Retrospective Analysis of the Development of Human Thyroglobulin during Pregnancy in Patients with Treated Non-Recurrent Differentiated Thyroid Cancer
Abstract
1. Introduction
2. Materials and Methods
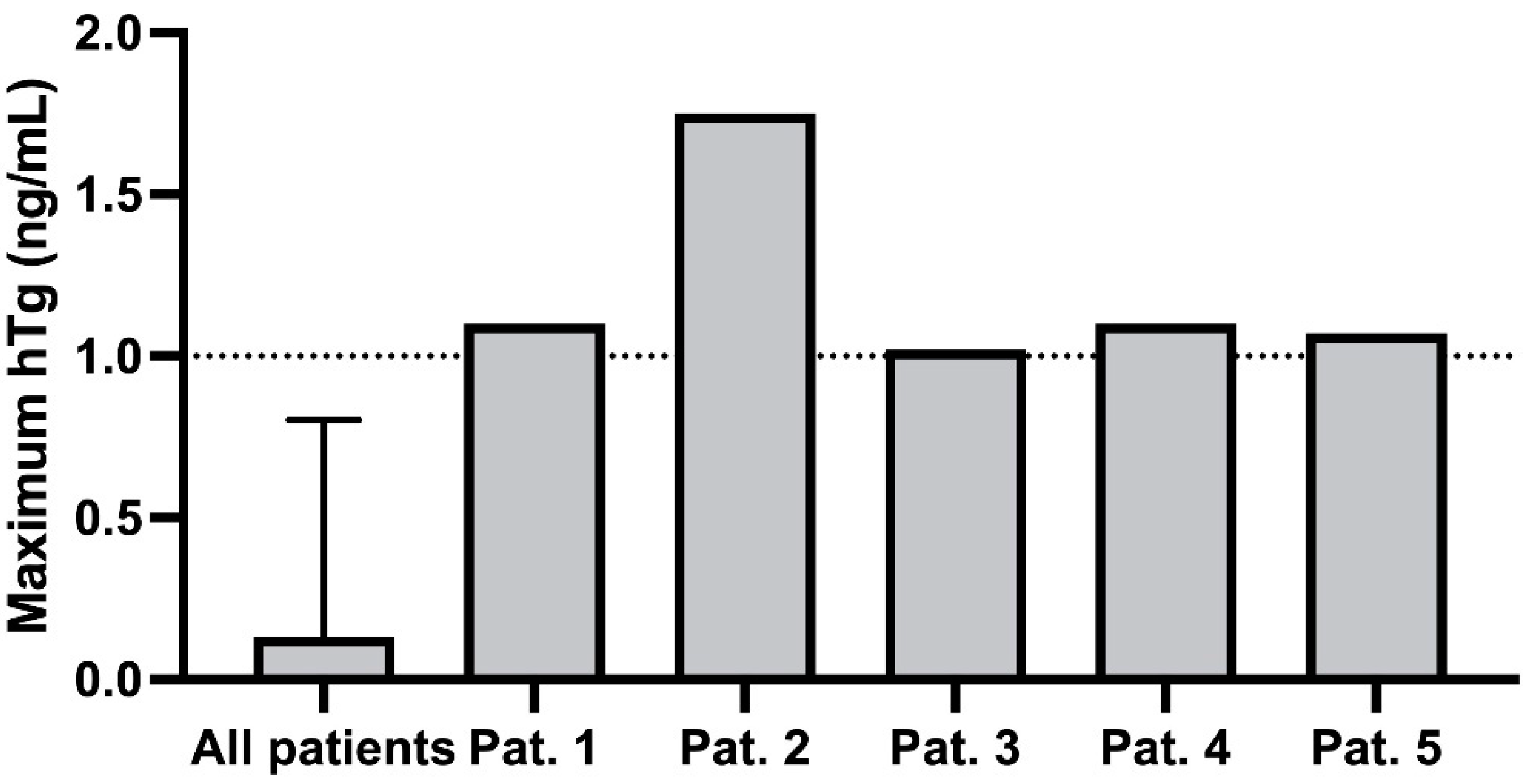
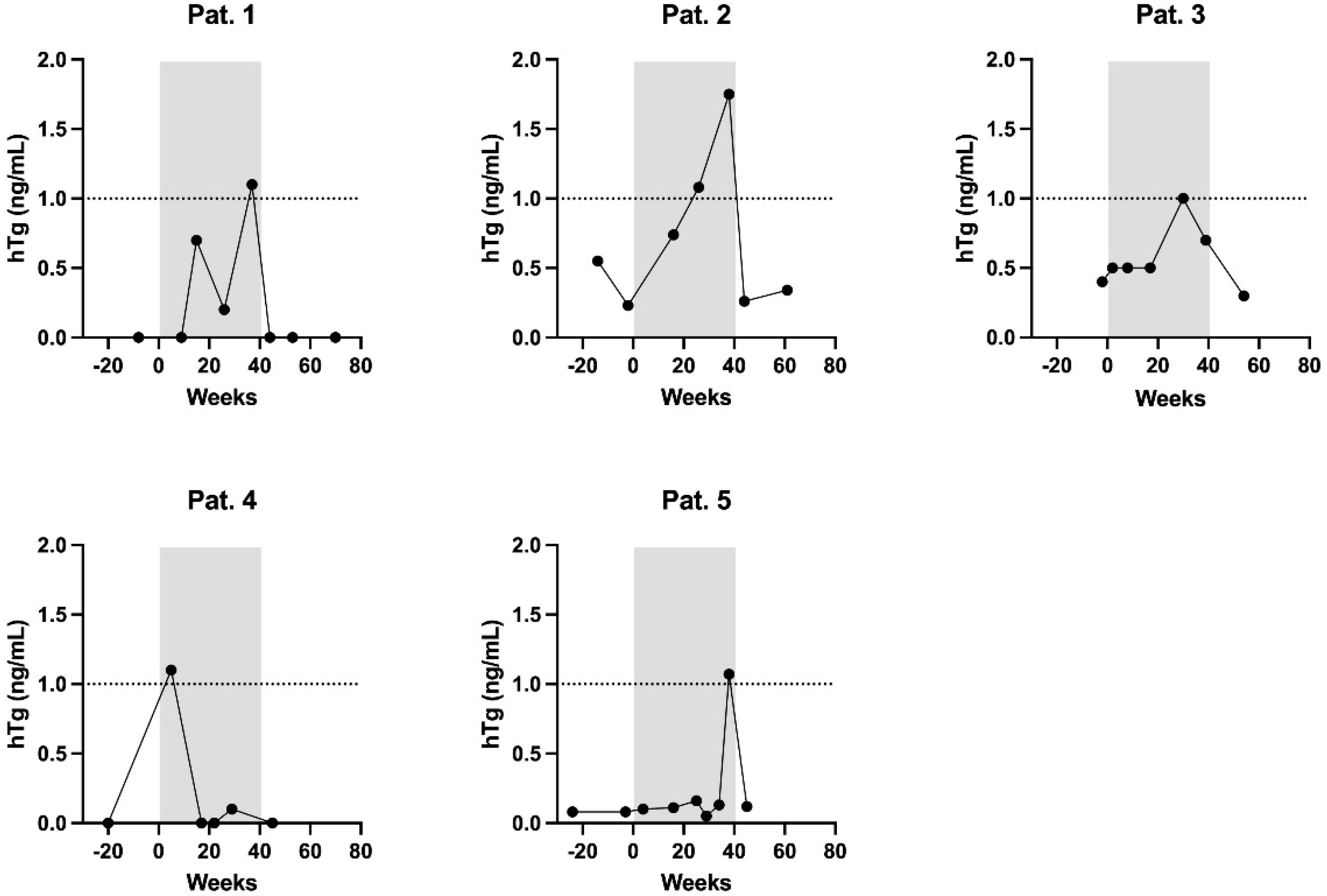
3. Results
4. Discussion
5. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dietlein, M.; Eschner, W.; Grünwald, F.; Lassmann, M.; Verburg, F.A.; Luster, M. Procedure guidelines for radioiodine therapy of differentiated thyroid cancer (Version 4). Nuklearmedizin 2016, 55, 77–89. [Google Scholar] [PubMed]
- Happel, C.; Kranert, W.T.; Gröner, D.; Bockisch, B.; Sabet, A.; Vardarli, I.; Görges, R.; Herrmann, K.; Grünwald, F. Correction for hyperfunctioning radiation-induced stunning (CHRIS) in benign thyroid diseases. Endocrine 2020, 69, 466–473. [Google Scholar] [CrossRef] [PubMed]
- Happel, C.; Kranert, W.T.; Gröner, D.; Baumgarten, J.; Halstenberg, J.; Bockisch, B.; Sabet, A.; Grünwald, F. Focus on radioiodine-131 biokinetics: The influence of methylprednisolone on intratherapeutic effective half-life of 131I during radioiodine therapy of Graves’ disease. Endocrine 2021, 73, 125–130. [Google Scholar] [CrossRef] [PubMed]
