Abstract
Treatment with bevacizumab is known to cause adverse events such as proteinuria and hypertension, amongst others. However, while bevacizumab-induced hypertension has been linked to increased overall survival (OS), data on proteinuria are controversial. We performed a retrospective analysis to observe the influence of adverse events developed during treatment with bevacizumab and chemotherapy on the OS in patients with metastatic colorectal cancer (mCRC). Kaplan–Meier and log-rank analyses were used to assess differences in OS, and hazard ratios (HR) were estimated using Cox models. Out of the 3497 mCRC patients admitted to our center between 2014 and 2019, 150 met the criteria for inclusion in our analysis. Out of these, 50.7% experienced proteinuria and had reached a longer OS (40 versus 25 months, p = 0.015) and progression-free survival (15 versus 12 months, p = 0.039). The following groups were identified as having a lower risk of death: patients with proteinuria (HR 0.589; 95% CI 0.402–0.863; p = 0.007), one metastatic site (HR 0.533; 95% CI 0.363–0.783; p = 0.001), and non-metastatic stage at diagnosis (HR 0.459; 95% CI 0.293–0.720; p = 0.001). Patients with anemia and diabetes had an increased risk of death. Proteinuria emerges as a useful prognostic factor in mCRC patients undergoing bevacizumab-based systemic therapy, and it could be easily integrated into the decision-making process, thus allowing physicians to further individualize systemic treatments.
1. Introduction
Colorectal cancer (CRC) has an increased incidence in both men and women [1]. If diagnosed at an early stage, it is associated with a good prognosis [2]. However, 20–25% of patients already have metastases at the time of diagnosis and about half of those diagnosed at an early stage will eventually develop metastatic disease [3]. Surgery and fluoropyrimidine-based chemotherapy continue to represent the treatment backbone of CRC, but the advent of molecular-targeted therapies has changed the treatment landscape and greatly influenced the prognosis of metastatic disease over the last 15 years [4].
One of the major targets of the biological therapies is the cell proliferation pathway, which in CRC depends on Epidermal Growth Factor Receptor signaling. Monoclonal antibodies such as cetuximab or panitumumab have been successfully used, in conjunction with chemotherapy, for the treatment of patients not harboring mutations in the RAS oncogenes (i.e., wild-type KRAS and NRAS). Moreover, the BRAF mutations such as V600E or V600K have shown prognostic but not predictive significance for this group of patients in various studies [5,6].
Angiogenesis has an important role in tumor proliferation and metastasis. Vascular Endothelial Growth Factor (VEGF) is a key mediator of this process, and, as such, it is also a major target for many biological therapies. Inhibition of angiogenesis has become the standard approach in certain types of cancers such as colorectal, bronchopulmonary, ovarian, renal, breast, and cervical cancer [7,8,9]. However, despite extensive research, one of the major drawbacks of antiangiogenic therapies continues to be the lack of predictive biomarkers.
A current global issue is the cost of anticancer drugs, for which more than USD 100 billion is spent annually worldwide [10]. The cost-effectiveness ratio of bevacizumab for mCRC is USD 571.240 per quality-adjusted life years in the first-line setting [11]. Identifying a prognostic or predictive marker for bevacizumab therapy would help individualize treatment and alleviate the burden of increased cost.
In combination with chemotherapy, bevacizumab (a humanized IgG monoclonal antibody that binds to VEGF-A and prevents activation of the tyrosine kinase domain of its receptors VEGFR1 and VEGFR2) has been shown to be effective in clinical trials by increasing overall survival (OS), progression-free survival (PFS), and response rate (RR) [8,12,13,14,15,16]. However, adverse events (AEs) of bevacizumab, in addition to those induced by chemotherapy, may negatively impact treatment outcomes. Hematological, digestive, and neurological toxicity have been reported in patients with CRC treated with chemotherapy [17,18,19]. Bevacizumab is also associated with several particular side events such as high blood pressure, risk of bleeding, proteinuria, fistulas, gastrointestinal perforations, thromboembolic events, impaired wound healing, and heart failure [12,20].
Bevacizumab was associated with the onset of proteinuria in 10 to 30% of the patients with CRC and up to 71% of the patients with renal cancer [21]. Although studies have shown a relationship between bevacizumab and the risk of developing proteinuria [22,23,24], the mechanism by which it occurs is not yet fully understood. Most of the time, AEs are reported in clinical trials to verify the treatment safety and not to evaluate their influence on OS. Studies have shown that the occurrence of proteinuria can be considered a predictive [25] or prognostic factor [26], but others have failed to demonstrate this relationship [21,27].
