Promising Therapeutic Impact of a Selective Estrogen Receptor Downregulator, Fulvestrant, as Demonstrated In Vitro upon Low-Grade Serous Ovarian Carcinoma Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
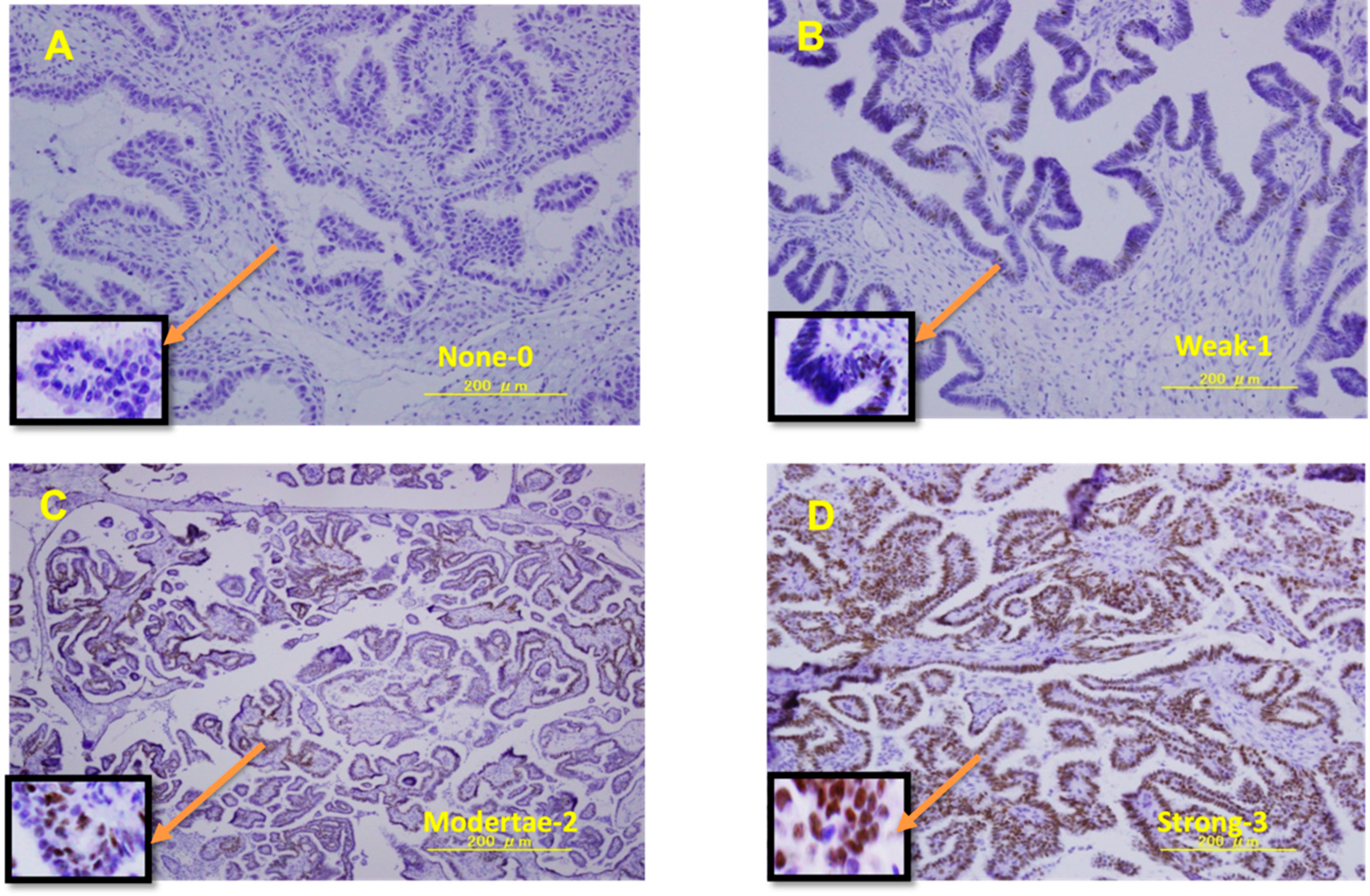
2.2. Immunohistochemistry
2.3. Cell Culture
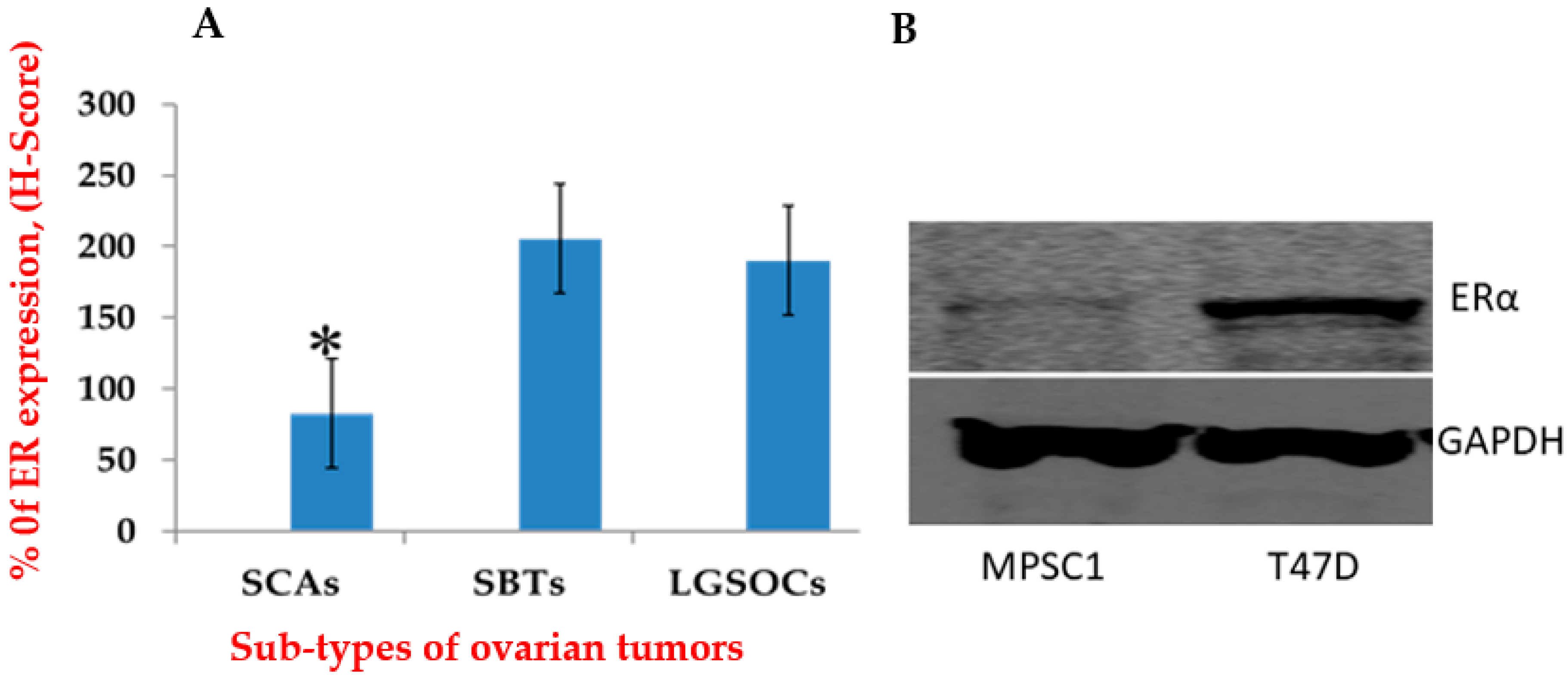
2.4. Western Blot Analysis
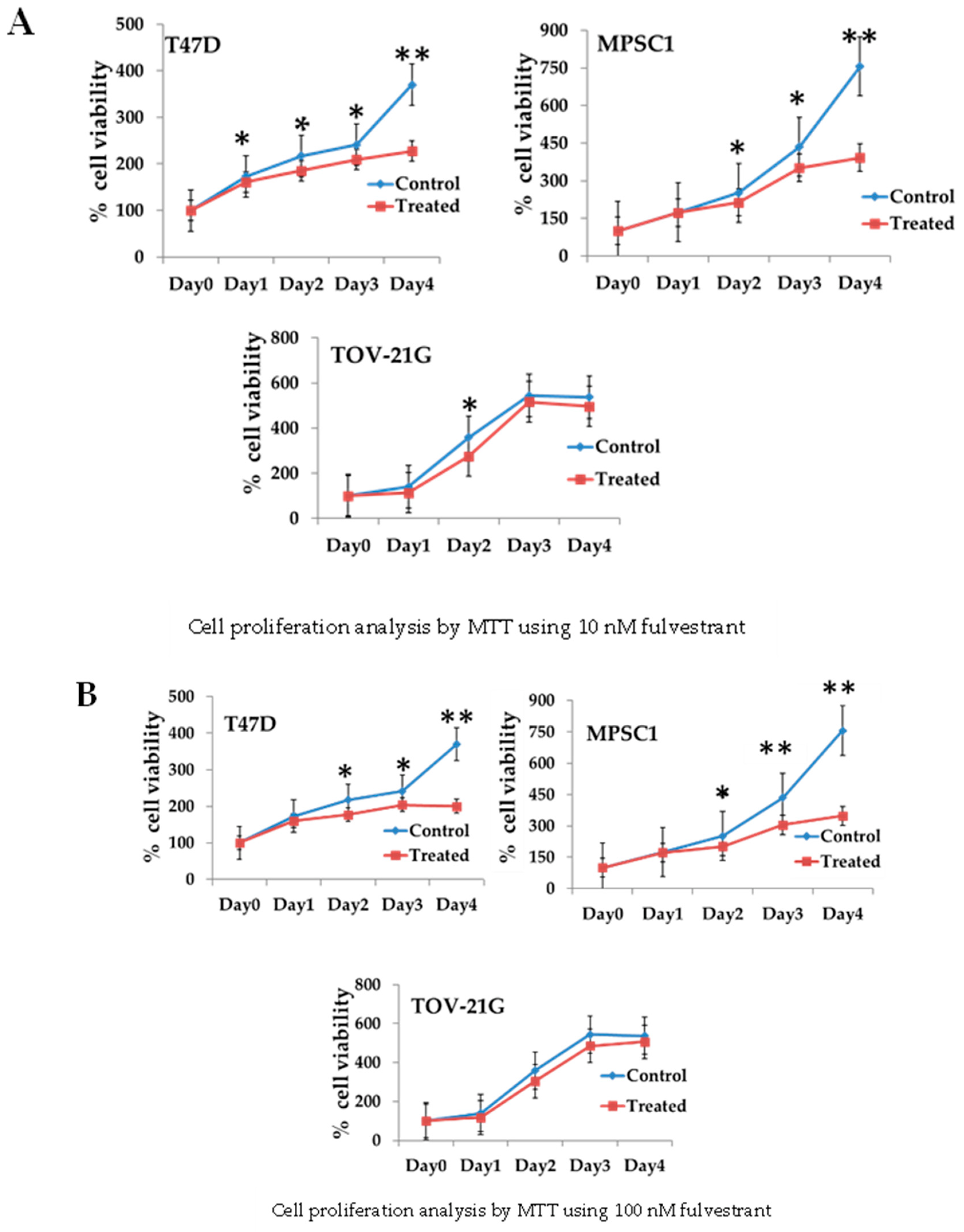
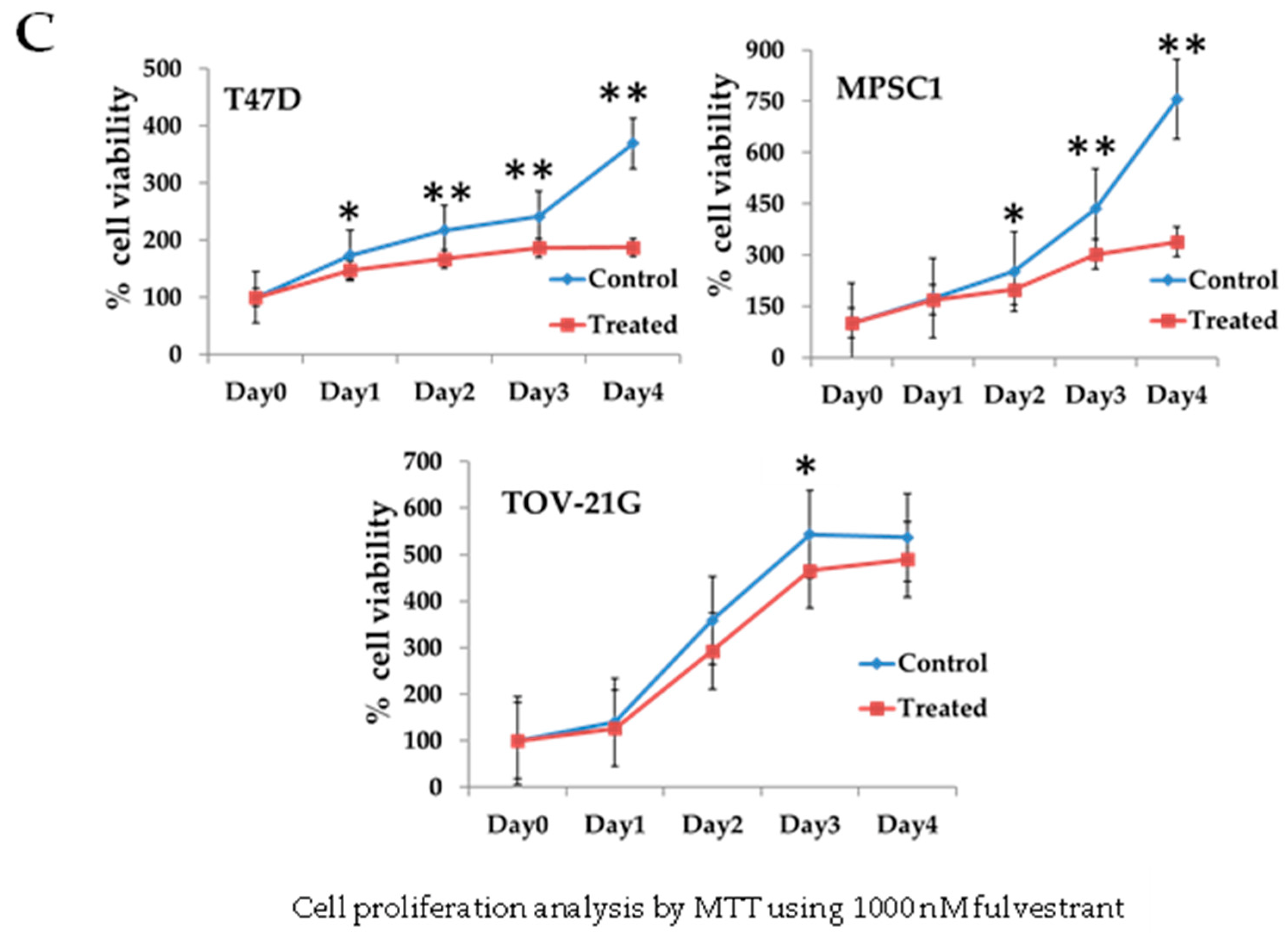
2.5. Cell Proliferation Assay
2.6. siRNA Transfection
2.7. Reverse Transcription Polymerase Chain Reaction
2.8. Statistcal Analysis
3. Results
3.1. ER Expression Was Higher in LGSOCs and SBTs Than in SCAs
3.2. ER Expressions in MPSC1
3.3. Regulation of Cell Growth Depends on ER Expression in MPSC1 Cells
3.4. Relationship between Different Oncogenic Mutations and Hormone Receptors
3.5. siRNA Knockdown of ERα Decreased ERα Expression in MPSC1 Cells as Shown by RT-PCR
3.6. ERα Knockdown Decreases Cell Proliferation in the MPSC1 Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Yang, J.; Jin, Y.; Cheng, S.; Wang, C.; Zhang, N.; Huang, S.; Zhao, Y.; Wang, Y. Clinical significance for combined coagulation indexes in epithelial ovarian cancer prognosis. J. Ovarian Res. 2021, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Hatano, Y.; Hatano, K.; Tamada, M.; Morishige, K.I.; Tomita, H.; Yanai, H.; Hara, A. A comprehensive review of ovarian serous carcinoma. Adv. Anat. Pathol. 2019, 26, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Padilla, I.; Malpica, A.L.; Minig, L.; Chiva, L.M.; Gershenson, D.M.; Gonzalez-Martin, A. Ovarian low-grade serous carcinoma: A comprehensive update. Gynecol. Oncol. 2012, 126, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Bodurka, D.C.; Deavers, M.T.; Tian, C.; Sun, C.C.; Malpica, A.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Birrer, M.J.; Ozols, R.; et al. Reclassification of Serous Ovarian Carcinoma by a 2-Tier System A Gynecologic Oncology Group Study. Cancer 2012, 118, 3087–3094. [Google Scholar] [CrossRef] [PubMed]
- Gilks, C.B.; Prat, J. Ovarian carcinoma pathology and genetics: Recent advances. Hum. Pathol. 2009, 40, 1213–1223. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Gershenson, D.M. Low-Grade Serous Ovarian Cancer: A Unique Disease. Curr. Oncol. Rep. 2008, 10, 519–523. [Google Scholar] [CrossRef]
- Escobar, J.; Klimowicz, A.C.; Dean, M.; Chu, P.; Nation, J.G.; Nelson, G.S.; Ghatage, P.; Kalloger, S.E.; Köbel, M. Gynecologic Oncology Quanti fi cation of ER/PR expression in ovarian low-grade serous carcinoma. Gynecol. Oncol. 2013, 128, 371–376. [Google Scholar] [CrossRef]