- McCready, V.R. Radioiodine—The success story of Nuclear Medicine: 75th Anniversary of the first use of Iodine-131 in humans. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 179–182. [Google Scholar] [CrossRef]
- Verburg, F.A.; Schmidt, M.; Kreissl, M.C.; Grünwald, F.; Lassmann, M.; Hänscheid, H.; Hohberg, M.; Luster, M.; Dietlein, M. Procedural guideline for Iodine-131 whole-body scintigraphy in differentiated thyroid carcinoma (version 5). Nuklearmedizin 2019, 58, 228–241. [Google Scholar]
- Qu, M.; Wan, S.; Ren, B.; Wu, H.; Liu, L.; Shen, H. Association between TSHR gene methylation and papillary thyroid cancer: A meta-analysis. Endocrine 2020, 69, 508–515. [Google Scholar] [CrossRef]
- Albano, D.; Bonacina, M.; Durmo, R.; Bertagna, F.; Giubbini, R. Efficacy of low radioiodine activity versus intermediate-high activity in the ablation of low-risk differentiated thyroid cancer. Endocrine 2020, 68, 124–131. [Google Scholar] [CrossRef]
- Haugen, B.; Alexander, E.; Bible, K.C.; Doherty, G.M.; Mandel, S.J.; Nikiforov, Y.E.; Schuff, K.G. 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 2016, 26, 1–133. [Google Scholar] [CrossRef]
- Dietlein, M.; Dressler, J.; Grünwald, F.; Joseph, K.; Leisner, B.; Moser, E.; Reiners, C.; Rendl, J.; Schicha, H.; Schneider, P.; et al. Leitlinien zur Schilddrüsendiagnostik. Nuklearmedizin 2003, 42, 109–115. [Google Scholar] [CrossRef]
- Spencer, C.A.; LoPresti, J.S.; Fatemi, S.; Nicoloff, J.T. Detection of residual and recurrent differentiated thyroid carcinoma by serum thyroglobulin measurement. Thyroid 1999, 9, 435–441. [Google Scholar] [CrossRef]
- Spencer, C.A. Challenges of serum thyroglobulin (Tg) measurement in the presence of Tg autoantibodies. J. Clin. Endocrinol. Metab. 2004, 89, 3702–3704. [Google Scholar] [CrossRef] [PubMed]
- IASON GmbH. RIASON Tg c.t. Immunoradioassay Kit Instruction. Available online: https://iason.eu/content/uploads/2021/02/R01-020-100X-RIASON-anti-hTg-c.t.pdf (accessed on 6 March 2022).