Several studies reported that febrile neutropenia requires a dose reduction of chemotherapy, leading further to decreased OS in cancer patients [28,29]. Anemia is frequently observed in CRC patients due to tumor bleeding, especially in rectal cancer. In patients with squamous cell carcinoma of the anal canal and anal margin, for example, hemoglobin concentration was an independent prognostic factor for OS, with anemic patients having a poor prognosis [30]. Several studies investigated the impact of preoperative anemia in CRC patients [31,32], but, to the best of our knowledge, there are no data regarding the impact of myelosuppression induced anemia or other adverse events of chemotherapy and bevacizumab.
The aim of this retrospective study was to analyze the influence of proteinuria, hematological, hepatic, renal, digestive, and neurological toxicity on the results of treatment with bevacizumab plus chemotherapy in patients with mCRC. Identifying a biomarker may help to select the mCRC patient’s subgroup that will have a favorable outcome following treatment with bevacizumab and chemotherapy.
2. Materials and Methods
2.1. Patients
We performed a retrospective analysis of patients diagnosed with mCRC treated with bevacizumab and chemotherapy in our center. Inclusion criteria were: age over 18 years, histologically confirmed colorectal cancer, first-line bevacizumab treatment, adequate baseline hematological, hepatic and renal function, and performed urinalysis before and during treatment. Patients with incomplete data were excluded.
Patients received bevacizumab 7.5 mg/kg every 3 weeks or 5 mg/kg every 2 weeks along with standard dose chemotherapy regimens: CapeOX (oxaliplatin 130 mg/m2 iv and capecitabine 1000 mg/m2 twice a day oral, day 1–14), mFOLFOX 6 (oxaliplatin 85 mg/m2 iv, fluorouracil 400 mg/m2 bolus and 2400 mg/m2 iv 46 h and leucovorin 400 mg/m2 iv), CapeIRI (irinotecan 240 mg/m2 iv and capecitabine 1000 mg/m2 twice a day oral, day 1–14), FOLFIRI (irinotecan 180 mg/m2 iv, fluorouracil 400 mg/m2 bolus and 2400 mg/m2 iv 46 h and leucovorin 400 mg/m2 iv), de Gramont (fluorouracil 400 mg/m2 bolus and 2400 mg/m2 iv 46 h and leucovorin 200 mg/m2 iv), or capecitabine monotherapy.
For each case, several types of data were collected by reviewing patients’ medical records: demographic characteristics, types of chemotherapy, pre-existing comorbidities, treatment-related AEs (including the onset of proteinuria), PFS, and OS. Hematological (anemia, neutropenia, thrombocytopenia), hepatic, and renal toxicity were classified according to Common Terminology Criteria for Adverse Events (CTCAE) v4.0 by analysis of complete blood count (CBC), differential liver function (GGT, gamma-glutamyl transpeptidase; ASAT, aspartate-aminotransferase; ALAT, alanine-aminotransferase), and creatinine. Proteinuria was assessed in the summary urine test and was noted to be present or absent, with a cut-off level of 30 mg/dL. Tumor response was evaluated after at least 6 months of treatment and interpreted according to the Response Evaluation Criteria in Solid Tumors (RECIST) v1.1 provisions [33]: complete response (CR, disappearance of all lesions), partial response (PR, at least a 30% decrease in the sum of diameters of target lesions), stationary disease (SD, decrease by less than 30% or increase by less than 20%), progressive disease (PD, at least a 20% increase in the sum of diameters of target lesions or the occurrence of new lesions).
2.2. Statistical Analysis
For statistical analysis, we used the SPSS v.16.0 software (SPSS Inc., Chicago, IL, USA). The qualitative and quantitative variables were characterized by frequency, mean, median, and standard deviation to describe the basic characteristics of the studied population. The Chi-square test and Wilcoxon rank-sum test were used to compare median values and proportions. The Kaplan–Meier curve was used to estimate PFS and OS, and the log-rank test was used to compare groups, with a p-value of <0.05 indicating statistical significance. A logistic regression analysis was performed using the development of proteinuria as the dependent variable and the following factors as independent variables: previous hypertension, diabetes, other cardiovascular comorbidities, age, gender, and first-line chemotherapy regimen. To identify toxicities influencing OS, a univariate analysis was performed, and statistically significant factors were included in the multivariate Cox analysis using OS as the dependent variable. Other independent variables included were proteinuria, anemia, age groups (less or more than 65 years), comorbidities, stage at diagnosis (metastatic versus non-metastatic), number of metastatic sites (one versus more than one), and tumor location (left versus right). Furthermore, in order to minimize the case selection bias within the two groups (patients with and without proteinuria), propensity score matching was also used (XLSTAT v.2022). Stratification was performed using the following confounders: age, gender, pre-existing hypertension, other cardiovascular comorbidities, diabetes, tumor location, stage at diagnosis, primary tumor resection, number of metastatic sites, chemotherapy regimen, and tumor response, as listed in Table 1.

