early breast cancer. Ann. Oncol. 2021, 32, S1283. [Google Scholar] [CrossRef]
- Chan, A.; Moy, B.; Mansi, J.; Ejlertsen, B.; Holmes, F.A.; Chia, S.; Iwata, H.; Gnant, M.; Loibl, S.; Barrios, C.H.; et al. Final Efficacy Results of Neratinib in HER2-positive Hormone Receptor-positive Early-stage Breast Cancer From the Phase III ExteNET Trial. Clin. Breast Cancer 2020, 21, 80–91.e7. [Google Scholar] [CrossRef]
- Barcenas, C.; Hurvitz, S.; Di Palma, J.; Bose, R.; Chien, A.; Iannotti, N.; Marx, G.; Brufsky, A.; Litvak, A.; Ibrahim, E.; et al. Improved tolerability of neratinib in patients with HER2-positive early-stage breast cancer: The CONTROL trial. Ann. Oncol. 2020, 31, 1223–1230. [Google Scholar] [CrossRef] [PubMed]
- Tolaney, S.M.; Barry, W.T.; Guo, H.; Dillon, D.; Dang, C.T.; Yardley, D.A.; Moy, B.; Marcom, P.K.; Albain, K.S.; Rugo, H.S.; et al. Seven-year (yr) follow-up of adjuvant paclitaxel (T) and trastuzumab (H) (APT trial) for node-negative, HER2-positive breast cancer (BC). J. Clin. Oncol. 2017, 35, 511. [Google Scholar] [CrossRef]
- Tolaney, S.M.; Tayob, N.; Dang, C.; Yardley, D.A.; Isakoff, S.J.; Valero, V.; Faggen, M.; Mulvey, T.; Bose, R.; Hu, J.; et al. Adjuvant Trastuzumab Emtansine Versus Paclitaxel in Combination with Trastuzumab for Stage I HER2-Positive Breast Cancer (ATEMPT): A Randomized Clinical Trial. J. Clin. Oncol. 2021, 39, 2375–2385. [Google Scholar] [CrossRef]
- Slamon, D.; Eiermann, W.; Robert, N.J.; Glermek, J. Ten-year follow-up of BCIRG-006 comparing doxorubicin plus cyclophos-phamide followed by docetaxel with doxorubicin plus cyclophosphamide followed by docetaxel and trastuzumab with docetaxel, carboplatin and trastuzumab in HER2-positive early breast cancer. In Oral Presentation at San Antonio Breast Cancer Symposium; Abstract S5-04; 2015. [Google Scholar]
- Fisher, B.; Brown, A.; Mamounas, E.; Wieand, S.; Robidoux, A.; Margolese, R.G.; Cruz, A.B.; Fisher, E.R.; Wickerham, D.L.; Wolmark, N.; et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: Findings from National Surgical Adjuvant Breast and Bowel Project B-18. J. Clin. Oncol. 1997, 15, 2483–2493. [Google Scholar] [CrossRef]
- Bear, H.D.; Anderson, S.; Brown, A.; Smith, R.; Mamounas, E.P.; Fisher, B.; Margolese, R.; Theoret, H.; Soran, A.; Wickerham, D.L.; et al. The Effect on Tumor Response of Adding Sequential Preoperative Docetaxel to Preoperative Doxorubicin and Cyclophosphamide: Preliminary Results From National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J. Clin. Oncol. 2003, 21, 4165–4174. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef]
- Gandhi, S.; Brackstone, M.; Hong, N.J.L.; Grenier, D.; Donovan, E.; Lu, F.-I.; Skarpathiotakis, M.; Lee, J.; Boileau, J.-F.; Perera, F.; et al. A Canadian national guideline on the neoadjuvant treatment of invasive breast cancer, including patient assessment, systemic therapy, and local management principles. Breast Cancer Res. Treat. 2022, 193, 1–20. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Untch, M.; Blohmer, J.-U.; Costa, S.D.; Eidtmann, H.; Fasching, P.A.; Gerber, B.; Eiermann, W.; Hilfrich, J.; Huober, J.; et al. Definition and Impact of Pathologic Complete Response on Prognosis After Neoadjuvant Chemotherapy in Various Intrinsic Breast Cancer Subtypes. J. Clin. Oncol. 2012, 30, 1796–1804. [Google Scholar] [CrossRef] [Green Version]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef] [Green Version]
- Yau, C.; van der Noordaa, M.; Wei, J.; Osdoit, M.; Reyal, F.; Hamy, A.-S.; Lae, M.; Martin, M.; del Monte, M.; Boughey, J.C.; et al. Abstract GS5-01: Residual cancer burden after neoadjuvant therapy and long-term survival outcomes in breast cancer: A multi-center pooled analysis. Cancer Res. 2020, 80, GS5-01. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services Food and Drug Administration. Pathological Complete Response in Neoad-juvant Treatment of High-Risk Early-Stage Breast Cancer: Use as an Endpoint to Support Accelerated Approval. FDA-2012-D-0432. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pathological-complete-response-neoadjuvant-treatment-high-risk-early-stage-breast-cancer-use (accessed on 11 March 2022).