- Shen, F.; Zhang, X.; Zhang, Y.; Ding, J.; Chen, Q. Hormone receptors expression in ovarian cancer taking into account menopausal status: A retrospective study in Chinese population. Oncotarget 2017, 8, 84019–84027. [Google Scholar] [CrossRef]
- Arias-pulido, H.; Smith, H.O.; Joste, N.E.; Bocklage, T.; Qualls, R.; Chavez, A.; Prossnitz, E.R.; Verschraegen, C.F. Estrogen and Progesterone Receptor Status and Outcome in Epithelial Ovarian Cancers and Low Malignant Potential Tumors. Gynecol. Oncol. 2010, 114, 480–485. [Google Scholar] [CrossRef]
- Shvartsman, H.S.; Schmandt, R.E.; Thornton, A.D.; Ascp, H.T.; Deavers, M.T.; Silva, E.G.; Gershenson, D.M. Significantly Greater Expression of ER, PR, and ECAD in Advanced-Stage Low-Grade Ovarian Serous Carcinoma as Revealed by Immunohistochemical Analysis. Int. J. Gynecol. Pathol. 2007, 26, 404–409. [Google Scholar]
- Sinn, B.V.; Darb-esfahani, S.; Wirtz, R.M.; Budczies, J.; Sehouli, J.; Chekerov, R.; Dietel, M.; Denkert, C. Evaluation of a hormone receptor-positive ovarian carcinoma subtype with a favourable prognosis by determination of progesterone receptor and oestrogen receptor 1 mRNA expression in formalin-fixed paraffin-embedded tissue. Histopathology 2011, 59, 918–927. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Brunello, A.; Maruzzo, M.; Basso, U.; Basso, S.M.M. Treatment of Estrogen Receptor-Positive Breast Cancer. Curr. Med. Chem. 2013, 20, 596–604. [Google Scholar] [CrossRef]
- Patch, A.M.; Christie, E.L.; Etemadmoghadam, D.; Garsed, D.W.; George, J.; Fereday, S.; Nones, K.; Cowin, P.; Alsop, K.; Bailey, P.J.; et al. Whole-genome characterization of chemoresistant ovarian cancer. Nature 2015, 521, 489–494. [Google Scholar] [CrossRef]
- Wong, K.; Tsang, Y.T.M.; Deavers, M.T.; Mok, S.C.; Zu, Z.; Sun, C.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Gershenson, D.M. BRAF Mutation Is Rare in Advanced-Stage Low-Grade Ovarian Serous Carcinomas,”BRAF Mutation Is Rare in Advanced-Stage Low-Grade Ovarian Serous Carcinomas. Am. J. Pathol. 2010, 177, 1611–1617. [Google Scholar] [CrossRef]
- Ishibashi, T.; Nakayama, K.; Razia, S.; Ishikawa, M.; Nakamura, K.; Yamashita, H.; Dey, P.; Idia, K.; Kurioka, H.; Nakayama, S.; et al. High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics 2019, 10, 13. [Google Scholar] [CrossRef]
- Saal, L.H.; Holm, K.; Maurer, M.; Memeo, L.; Su, T.; Wang, X.; Mansukhani, M.; Enoksson, J.; Hibshoosh, H.; Yu, J.S.; et al. PIK3CA Mutations Correlate with Hormone Receptors, Node Metastasis, and ERBB2, and Are Mutually Exclusive with PTEN Loss in Human Breast Carcinoma. Cancer Res. 2005, 65, 2554–2560. [Google Scholar] [CrossRef]