Table 1.
Patients’ characteristics.
3. Results
3.1. Baseline Patient Disposition and Disease Characteristics
A total of 150 mCRC patients undergoing first-line chemotherapy concomitant with bevacizumab between 2014 and 2019 were included in the analysis (Figure 1). The median age of the patients was 64 ± 9.6 years. Most of the tumors (67%) were located on the descending colon. Mutations in the RAS (KRAS, NRAS) and BRAF (V600E) genes were present in 60 patients out of the 107 for whom these data were available. The most common site for metastasis was the liver (63%), followed by the lung (17%) and bone (5%), while 51 patients presented more than one site of metastasis. The median follow up was 27 months. Baseline patient disposition and disease characteristics according to proteinuria are summarized in Table 1. There were no significant differences between groups in terms of gender (p = 0.25), age (p = 0.28), cardiovascular comorbidities (p = 0.58), diabetes (p = 0.47), primary tumor location (p = 0.44), or associated chemotherapy regimen (p = 0.97).
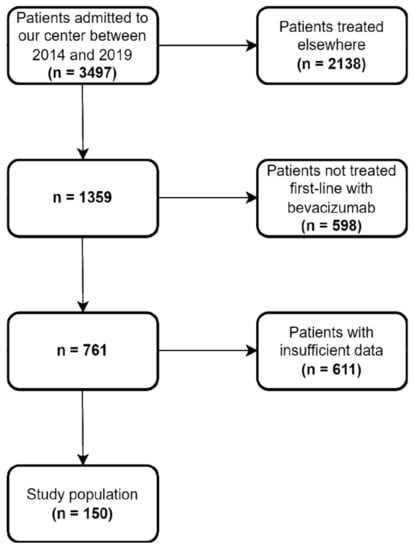
Figure 1.
Flowchart detailing patient selection for the study.
3.2. Adverse Events
We analyzed both bevacizumab- and chemotherapy-related toxicities. The most common adverse events and the incidence of grade 3 or higher AEs during the treatment period are shown in Table 2. Hepatic toxicity and anemia were the most common AEs of any grade; hepatic toxicity and neutropenia were the most common grade 3 and 4 AEs. Grade 3 or higher oxaliplatin-related neurological toxicity (peripheral neuropathy) occurred in 11 patients.

Table 2.
Adverse events of bevacizumab and chemotherapy 1.
3.2.1. Proteinuria
Proteinuria was present in 50.7% of patients. The median time to the onset of the proteinuria was 10 (range 1–32) months. None of the factors analyzed using the logistic regression method were related to the development of proteinuria: pre-existing hypertension (p = 0.08), presence of diabetes (p = 0.477), other cardiovascular comorbidities (p = 0.589), gender (p = 0.259), age (p = 0.383), or chemotherapy regimen (oxaliplatin-based, p = 0.965; irinotecan-based, p = 0.835; fluorouracil/capecitabine-based, p = 0.976). Median PFS was 13 months (95% CI 11.9–14.0) in the entire study population, and median OS was 35 months (95% CI 30.9–39.0). Patients who developed proteinuria during treatment had a longer PFS (15 versus 12 months, p = 0.039) and OS (40 versus 25 months, p = 0.015) compared with those without proteinuria (Figure 2). The disease control rate (DCR) was also higher in patients with proteinuria (76.3% versus 68.9%), but the difference was not statistically significant (p = 0.309).
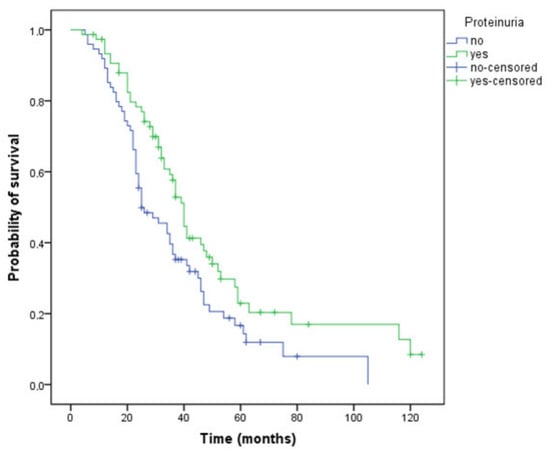
Figure 2.