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Roman, L.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Staroslawska, E.; De La Haba-Rodriguez, J.; Im, S.-A.; et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): A randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012, 13, 25–32. [Google Scholar] [CrossRef]
- Gianni, L.; Pienkowski, T.; Im, Y.-H.; Tseng, L.-M.; Liu, M.-C.; Lluch, A.; Starosławska, E.; De La Haba-Rodríguez, J.R.; Im, S.-A.; Pedrini, J.L.; et al. 5-year analysis of neoadjuvant pertuzumab and trastuzumab in patients with locally advanced, inflammatory, or early-stage HER2-positive breast cancer (NeoSphere): A multicentre, open-label, phase 2 randomised trial. Lancet Oncol. 2016, 17, 791–800. [Google Scholar] [CrossRef]
- Schneeweiss, A.; Chia, S.; Hickish, T.; Harvey, V.; Eniu, A.; Hegg, R.; Tausch, C.; Seo, J.H.; Tsai, Y.-F.; Ratnayake, J.; et al. Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: A randomized phase II cardiac safety study (TRYPHAENA). Ann. Oncol. 2013, 24, 2278–2284. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Ewer, M.S.; Viale, G.; Delaloge, S.; Ferrero, J.-M.; Verrill, M.; Colomer, R.; Vieira, C.; Werner, T.L.; Douthwaite, H.; et al. Pertuzumab, Trastuzumab, and Standard Anthracy-Cline- and Taxane-Based Chemotherapy for the Neoadjuvant Treatment of Patients with HER2-Positive Localized Breast Cancer (BERENICE): A Phase II, Open-Label, Multicenter, Multinational Cardiac Safety Study. Ann. Oncol. 2018, 29, 646–653. [Google Scholar] [CrossRef] [PubMed]
- Guarneri, V.; Frassoldati, A.; Bottini, A.; Cagossi, K.; Bisagni, G.; Sarti, S.; Ravaioli, A.; Cavanna, L.; Giardina, G.; Musolino, A.; et al. Preoperative Chemotherapy Plus Trastuzumab, Lapatinib, or Both in Human Epidermal Growth Factor Receptor 2–Positive Operable Breast Cancer: Results of the Randomized Phase II CHER-LOB Study. J. Clin. Oncol. 2012, 30, 1989–1995. [Google Scholar] [CrossRef]
- Robidoux, A.; Tang, G.; Rastogi, P.; Geyer, C.E.; Azar, C.A.; Atkins, J.N.; Fehrenbacher, L.; Bear, H.D.; Baez-Diaz, L.; Sarwar, S.; et al. Lapatinib as a component of neoadjuvant therapy for HER2-positive operable breast cancer (NSABP protocol B-41): An open-label, randomised phase 3 trial. Lancet Oncol. 2013, 14, 1183–1192. [Google Scholar] [CrossRef]
- Baselga, J.; Bradbury, I.; Eidtmann, H.; Di Cosimo, S.; de Azambuja, E.; Aura, C.; Gómez, H.; Dinh, P.; Fauria, K.; Van Dooren, V.; et al. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): A randomised, open-label, multicentre, phase 3 trial. Lancet 2012, 379, 633–640. [Google Scholar] [CrossRef] [Green Version]