- Banerji, S.; Cibulskis, K.; Rangel-escareno, C.; Brown, K.K.; Carter, S.L.; Frederick, A.M.; Lawrence, M.S.; Sivachenko, A.Y.; Sougnez, C.; Zou, L.; et al. Sequence analysis of mutations and translocations across breast cancer subtypes. Nature 2012, 486, 6–10. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network, G. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Isakoff, S.J.; Engelman, J.A.; Irie, H.Y.; Luo, J.; Brachmann, S.M.; Pearline, R.V.; Cantley, L.C.; Brugge, J.S. Breast cancer-associated PIK3CA mutations are oncogenic in mammary epithelial cells. Cancer Res. 2005, 65, 10992–11000. [Google Scholar] [CrossRef]
- Berns, K.; Horlings, H.M.; Hennessy, B.T.; Madiredjo, M.; Hijmans, E.M.; Beelen, K.; Linn, S.C.; Gonzalez-angulo, A.M.; Stemke-hale, K.; Hauptmann, M.; et al. A Functional Genetic Approach Identifies the PI3K Pathway as a Major Determinant of Trastuzumab Resistance in Breast Cancer. Cancer Cell 2007, 12, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Molpus, K.L.; Wu, H.; Fuller, A.F. CASE REPORT Recurrent Psammocarcinoma of the Peritoneum with Complete Response to Tamoxifen Therapy. Gynecol Oncol. 1998, 209, 206–209. [Google Scholar] [CrossRef] [PubMed]
- Crowder, R.J.; Phommaly, C.; Tao, Y.; Hoog, J.; Luo, J.; Perou, M.; Parker, J.S.; Miller, M.A.; Huntsman, D.G.; Lin, L.; et al. breast cancer. Cancer Res. 2010, 69, 3955–3962. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Nakayama, K.; Shanta, K.; Razia, S.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Sato, S.; Iida, K.; Kanno, K. Establishment of a Novel In Vitro Model of Endometriosis with Oncogenic KRAS and PIK3CA Mutations for Understanding the Underlying Biology and Molecular Pathogenesis. Cancers 2021, 13, 3174. [Google Scholar] [CrossRef]
- Ciucci, A.; Zannoni, G.F.; Buttarelli, M.; Lisi, L.; Martinelli, E.; Scambia, G.; Gallo, D. Multiple direct and indirect mechanisms drive estrogen-induced tumor growth in high grade serous ovarian cancers. Oncotarget 2016, 7, 8155–8171. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, D.; Okubo, T.; Kao, Y.C.; Yang, C. Breast tumor aromatase: Functional role and transcriptional regulation. Endocr. Relat. Cancer 1999, 6, 149–156. [Google Scholar] [CrossRef]
- Heo, S.; Kim, J.W.; Shin, S.; Jeong, S.; Lim, H.; Choi, Y.; Lee, K.; Kang, W.; Jeong, Y.; Kang, H. Review of Ovarian Tumors in Children and Adolescents: Radiologic-Pathologic Correlation. Radiographics 2014, 34, 2039–2055. [Google Scholar] [CrossRef]