Kaplan–Meier curve of overall survival for patients who have or have not developed proteinuria during treatment (OS, 40 versus 25 months, p = 0.015).
3.2.2. Anemia
Patients who had anemia during treatment, regardless of grade, had a 20-month shorter survival (Figure 3) compared with those not experiencing this AE (32 versus 52 months, p < 0.001). The DCR was higher in patients without anemia (73.8% versus 72.2%), but the difference did not reach statistical significance (p = 0.84).
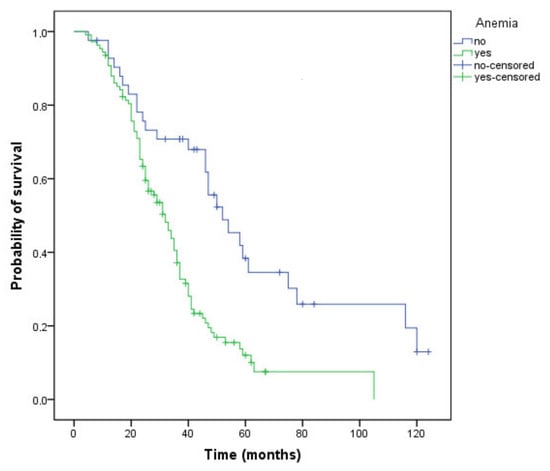
Figure 3.
Kaplan–Meier curve of overall survival for patients who have or have not developed anemia during treatment (OS, 32 versus 52 months, p < 0.001).
3.3. Disease Control Achievement and Stage at Diagnosis
Patients who achieved disease control with first-line chemotherapy plus bevacizumab treatment had a significantly longer survival: 40 versus 23 months (Figure 4) compared to those with progressive disease (p < 0.001).
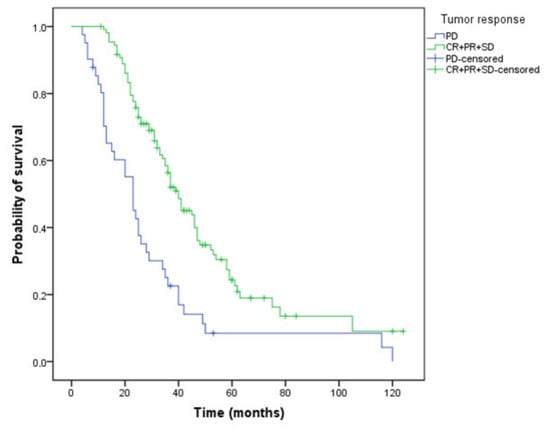
Figure 4.
Kaplan–Meier curve of overall survival for patients who have or have not obtained a tumor response (OS, 40 versus 23 months, p < 0.001, PD—progressive disease, CR—complete response, PR- partial response, SD—stabile disease).
Patients with the metastatic stage at diagnosis had a 31-month OS. Survival of those who had progressed in less than 12 months after completion of adjuvant chemotherapy was 37 months, while patients progressing after more than 12 months from completion of adjuvant treatment achieved the best OS, 50 months (p = 0.002) (Figure 5). Patients with a single metastatic site, regardless of location, had better survival rates compared to patients with at least two metastatic sites (39 versus 29 months, p = 0.017).
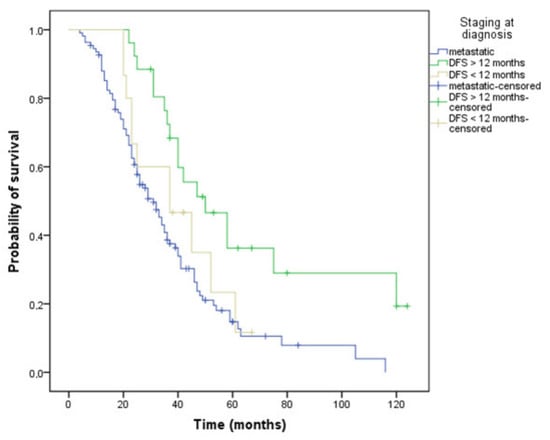
Figure 5.
Kaplan–Meier curve of overall survival depending on the stage at diagnosis: metastatic or non-metastatic: DFS (disease-free survival) less or more than 12 months (OS, 31 versus 37 versus 50 months, p = 0.002).
3.4. Prognostic Factors
The following two toxicities were significantly associated with OS in the univariate analysis: proteinuria (p = 0.017) and anemia (p = 0.001). The other adverse events affected quality of life but not survival: neutropenia (p = 0.446), thrombocytopenia (p = 0.259), hepatic toxicity (p = 0.169), renal toxicity (p = 0.164), neurological toxicity (p = 0.364), and digestive toxicity (p = 0.224).