- Carey, L.A.; Berry, D.A.; Cirrincione, C.T.; Barry, W.T.; Pitcher, B.N.; Harris, L.N.; Ollila, D.W.; Krop, I.E.; Henry, N.L.; Weckstein, D.J.; et al. Molecular Heterogeneity and Response to Neoadjuvant Human Epidermal Growth Factor Receptor 2 Targeting in CALGB 40601, a Randomized Phase III Trial of Paclitaxel Plus Trastuzumab with or without Lapatinib. J. Clin. Oncol. 2016, 34, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Guarneri, V.; Griguolo, G.; Miglietta, F.; Conte, P.; Dieci, M.; Girardi, F. Survival after neoadjuvant therapy with trastuzumab–lapatinib and chemotherapy in patients with HER2-positive early breast cancer: A meta-analysis of randomized trials. ESMO Open 2022, 7, 100433. [Google Scholar] [CrossRef]
- van Ramshorst, M.S.; van der Voort, A.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Dezentjé, V.O.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Neoadjuvant Chemo-Therapy with or without Anthracyclines in the Presence of Dual HER2 Blockade for HER2-Positive Breast Cancer (TRAIN-2): A Multicentre, Open-Label, Randomised, Phase 3 Trial. Lancet Oncol. 2018, 19, 1630–1640. [Google Scholar]
- van der Voort, A.; van Ramshorst, M.S.; van Werkhoven, E.D.; Mandjes, I.A.; Kemper, I.; Vulink, A.J.; Oving, I.M.; Honkoop, A.H.; Tick, L.W.; van de Wouw, A.J.; et al. Three-Year Follow-up of Neoadjuvant Chemotherapy with or without Anthracyclines in the Presence of Dual ERBB2 Blockade in Patients with ERBB2 -Positive Breast Cancer. JAMA Oncol. 2021, 7, 978. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer, Version 2.2022. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 24 October 2021).
- Hofmann, D.; Nitz, U.; Gluz, O.; Kates, R.E.; Schinkoethe, T.; Staib, P.; Harbeck, N. WSG ADAPT—Adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: Study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trial. Trials 2013, 14, 261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitz, U.A.; Gluz, O.; Christgen, M.; Grischke, E.M.; Augustin, D.; Kuemmel, S.; Braun, M.; Potenberg, J.; Kohls, A.; Krauss, K.; et al. De-Escalation Strategies in HER2-Positive Early Breast Cancer (EBC): Final Analysis of the WSG-ADAPT HER2+/HR− Phase II Trial: Efficacy, Safety, and Predictive Markers for 12 Weeks of Neoadjuvant Dual Blockade with Trastuzumab and Pertuzumab ± Weekly Pacl. Ann. Oncol. 2017, 28, 2768–2772. [Google Scholar] [CrossRef]
- Harbeck, N.; Gluz, O.; Christgen, M.; Kuemmel, S.; Grischke, E.-M.; Braun, M.; Potenberg, J.; Krauss, K.; Schumacher, C.; Forstbauer, H.; et al. De-escalated neoadjuvant pertuzumab+trastuzumab with or without paclitaxel weekly in HR-/HER2+ early breast cancer: ADAPT-HR-/HER2+ biomarker and survival results. J. Clin. Oncol. 2021, 39, 503. [Google Scholar] [CrossRef]
- Von Minckwitz, G.; Huang, C.-S.; Mano, M.S.; Loibl, S.; Mamounas, E.P.; Untch, M.; Wolmark, N.; Rastogi, P.; Schneeweiss, A.; Redondo, A.; et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N. Engl. J. Med. 2019, 380, 617–628. [Google Scholar] [CrossRef]
- National Institutes of Health (NIH). Available online: ClinicalTrials.gov (accessed on 11 March 2022).