- Terzic, M.; Rapisarda, A.M.C.; Della Corte, L.; Manchanda, R.; Aimagambetova, G.; Norton, M.; Garzon, S.; Riemma, G.; King, C.R.; Chiofalo, B.; et al. Diagnostic work-up in paediatric and adolescent patients with adnexal masses: An evidence-based approach. J. Obstet. Gynaecol. 2021, 41, 503–515. [Google Scholar] [CrossRef]
- Shabani, N.; Kuhn, C.; Kunze, S.; Schulze, S.; Mayr, D.; Dian, D.; Gingelmaier, A.; Schindlbeck, C.; Willgeroth, F.; Sommer, H.; et al. Prognostic significance of oestrogen receptor alpha (ERα) and beta (ERβ), progesterone receptor A (PR-A) and B (PR-B) in endometrial carcinomas. Eur. J. Cancer 2007, 43, 2434–2444. [Google Scholar] [CrossRef]
- Asgari, M.; Morakabati, A. Estrogen receptor beta expression in prostate adenocarcinoma. Diagn. Pathol. 2011, 6, 61. [Google Scholar] [CrossRef]
- Lee, P.; Rosen, D.G.; Zhu, C.; Silva, E.G.; Liu, J. Expression of progesterone receptor is a favorable prognostic marker in ovarian cancer. Gynecol. Oncol. 2005, 96, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Longacre, T.A.; McKenney, J.K.; Tazelaar, H.D.; Kempson, R.L.; Hendrickson, M.R. Ovarian Serous Tumors of Low Malignant Potential (Borderline Tumors). Am. J. Surg. Pathol. 2005, 29, 707–723. [Google Scholar] [CrossRef] [PubMed]
- Abu-Jawdeh, G.M.; Jacobs, T.W.; Niloff, J.; Cannistra, S.A. Estrogen receptor expression is a common feature of ovarian borderline tumors. Gynecol. Oncol. 1996, 60, 301–307. [Google Scholar] [CrossRef]
- Buttarelli, M.; Mascilini, F.; Zannoni, G.F.; Ciucci, A.; Martinelli, E.; Filippetti, F.; Scambia, G.; Ferrandina, G.; Gallo, D. Hormone receptor expression profile of low-grade serous ovarian cancers. Gynecol. Oncol. 2017, 145, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Chaudhri, R.A.; Hadadi, A.; Lobachev, K.S.; Schwartz, Z.; Boyan, B.D. Estrogen receptor-alpha 36 mediates the anti-apoptotic effect of estradiol in triple negative breast cancer cells via a membrane-associated mechanism. Public Health Nutr. 2014, 1843, 2796–2806. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Bodurka, D.C.; Lu, K.H.; Nathan, L.C.; Milojevic, L.; Wong, K.K.; Malpica, A.; Sun, C.C. Impact of age and primary disease site on outcome in women with low-grade serous carcinoma of the ovary or peritoneum: Results of a large single-institution registry of a rare tumor. J. Clin. Oncol. 2015, 33, 2675–2682. [Google Scholar] [CrossRef] [PubMed]