In the multivariate analysis (Table 3), the following groups had a lower risk of death: patients with proteinuria (HR 0.589; 95% CI 0.402–0.863; p = 0.007), one metastatic site (HR 0.533; 95% CI 0.363–0.783; p = 0.001), and non-metastatic stage at diagnosis (HR 0.459; 95% CI 0.293–0.720; p = 0.001). Patients with anemia (HR 2.437; 95% CI 1.531–3.881; p < 0.001) and diabetes (HR 1.828; 95% CI 1.002–3.337; p = 0.049) had an increased risk of death.

Table 3.
Univariate and multivariate prognostic factors for longer OS in metastatic colorectal patients treated with bevacizumab and chemotherapy.
3.5. Propensity Score Matching
Additionally, the XLSTAT v.2022 version of the propensity score matching was employed to further minimize the case selection bias within the two groups (patients who have or have not developed proteinuria during treatment with bevacizumab). We have thus identified 64 pairs of patients, representing 85% of the total analyzed cases (Table 4 and Supplementary Table S1-PSM). In this context, patients who developed proteinuria had a longer OS (40 versus 25 months, p = 0.028) as compared with those without proteinuria (Figure 6A). Patients who maintained a normal hemoglobin value had a longer OS compared with anemic patients (54 versus 31 months, p < 0.001) (Figure 6B). Moreover, patients who achieved disease control with first-line therapy had a longer OS (40 versus 23 months, p < 0.001) (Figure 6C) compared to those with progressive disease. Patients with a metastatic stage at diagnosis had a 29-month OS, significantly shorter than those with a non-metastatic stage at diagnosis with DFS greater than 12 months or less than 12 months, respectively (29 versus 37 versus 50 months, p = 0.006) (Figure 6D). To further verify these results, we performed a multivariate Cox analysis for the above-mentioned subset of patients. All the parameters we have considered (proteinuria, anemia, diabetes, stage at diagnosis, and the number of metastatic sites) generated consistent significant results, thus strengthening our previous conclusions regarding their influence on OS (Table 5).

Table 4.
Summary of the matched observations according to the presence of proteinuria.
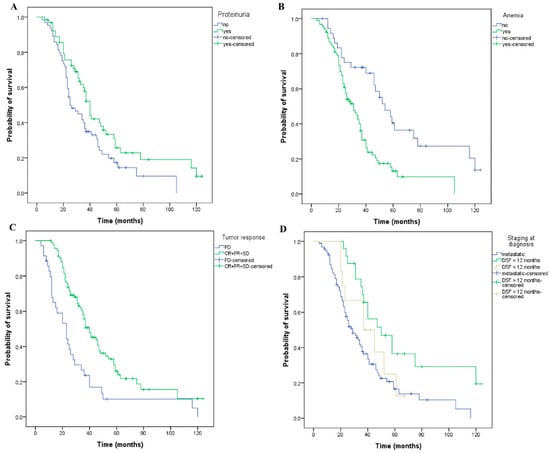
Figure 6.
Kaplan–Meier curves of overall survival for matched patients using the propensity score matching method. The patients have or have not developed: (A) proteinuria (OS, 40 versus 25 months, p = 0.028), and (B) anemia (OS, 31 versus 54 months, p < 0.001) during treatment. (C) The patients have or have not obtained a tumor response (OS, 40 versus 23 months, p < 0.001, PD—progressive disease, CR—complete response, PR—Partial response, SD-stabile disease). (D) The patients had or had no metastases at the stage of diagnosis, DFS = disease-free survival less or more than 12 months (OS, 29 versus 37 versus 50 months, p = 0.006).

Table 5.
Univariate and multivariate prognostic factors for longer OS in metastatic colorectal patients treated with bevacizumab and chemotherapy after performing propensity score matching.
4. Discussion
Numerous studies and retrospective analyses have been performed to identify novel prognostic factors that could be readily used in the clinical setting for CRC patients. Factors such as the location of the primary tumor, histologic grade, history of primary surgery, metastasectomy, performance status, peritoneal metastases, lactate dehydrogenase, PFS interval prior to liver surgery, carcinoembryonic antigen levels, liver toxicity (transaminases), and the size of the two largest lesions on CT scans have been evaluated in several prospective and retrospective studies [34,35,36]. However, no prognostic or predictive biomarkers specific to patients undergoing antiangiogenic systemic therapy have been identified to date. Although VEGF is one of the most studied biomarkers in clinical trials [37,38,39], the data available so far are still contradictory.