- Lin, N.U.; Borges, V.; Anders, C.; Murthy, R.K.; Paplomata, E.; Hamilton, E.; Hurvitz, S.; Loi, S.; Okines, A.; Abramson, V.; et al. Intracranial Efficacy and Survival with Tucatinib Plus Trastuzumab and Capecitabine for Previously Treated HER2-Positive Breast Cancer with Brain Metastases in the HER2CLIMB Trial. J. Clin. Oncol. 2020, 38, 2610–2619. [Google Scholar] [CrossRef] [PubMed]
- Basik, M.; Cecchini, R.S.; De Los Santos, J.F.; Umphrey, H.R.; Julian, T.B.; Mamounas, E.P.; White, J.; Lucas, P.C.; Balanoff, C.; Tan, A.R.; et al. Abstract GS5-05: Primary Analysis of NRG-BR005, a Phase II trial Assessing Accuracy of Tumor Bed Biopsies in Predicting Pathologic Complete Response (pCR) in Patients with Clinical/Radiological Complete Response after Neoadjuvant Chemotherapy (NCT) to Exp. Cancer Res. 2020, 80, GS5-05. [Google Scholar]
- Fatayer, H.; Sharma, N.; Manuel, D.; Kim, B.; Keding, A.; Perren, T.; Velikova, G.; Lansdown, M.; Shaaban, A.M.; Dall, B. Serial MRI Scans Help in Assessing Early Response to Neo-Adjuvant Chemotherapy and Tailoring Breast Cancer Treatment. Eur. J. Surg. Oncol. 2016, 42, 965–972. [Google Scholar] [CrossRef]
- Robinson, A.; Souied, O.; Bota, A.B.; Levasseur, N.; Stober, C.; Hilton, J.; Kamel, D.; Hutton, B.; Vandermeer, L.; Mazzarello, S.; et al. Optimal vascular access strategies for patients receiving chemotherapy for early-stage breast cancer: A systematic review. Breast Cancer Res. Treat. 2018, 171, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Clemons, M.; Stober, C.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.-C.; Saunders, D.; Vandermeer, L.; Mazzarello, S.; Awan, A.; et al. A randomized trial comparing vascular access strategies for patients receiving chemotherapy with trastuzumab for early-stage breast cancer. Support. Care Cancer 2020, 28, 4891–4899. [Google Scholar] [CrossRef]
- Robinson, A.; Stober, C.; Fergusson, D.; Kehoe, A.; Bedard, D.; MacDonald, F.; Brunet, M.-C.; Saunders, D.; Mazzarello, S.; Vandermeer, L.; et al. A multicentre, randomized pilot trial comparing vascular access strategies for early stage breast cancer patients receiving non-trastuzumab containing chemotherapy. Breast Cancer Res. Treat. 2019, 178, 337–345. [Google Scholar] [CrossRef]
- Dang, C.T.; Yu, A.F.; Jones, L.W.; Liu, J.; Steingart, R.M.; Argolo, D.F.; Norton, L.; Hudis, C.A. Cardiac Surveillance Guidelines for Trastuzumab-Containing Therapy in Early-Stage Breast Cancer: Getting to the Heart of the Matter. J. Clin. Oncol. 2016, 34, 1030–1033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dent, S.; Fergusson, D.; Aseyev, O.; Stober, C.; Pond, G.; Awan, A.A.; McGee, S.F.; Ng, T.L.; Simos, D.; Vandermeer, L.; et al. A Randomized Trial Comparing 3- versus 4-Monthly Cardiac Monitoring in Patients Receiving Trastuzumab-Based Chemotherapy for Early Breast Cancer. Curr. Oncol. 2021, 28, 5073–5083. [Google Scholar] [CrossRef]
- Ewer, M.S.; Vooletich, M.T.; Durand, J.-B.; Woods, M.L.; Davis, J.R.; Valero, V.; Lenihan, D.J. Reversibility of Trastuzumab-Related Cardiotoxicity: New Insights Based on Clinical Course and Response to Medical Treatment. J. Clin. Oncol. 2005, 23, 7820–7826. [Google Scholar] [CrossRef]
- Earl, H.; Hiller, L.; Vallier, A.L.; Loi, S.; McAdam, K.; Hughes-Davies, L.; Rea, D.; Howe, D.; Raynes, K.; Higgins, H.B.; et al. Six Versus 12 Months’ Adjuvant Trastuzumab in Patients with HER2-Positive Early Breast Cancer: The PERSEPHONE Non-Inferiority RCT. Health Technol. Assess 2020, 24, 1–190. [Google Scholar] [CrossRef] [PubMed]