- Gershenson, D.M.; Sun, C.C.; Bodurka, D.; Coleman, R.L.; Lu, K.H.; Sood, A.K.; Deavers, M.; Malpica, A.L.; Kavanagh, J.J. Recurrent low-grade serous ovarian carcinoma is relatively chemoresistant. Gynecol. Oncol. 2009, 114, 48–52. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Lu, K.H.; Coleman, R.L.; Sood, A.K.; Malpica, A.; Deavers, M.T.; Silva, E.G.; Bodurka, D.C. Clinical behavior of stage II-IV low-grade serous carcinoma of the ovary. Obstet. Gynecol. 2006, 108, 361–368. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Sun, C.C.; Malpica, A.; Deavers, M.T.; Bodurka, D.C.; Gershenson, D.M. Low-grade serous primary peritoneal carcinoma. Gynecol. Oncol. 2011, 121, 482–486. [Google Scholar] [CrossRef]
- Schmeler, K.M.; Sun, C.C.; Bodurka, D.C.; Deavers, M.T.; Malpica, A.; Coleman, R.L.; Ramirez, P.T.; Gershenson, D.M. Neoadjuvant chemotherapy for low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2008, 108, 510–514. [Google Scholar] [CrossRef]
- Grabowski, J.P.; Harter, P.; Heitz, F.; Pujade-Lauraine, E.; Reuss, A.; Kristensen, G.; Ray-Coquard, I.; Heitz, J.; Traut, A.; Pfisterer, J.; et al. Operability and chemotherapy responsiveness in advanced low-grade serous ovarian cancer. An analysis of the AGO Study Group metadatabase. Gynecol. Oncol. 2016, 140, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Fader, A.N.; Java, J.; Krivak, T.C.; Bristow, R.E.; Tergas, A.I.; Bookman, M.A.; Armstrong, D.K.; Tanner, E.J.; Gershenson, D.M. The prognostic significance of pre- and post-treatment CA-125 in grade 1 serous ovarian carcinoma: A Gynecologic Oncology Group study. Gynecol. Oncol. 2014, 132, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.F.; Brady, M.F.; Matulonis, U.A.; Miller, A.; Kohn, E.C.; Swisher, E.M.; Cella, D.; Tew, W.P.; Cloven, N.G.; Muller, C.Y.; et al. Olaparib with or without Cediranib Versus Platinum-Based Chemotherapy in Recurrent Platinum-Sensitive Ovarian Cancer (NRG-GY004): A Randomized, Open-Label, Phase III Trial. J. Clin. Oncol. 2022, JCO-21. [Google Scholar] [CrossRef] [PubMed]
- Cianci, S.; Riemma, G.; Ronsini, C.; De Franciscis, P.; Torella, M.; Schiattarella, A.; La Verde, M.; Colacurci, N. Hyperthermic intraperitoneal chemotherapy (HIPEC) for ovarian cancer recurrence: Systematic review and meta-analysis. Gland Surg. 2020, 9, 1140–1148. [Google Scholar] [CrossRef]