The main purpose of this study was to analyze the putative relationship between the occurrence of treatment-related adverse events, specifically bevacizumab-induced proteinuria, and OS. Results showed that the category of patients who developed proteinuria had a significantly better OS and PFS compared to those who did not experience this AE.
Previous studies have shown a close correlation between the use of bevacizumab and the development of proteinuria [22,23,24]. Proteinuria has also been studied as a predictive factor, but no consensus was reached. Zee et al. reported significantly lower survival rates in patients with colorectal cancer treated with antiangiogenic therapy if they developed proteinuria grade 2 or higher, as opposed to grade 0–1 (OS 4.2 months versus 23.9 months) [40]. In another study, no correlation was found between the severity of proteinuria and survival in patients with mCRC treated with bevacizumab [21]. Feliu et al. demonstrated that the occurrence of proteinuria is correlated with the response rate. However, they included only elderly patients in the study. Patients with moderate and severe proteinuria had a response rate of 56% and an OS of 22 months compared to 37% and 20.1 months, respectively, in patients with grade 0–1 proteinuria, but the survival advantage was not statistically significant [27]. Another study showed that the early development of both hypertension and proteinuria after the initiation of bevacizumab in patients with breast cancer is associated with tumor response rate, and the authors suggested that these two side effects could be considered predictive [25].
Our results differ from the mentioned studies due in part to variations between target populations and also to differences in methods and parameter definitions. Another very important source of bias is the small patient numbers in all these studies, which we have also emphasized. For example, Zee et al. conclude that grade 2 proteinuria represents a pejorative factor for OS but not for PFS or RR in their subjects. In our slightly larger sample, we have found the presence of proteinuria to carry a better prognosis for both OS and PFS, and we hypothesized it might be regarded as a surrogate marker for higher efficacy of anti-angiogenic therapies. While qualitative analysis is definitely more error-prone, it offers a more affordable and feasible evaluation of proteinuria than quantitative methods. We only performed a qualitative evaluation (yes versus no) of proteinuria and identified it in 76 patients in our sample, while the more quantitative analysis of Zee et al. (though also based on dipstick urine protein level) found grade 1 proteinuria in 12 patients and grade 2 in only 4 of their patients, respectively. Further, they also noted that proteinuria (at any grade) has not been associated with kidney dysfunction, hence the grade 2 cut-off might indeed be considered somewhat arbitrary.
Other authors correlated the development of proteinuria with the cumulative dose of bevacizumab, the number of cycles administered [27,41], systolic blood pressure values above 130 mmHg [42], or the presence of diabetes [43]. Out of all these, only the presence of diabetes was analyzed in the present study, but neither this nor any other variable appeared to significantly influence the development of proteinuria; 10 of 17 diabetic patients included in our cohort developed proteinuria during treatment.
A meta-analysis that included data from 16 studies showed that adding bevacizumab to chemotherapy increases 4.79-fold the median risk of grade 3–4 proteinuria. This increase varied with cancer type (e.g., 2.52 for colorectal cancer; 48.7 for kidney cancer) and showed a linear relationship with the dose of bevacizumab (e.g., 2.62 at a dose of 2.5 mg/kg and 8.56 at 5 mg/kg, compared to chemotherapy alone) [22].
Several potential angiogenesis-related mechanisms have been proposed for the induction of proteinuria. As a response to hypoxia and decreased proteasomal degradation of hypoxia-inducible factor 1-alpha (HIF-1-alpha), both production of VEGF by podocytes and consecutive activation of the VEGF-2 receptor on glomerular capillary endothelial cells are increased. Conversely, VEGF/VEGFR-2 inhibition causes a loss of podocytes, endothelial fenestration, glomerulosclerosis, and tubulointerstitial fibrosis [44]. In addition, inhibition of VEGF may cause glomerular thrombotic microangiopathy and membranoproliferative changes [45].
The correlation between bevacizumab-induced toxicity and outcome may have a genetic explanation. Studies identified genetic variants of VEGF and VEGFR having potentially predictive value for antiangiogenic therapy [46,47]. Hansen et al. reported that VEGFR-1 319 C/A single nucleotide polymorphism was associated with the response rate in mCRC patients treated with bevacizumab and chemotherapy [48]. Another study suggested that genetic variants of VEGF may be linked to the risk of toxicity. Breast cancer patients treated with bevacizumab and chemotherapy carrying VEGF-634 CC and VEGF-1498 TT genotypes had a lower incidence of grade 3 or 4 hypertension [49]. Nikzamir et al. reported that VEGF + 405 GG genotype was a predictive factor for albuminuria in patients with type 2 diabetes [50]. Patients developing proteinuria during bevacizumab treatment may be carriers of such variants. However, the role of genetic variants of VEGF in the development of bevacizumab-related proteinuria has not been studied yet.