- Alexander, E.K.; Pearce, E.N.; Brent, G.A.; Brown, R.S.; Chen, H.; Dosiou, C.; Grobman, W.A.; Laurberg, P.; Lazarus, J.H.; Mandel, S.J.; et al. 2017 Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease During Pregnancy and the Postpartum. Thyroid 2017, 27, 315–389. [Google Scholar] [CrossRef] [PubMed]
- Staudt, J.; Happel, C.; Middendorp, M.; Grünwald, F. Nachsorge des Schilddrüsenkarzinoms in der Schwangerschaft: hTg-Verlauf. Nuklearmedizin 2010, 49, N38–N40. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.S.; Kim, W.G.; Park, S.; Kim, M.; Kwon, H.; Jeon, M.J.; Lee, J.H.; Baek, J.H.; Song, D.E.; Kim, T.Y.; et al. Serial neck ultrasonographic evaluation of changes in papillary thyroid carcinoma during pregnancy. Thyroid 2017, 27, 773–777. [Google Scholar] [CrossRef]
- Murray, J.; Williams, G.; Harrington, K.; Newbold, K.; Nutting, C. Rising Thyroglobulin Tumour Marker During Pregnacy in a Thyroid Cancer Patient: No Cause for Alarm? Clin. Endocrinol. 2012, 77, 155–157. [Google Scholar] [CrossRef]
- Pomorski, L.; Bartos, M.; Narebski, J. Pregnancy following operative and complementary treatment of thyroid cancer. Zentralbl. Gynakol. 2000, 122, 383–386. [Google Scholar]
- Rosvoll, R.V.; Winship, T. Thyroid carcinoma and pregnancy. Surg. Gynecol. Obstet. 1965, 121, 1039–1042. [Google Scholar]
- Hill, C.S.; Clark, R.L.; Wolf, M. The effect of subsequent pregnancy on thyroid cancer. Surg. Gynecol. Obstet. 1966, 122, 1219–1222. [Google Scholar]
- Leboeuf, R.; Emerick, L.E.; Martorella, A.J.; Tuttle, R.M. Impact of pregnancy on serum thyroglobulin an detection of recurrent disease shortly after delivery in thyroid cancer survivors. Thyroid 1993, 17, 543–547. [Google Scholar] [CrossRef]
- Tyndall, C.; Cuzzilla, R.; Kane, S.C. The rhesus incompatible pregnancy and its consequences for affected fetuses and neonates. Transfus. Apher. Sci. 2020, 59, 102948. [Google Scholar] [CrossRef]
- Szinnai, G.; Lacroix, L.; Carre, A.; Guimiot, F.; Talbot, M.; Martinovic, J.; Delezoide, A.L.; Vekemans, M.; Michiels, S.; Caillou, B.; et al. Sodium/iodide symporter (NIS) gene expression is the limiting step for the onset of thyroid function in the human fetus. J. Clin. Endocrinol. Metab. 2007, 92, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Sekizawa, A.; Yokokawa, K.; Sugito, Y.; Iwasaki, M.; Yukimoto, Y.; Ichizuka, K.; Saito, H.; Okai, T. Evaluation of bidirectional transfer of plasma DNA through placenta. Hum. Genet. 2003, 113, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Breveglieri, G.; D’Aversa, E.; Finotti, A.; Borgatti, M. Non-invasive prenatal testing using fetal DNA. Mol. Diagnost. Therap. 2019, 23, 291–299. [Google Scholar] [CrossRef]
- Ben-Rafael, Z.; Struass, J.F.; Arendash-Durand, B.; Mastroianni, L.; Flickinger, G.L. Changes in thyroid function tests and sex hormone binding globulin associated with treatment by gonadotropin. Fertil. Steril. 1987, 48, 318–320. [Google Scholar] [CrossRef]
- Cacciatore, B.; Tiitinen, A.; Stenman, U.H.; Ylöstalo, P. Normal early pregnancy: Serum hCG levels and vaginal ultrasonography findings. Brit. J. Obstetr. Gynaecol. 1990, 97, 899–903. [Google Scholar] [CrossRef]
- Kimura, M.; Amino, N.; Tamaki, H.; Mitsuda, N.; Miyai, K.; Tanizawa, O. Physiologic thyroid activation in normal early pregnancy is induced by circulating hCG. Obstet. Gynecol. 1990, 75, 775–778. [Google Scholar]