- Fader, A.N.; Bergstrom, J.; Jernigan, A.; Tanner, E.J.; Roche, K.L.; Stone, R.L.; Levinson, K.L.; Ricci, S.; Wethingon, S.; Wang, T.L.; et al. Primary cytoreductive surgery and adjuvant hormonal monotherapy in women with advanced low-grade serous ovarian carcinoma: Reducing overtreatment without compromising survival? Gynecol. Oncol. 2017, 147, 85–91. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, R.; El Jamal, L.; El Iskandarani, S.; Kort, J.; Abdel Salam, M.; Assi, H. Endocrine and Targeted Therapy for Hormone-Receptor-Positive, HER2-Negative Advanced Breast Cancer: Insights to Sequencing Treatment and Overcoming Resistance Based on Clinical Trials. Front. Oncol. 2019, 9, 510. [Google Scholar] [CrossRef]
- Gershenson, D.M.; Sun, C.C.; Iyer, R.B.; Malpica, A.L.; Kavanagh, J.J.; Bodurka, D.C.; Schmeler, K.; Deavers, M. Hormonal therapy for recurrent low-grade serous carcinoma of the ovary or peritoneum. Gynecol. Oncol. 2012, 125, 661–666. [Google Scholar] [CrossRef]
- Zhou, B.; Sun, Q.; Cong, R.; Gu, H.; Tang, N.; Yang, L.; Wang, B. Hormone replacement therapy and ovarian cancer risk: A meta-analysis. Gynecol. Oncol. 2008, 108, 641–651. [Google Scholar] [CrossRef]
- Silva, E.G.; Tornos, C.; Deavers, M.; Kaisman, K.; Gray, K.; Gershenson, D. Induction of epithelial neoplasms in the ovaries of guinea pigs by estrogenic stimulation. Gynecol. Oncol. 1998, 71, 240–246. [Google Scholar] [CrossRef]
- Neven, P.; Paridaens, R.; Pelgrims, G.; Martens, M.; Bols, A.; Goeminne, J.C.; Vindevoghel, A.; Demol, J.; Stragier, B.; De Greve, J.; et al. Fulvestrant (FaslodexTM) in advanced breast cancer: Clinical experience from a Belgian cooperative study. Breast Cancer Res. Treat. 2008, 109, 59–65. [Google Scholar] [CrossRef][Green Version]
- Howell, A.; Robertson, J.F.R.; Albano, J.Q.; Aschermannova, A.; Mauriac, L.; Kleeberg, U.R.; Vergote, I.; Erikstein, B.; Webster, A.; Morris, C. Fulvestrant, formerly ICI 182,780, is as effective as anastrozole in postmenopausal women with advanced breast cancer progressing after prior endocrine treatment. J. Clin. Oncol. 2002, 20, 3396–3403. [Google Scholar] [CrossRef]
- Osborne, C.K.; Pippen, J.; Jones, S.E.; Parker, L.M.; Ellis, M.; Come, S.; Gertler, S.Z.; May, J.T.; Burton, G.; Dimery, I.; et al. Double-blind, randomized trial comparing the efficacy and tolerability of fulvestrant versus anastrozole in postmenopausal women with advanced breast cancer progressing on prior endocrine therapy: Results of a North American trial. J. Clin. Oncol. 2002, 20, 3386–3395. [Google Scholar] [CrossRef] [PubMed]
- Mishra, A.K.; Abrahamsson, A.; Dabrosin, C. Fulvestrant inhibits growth of triple negative breast cancer and synergizes with tamoxifen in ERα positive breast cancer by up-regulation of ERβ. Oncotarget 2016, 7, 56876–56888. [Google Scholar] [CrossRef]