No guidelines are currently available for the management of bevacizumab-induced proteinuria, although there is general consensus on the necessity to prevent subsequent renal failure, cardiovascular complications, as well as tumor progression due to permanent discontinuation of biologic therapy if proteinuria exceeds 2 g/24 h or nephrotic syndrome occurs, respectively.
In the present study, the occurrence of at least one episode of anemia during treatment was a negative prognostic factor for OS. Survival decreased significantly according to the grade of anemia (20 months for grade 3 versus 31 months for grade 2 versus 34 months for grade 1; no grade 4 or 5 anemia was reported). This is in accord with the conclusions of a meta-analysis reporting that anemia at any point during the course of the disease increases the risk of death in cancer patients. When presenting anemia, the relative risk of death was increased by 19% in lung cancer, by 75% in head and neck carcinomas, and by 47% in prostate cancer patients [51]. Anemia during chemotherapy also affects OS by the deriving necessity to delay or reduce the dose of chemotherapy. In addition, anemia produces tumor hypoxia that reduces the effectiveness of chemotherapy and bevacizumab. Although anemia can be corrected, there is no evidence to improve long-term prognosis after performing therapeutic procedures (transfusions, stimulation of erythropoiesis).
Another factor influencing both OS and disease-free survival (DFS) is the tumor stage at diagnosis [52,53]. In multivariate analyses, we have split our study population into two groups (upfront metastatic, n = 109; and non-metastatic, n = 41) and found that initially non-metastatic patients had significantly better survival rates. Patients with a single metastatic site had a 10-month longer survival than patients with at least two metastatic sites. The present study also showed that tumor volume has a negative impact on the prognosis. Köhne et al. analyzed a panel of clinical, hematological, and biochemical factors to identify prognostic markers of CRC patients treated with fluorouracil-based chemotherapy, and the results showed that the number of metastatic sites, along with other factors, classified patients with mCRC into different risk categories. The most unfavorable risk was for patients with ECOG performance status 0–1, more than one metastatic site, and alkaline phosphatase over 300 U/L. Platelets (>400 × 109/L), alkaline phosphatase level (>300 IU/L), WBC count (>10 × 109/L), and hemoglobin (<11 g/dL) predicted an inferior survival probability. Lactate dehydrogenase, bilirubin, ALAT, ASAT, total protein, albumin, and carcinoembryonic antigen (CEA) levels were not significant [54]. The number of metastatic sites is considered a negative prognostic factor not only for CRC [55] but also for lung cancer [56], esogastric cancer [57], and for endometrial carcinoma [58].
Another multivariate analysis concluded that primary tumor location, performance status, number of metastatic sites, baseline CEA level, and platelets may be considered prognostic factors in patients with mCRC treated with oxaliplatin and bevacizumab [59]. In the current analysis, renal, hepatic, digestive, and neurological toxicities affected the quality of life to various degrees but did not influence OS.
Our research is subject to several limitations. We have included in our study 150 carefully chosen patients, a number that precludes definitive conclusions or recommendations based on the results above. However, our results provide additional data on the prognostic role of proteinuria and warrant more extensive prospective studies in order to validate the present findings. Another study limitation is the retrospective nature of the study and selection bias. For example, about 20% of patients present with de novo metastatic colon cancer. In the present study, the number of de novo metastatic diseases is >70%, which suggests patients with a previous diagnosis of early-stage colorectal cancer are not reflecting the source population, and the finding that previous history of an early-stage disease has a better prognosis could merely be due to selection bias. In addition, some of the known prognostic factors including BRAF status, metastasectomy, subsequent line of chemotherapy, and baseline performance status were not examined in the analysis. The study might have a potentially short follow-up bias. Studies reported a wide range time-to-onset of proteinuria, from 3 weeks to 37 months, with a median of 5.6 months from the start of bevacizumab. The median follow up for the present study was 27 months, so several additional cases of late proteinuria occurring in our population were thus not considered. It is common knowledge that hypertension influences the development of proteinuria through various mechanisms. In the logistic regression analysis, we included only pre-existing hypertension without considering whether blood pressure had been controlled by anti-hypertensive treatment. It is also possible that some patients might have developed hypertension during treatment, and this might have influenced the occurrence of proteinuria. Another study limitation is the heterogeneity of the patients included, whether synchronous or metachronous metastases, and the lack of information about the metastatic disease characteristics that may influence the carcinological results.