- Pivot, X.; Romieu, G.; Debled, M.; Pierga, J.-Y.; Kerbrat, P.; Bachelot, T.; Lortholary, A.; Espié, M.; Fumoleau, P.; Serin, D.; et al. 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): A randomised phase 3 trial. Lancet Oncol. 2013, 14, 741–748. [Google Scholar] [CrossRef]
| Trial Name, NCT Number and Sponsor | Main Eligibility Criteria/Study Population | Study Design | Intervention | Control |
|---|---|---|---|---|
| Decrescendo, NCT04675827, Jules Bordet Institute |
| Phase 2, open-label, multicenter, non-randomized, sequential assignment |
| N/A |
| CompassHER2-pCR, NCT04266249, ECOG-ACRIN Cancer Research Group |
| Phase 2, open-label, multicenter, non-randomized, parallel assignment |
| N/A |
| PHERGAIN-2, NCT04733118 MedSIR |
| Phase 2 open-label, single-group assignment |
| N/A |
| TRAIN-3 study, NCT03820063, Borstkanker Onderzoek Groep |
| Phase 2, multicenter, single-arm | Image-guided de-escalated neoadjuvant treatment | N/A |
| NCT04419181, University of Rochester |
| Phase 2, open-label, non-randomized, parallel assignment |
| |
| DAPHNe, NCT03716180, Dana-Farber Cancer Institute |
| Phase 1, Open-label, single-group assignment | Adjuvant trastuzumab and pertuzumab w/o further chemotherapy if pCR achieved | |
| IRIS-C/D, NCT04383275, Fudan University |
| Phase 2, open-label, single-group assignment | IRIS-C: Oral capecitabine for 4 cycles with standard trastuzumab for 1 yr IRIS-D: Oral vinorelbine for 4 cycles with standard trastuzumab for 1 yr | N/A |
| IRIS, NCT04383275, Fudan University |
| Phase 2, open-label, single-group assignment | IRIS-A: capecitabine for 6 cycles with standard trastuzumab for 1 yr IRIS-B: endocrine therapy combined with standard trastuzumab for 1 yr | |
| REaCT-HER-TIME, NCT04928261, Ottawa Hospital Research Institute |
| Phase 4, open-label, single-group assignment | Adjuvant trastuzumab for a total of 9 cycles every 3 weeks (or its equivalent if administered weekly), including the treatment received preoperatively | |
| REaCT-LOW RISK HER2, NCT03705429, Lawson Health Research Institute | Early-stage breast cancer for whom study regimens are being considered | Phase 3, open-label, multicenter, randomized | Docetaxel plus cyclophosphamide for 4 cycles and trastuzumab for 1 year | Weekly paclitaxel (× 12 weeks) and trastuzumab for 1 year |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McGee, S.F.; Clemons, M.; Savard, M.-F. Evolving Role of Risk Tailored Therapy in Early Stage HER2-Positive Breast Cancer: A Canadian Perspective. Curr. Oncol. 2022, 29, 4125-4137. https://doi.org/10.3390/curroncol29060329
McGee SF, Clemons M, Savard M-F. Evolving Role of Risk Tailored Therapy in Early Stage HER2-Positive Breast Cancer: A Canadian Perspective. Current Oncology. 2022; 29(6):4125-4137. https://doi.org/10.3390/curroncol29060329
Chicago/Turabian StyleMcGee, Sharon F., Mark Clemons, and Marie-France Savard. 2022. "Evolving Role of Risk Tailored Therapy in Early Stage HER2-Positive Breast Cancer: A Canadian Perspective" Current Oncology 29, no. 6: 4125-4137. https://doi.org/10.3390/curroncol29060329
APA StyleMcGee, S. F., Clemons, M., & Savard, M.-F. (2022). Evolving Role of Risk Tailored Therapy in Early Stage HER2-Positive Breast Cancer: A Canadian Perspective. Current Oncology, 29(6), 4125-4137. https://doi.org/10.3390/curroncol29060329