- Nukatsuka, M.; Saito, H.; Noguchi, S.; Takechi, T. Estrogen down-regulator fulvestrant potentiates antitumor activity of fluoropyrimidine in estrogen-responsive MCF-7 human breast cancer cells. In Vivo 2019, 33, 1439–1445. [Google Scholar] [CrossRef]
- Loi, S.; Haibe-Kains, B.; Majjaj, S.; Lallemand, F.; Durbecq, V.; Larsimont, D.; Gonzalez-Angulo, A.M.; Pusztai, L.; Symmans, W.F.; Bardelli, A.; et al. PIK3CA mutations associated with gene signature of low mTORC1 signaling and better outcomes in estrogen receptor-positive breast cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 10208–10213. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Tenorio, G.; Stål, O.; Arnesson, L.G.; Malmström, A.; Nordenskjöld, B.; Nordenskjöld, K.; Bång, H.; Källström, A.C.; Einarsson, E.; Norberg, B.; et al. Activation of Akt/PKB in breast cancer predicts a worse outcome among endocrine treated patients. Br. J. Cancer 2002, 86, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Toral-Barza, L.; Discafani, C.; Zhang, W.G.; Skotnicki, J.; Frost, P.; Gibbons, J.J. mTOR, a novel target in breast cancer: The effect of CCI-779, an mTOR inhibitor, in preclinical models of breast cancer. Endocr. Relat. Cancer 2001, 8, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Blancas, I.; Olier, C.; Conde, V.; Bayo, J.L.; Herrero, C.; Zarcos-Pedrinaci, I.; Carabantes, F.; Baena-Cañada, J.M.; Cruz, J.; Ruiz-Borrego, M. Real-world data of fulvestrant as first-line treatment of postmenopausal women with estrogen receptor-positive metastatic breast cancer. Sci. Rep. 2021, 11, 4274. [Google Scholar] [CrossRef]
- Bedard, P.L.; Freedman, O.C.; Howell, A.; Clemons, M. Overcoming endocrine resistance in breast cancer—Are signal transduction inhibitors the answer? Breast Cancer Res. Treat. 2008, 108, 307–317. [Google Scholar] [CrossRef]
- Breast International Group (BIG) 1-98 Collaborative Group; Thürlimann, B.; Keshaviah, A.; Coates, A.S.; Mouridsen, H.; Mauriac, L.; Forbes, J.F.; Paridaens, R.; Castiglione-Gertsch, M.; Gelber, R.D.; et al. A comparison of letrozole and tamoxifen in postmenopausal women with early breast cancer. N. Engl. J. Med. 2005, 353, 2747–2757. [Google Scholar]
- Argenta, P.A.; Thomas, S.G.; Judson, P.L.; Downs, L.S.; Geller, M.A.; Carson, L.F.; Jonson, A.L.; Ghebre, R. A phase II study of fulvestrant in the treatment of multiply-recurrent epithelial ovarian cancer. Gynecol. Oncol. 2009, 113, 205–209. [Google Scholar] [CrossRef] [PubMed]
| No | Age | FIGO Stage | ER Expression | PIK3CA E9 | PIK3CA E20 | ERBB2 | BRAF |
|---|---|---|---|---|---|---|---|