- Glinoer, D.; de Nayer, P.; Robyn, C.; Lejeune, B.; Kinthaert, J.; Meuris, S. Serum levels of intact chorionic gonadortropin (HCG) an its free alpha and beta subunits, in relation to maternal thyroid stimulation during normal pregnancy. J. Endocrinol. Investig. 1993, 16, 881–888. [Google Scholar] [CrossRef]
- Kennedy, R.L.; Darne, J. The role of hCG in regulation of the thyroid gland in normal an abnormal pregnancy. Obstet. Gynecol. 1991, 78, 298–307. [Google Scholar]
| Variable | |
|---|---|
| Age at time of diagnosis (years) | 24 (14–37) |
| Age at time of conception (years) | 31 (25–41) |
| Time to conception after last RIT (months) | 50 (12–190) |
| Time to conception after last negative stimulated hTg (months) | 21 (6–142) |
| Histological tumor type | |
| Papillary carcinoma | 45 (96) |
| Follicular carcinoma | 2 (4) |
| Risk stratification according to ATA guidelines | |
| Low Risk | 11 (23) |
| High Risk | 35 (75) |
| Unknown | 1 (2) |
| Patient | hTg [ng/mL] | Tg-AB [U/mL] | hTg Recovery [%] | TSH [mIU/L | Week of Pregnancy |
|---|---|---|---|---|---|
| 1 | 0 | 2 | 98 | 17.44 | 9 |
| 0.7 | 29 | 98 | 4.25 | 15 | |
| 0.2 | 11 | 102 | 1.66 | 26 | |
| 1.1 | 30 | 127 | 1.29 | 37 | |
| 2 | 0.74 | 2247 | 89 | 0.20 | 16 |
| 1.08 | 1670 | 96 | 0.05 | 26 | |
| 1.75 | 1022 | 96 | 0.03 | 38 | |
| 3 | 0.5 | - | 104 | 1.41 | 8 |
| 0.5 | 41 | 96 | 12.44 | 17 | |
| 1 | - | 106 | 3.09 | 30 | |
| 0.7 | - | 103 | 1.60 | 39 | |
| 4 | 1.1 | 16 | 94 | 0.04 | 9 |
| 0 | 33 | 97 | 0.05 | 17 | |
| 0 | 44 | 136 | 0.43 | 22 | |
| 0.1 | 45 | 100 | 1.93 | 29 | |
| 5 | 0.1 | 5 | 106 | 0.31 | 4 |
| 0.11 | 7 | 100 | 0.42 | 16 | |
| 0.16 | 15 | 98 | 0.24 | 25 | |
| 0.05 | 5 | 103 | 0.11 | 29 | |
| 0.13 | 12 | 102 | 0.12 | 33 | |
| 1.07 | 8 | 98 | 1.68 | 38 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baumgarten, J.; Happel, C.; Groener, D.; Staudt, J.; Bockisch, B.; Sabet, A.; Grünwald, F.; Rink, T. Retrospective Analysis of the Development of Human Thyroglobulin during Pregnancy in Patients with Treated Non-Recurrent Differentiated Thyroid Cancer. Curr. Oncol. 2022, 29, 4012-4019. https://doi.org/10.3390/curroncol29060320
Baumgarten J, Happel C, Groener D, Staudt J, Bockisch B, Sabet A, Grünwald F, Rink T. Retrospective Analysis of the Development of Human Thyroglobulin during Pregnancy in Patients with Treated Non-Recurrent Differentiated Thyroid Cancer. Current Oncology. 2022; 29(6):4012-4019. https://doi.org/10.3390/curroncol29060320
Chicago/Turabian StyleBaumgarten, Justus, Christian Happel, Daniel Groener, Jennifer Staudt, Benjamin Bockisch, Amir Sabet, Frank Grünwald, and Thomas Rink. 2022. "Retrospective Analysis of the Development of Human Thyroglobulin during Pregnancy in Patients with Treated Non-Recurrent Differentiated Thyroid Cancer" Current Oncology 29, no. 6: 4012-4019. https://doi.org/10.3390/curroncol29060320
APA StyleBaumgarten, J., Happel, C., Groener, D., Staudt, J., Bockisch, B., Sabet, A., Grünwald, F., & Rink, T. (2022). Retrospective Analysis of the Development of Human Thyroglobulin during Pregnancy in Patients with Treated Non-Recurrent Differentiated Thyroid Cancer. Current Oncology, 29(6), 4012-4019. https://doi.org/10.3390/curroncol29060320