| 1 | 37 | Ⅲc | + | G1633C (E545Q) | WT | WT | WT |
| 2 | 61 | Ⅰc | + | WT | WT | A2384G (Q795R) | WT |
| 3 | 61 | IVb | + | G1633A (E545K) | WT | WT | WT |
| 4 | 83 | Ⅰa | + | A1634C (E545A) | WT | WT | WT |
| 5 | 27 | Ⅰc | + | A1634C (E545A) | WT | A2384G (Q795R) | T1796A (V600E) |
| 6 | 61 | Ⅲc | - | WT | WT | WT | WT |
| 7 | 40 | Ⅰc | + | A1634C (E545A) | WT | WT | T1796A (V600E) |
| 8 | 48 | Ⅰc | - | WT | WT | WT | WT |
| 9 | 26 | Ⅲc | + | G1633C (E545Q) | WT | WT | WT |
| 10 | 37 | IIc | - | WT | WT | A2384G (Q795R) | WT |
| Factors | ER-Positive | ER-Negative | p Value |
|---|---|---|---|
| n = 7 | n = 3 | ||
| PIK3CA | |||
| WT | 1 | 3 | 0.0113 |
| MT | 6 | 0 | |
| BRAF | |||
| WT | 5 | 3 | 0.300 |
| MT | 2 | 0 | |
| ERBB | |||
| WT | 6 | 1 | 0.097 |
| MT | 1 | 2 | |
| Age | |||
| <60 | 5 | 1 | 0.259 |
| >60 | 2 | 2 | |
| FIGO stage | |||
| I | 4 | 1 | 0.491 |
| II, III, IV | 3 | 2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shanta, K.; Nakayama, K.; Hossain, M.M.; Razia, S.; Ishibashi, T.; Ishikawa, M.; Yamashita, H.; Kanno, K.; Sato, S.; Nakayama, S.; et al. Promising Therapeutic Impact of a Selective Estrogen Receptor Downregulator, Fulvestrant, as Demonstrated In Vitro upon Low-Grade Serous Ovarian Carcinoma Cell Lines. Curr. Oncol. 2022, 29, 4020-4033. https://doi.org/10.3390/curroncol29060321
Shanta K, Nakayama K, Hossain MM, Razia S, Ishibashi T, Ishikawa M, Yamashita H, Kanno K, Sato S, Nakayama S, et al. Promising Therapeutic Impact of a Selective Estrogen Receptor Downregulator, Fulvestrant, as Demonstrated In Vitro upon Low-Grade Serous Ovarian Carcinoma Cell Lines. Current Oncology. 2022; 29(6):4020-4033. https://doi.org/10.3390/curroncol29060321
Chicago/Turabian StyleShanta, Kamrunnahar, Kentaro Nakayama, Mohammad Mahmud Hossain, Sultana Razia, Tomoka Ishibashi, Masako Ishikawa, Hitomi Yamashita, Kosuke Kanno, Seiya Sato, Satoru Nakayama, and et al. 2022. "Promising Therapeutic Impact of a Selective Estrogen Receptor Downregulator, Fulvestrant, as Demonstrated In Vitro upon Low-Grade Serous Ovarian Carcinoma Cell Lines" Current Oncology 29, no. 6: 4020-4033. https://doi.org/10.3390/curroncol29060321
APA StyleShanta, K., Nakayama, K., Hossain, M. M., Razia, S., Ishibashi, T., Ishikawa, M., Yamashita, H., Kanno, K., Sato, S., Nakayama, S., Otsuki, Y., & Kyo, S. (2022). Promising Therapeutic Impact of a Selective Estrogen Receptor Downregulator, Fulvestrant, as Demonstrated In Vitro upon Low-Grade Serous Ovarian Carcinoma Cell Lines. Current Oncology, 29(6), 4020-4033. https://doi.org/10.3390/curroncol29060321