5. Conclusions
The results of our study suggest that, in addition to the non-metastatic stage at diagnosis and one metastatic site, the development of proteinuria during first-line treatment with bevacizumab and chemotherapy of patients with mCRC was an independent prognostic factor for OS and correlates with a better prognosis. Despite the fact that literature data are controversial in terms of the prognostic role of proteinuria, the results of our study argue in favor of it. The presence of diabetes, pre-existing hypertension, and other cardiovascular conditions did not increase proteinuria risk in the studied group. Neutropenia, thrombocytopenia, hepatic, renal, and neurological toxicity do not influence OS. The presence of anemia during treatment and diabetes were negative prognostic factors.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/curroncol29060319/s1, Table S1: SPM.
Author Contributions
Conceptualization, D.C.M. and M.V.M.; methodology, M.V.M., T.A.-S., M.P.-T. and P.C.; formal analysis, D.C.M., B.G., M.P.-T. and P.C.; Resources, B.G. and T.A.-S.; writing—original draft preparation, D.C.M. and M.V.M.; writing—review and editing, all authors; Supervision, P.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Regional Institute of Oncology Iasi (77/04.04.2019).
Informed Consent Statement
Patient consent was waived in accordance with the ethical standards of the Ethics Committee of the Regional Institute of Oncology Iasi.
Data Availability Statement
The datasets generated and analyzed during the current study are available from the corresponding author and can be shared with the journal for review if needed.
Conflicts of Interest
D.C.M. and P.C. have no competing financial interests. M.V.M. and B.G. received honoraria from and were investigators in F. Hofmann-LaRoche-sponsored trials. T.A.S. received honoraria from F. Hofmann-LaRoche. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Siegel, R.; Desantis, C.; Jemal, A. Colorectal cancer statistics, 2014. CA Cancer J. Clin. 2014, 64, 104–117. [Google Scholar] [CrossRef]
- Aguiar Junior, S.; Oliveira, M.M.; Silva, D.R.M.E.; Mello, C.A.L.; Calsavara, V.F.; Curado, M.P. Survival of patients with colorectal cancer in a cancer center. Arq. Gastroenterol. 2020, 57, 172–177. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Cervantes, A.; Nordlinger, B.; Arnold, D. Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014, 25, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Piawah, S.; Venook, A.P. Targeted therapy for colorectal cancer metastases: A review of current methods of molecularly targeted therapy and the use of tumor biomarkers in the treatment of metastatic colorectal cancer. Cancer 2019, 125, 4139–4147. [Google Scholar] [CrossRef] [PubMed]
- Pikouli, A.; Papaconstantinou, D.; Wang, J.; Kavezou, F.; Pararas, N.; Nastos, C.; Pikoulis, E.; Margonis, G.A. Reevaluating the prognostic role of BRAF mutation in colorectal cancer liver metastases. Am. J. Surg. 2021, 223, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Grothey, A.; Fakih, M.; Tabernero, J. Management of BRAF-mutant metastatic colorectal cancer: A review of treatment options and evidence-based guidelines. Ann. Oncol. 2021, 32, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Hegewisch-Becker, S.; Graeven, U.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Steffens, C.C.; Al-Batran, S.E.; Lange, T.; Dietrich, G.; Stoehlmacher, J.; et al. Maintenance strategies after first-line oxaliplatin plus fluoropyrimidine plus bevacizumab for patients with metastatic colorectal cancer (AIO 0207): A randomised, non-inferiority, open-label, phase 3 trial. Lancet Oncol. 2015, 16, 1355–1369. [Google Scholar] [CrossRef]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Aghajanian, C.; Goff, B.; Nycum, L.R.; Wang, Y.V.; Husain, A.; Blank, S.V. Final overall survival and safety analysis of OCEANS, a phase 3 trial of chemotherapy with or without bevacizumab in patients with platinum-sensitive recurrent ovarian cancer. Gynecol. Oncol. 2015, 139, 10–16. [Google Scholar] [CrossRef] [Green Version]
- Prasad, V.; De Jesús, K.; Mailankody, S. The high price of anticancer drugs: Origins, implications, barriers, solutions. Nat. Rev. Clin. Oncol. 2017, 14, 381–390. [Google Scholar] [CrossRef]
- Goldstein, D.A.; Zeichner, S.B.; Bartnik, C.M.; Neustadter, E.; Flowers, C.R. Metastatic Colorectal Cancer: A Systematic Review of the Value of Current Therapies. Clin. Color. Cancer 2016, 15, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cassidy, J.; Saltz, L.B.; Giantonio, B.J.; Kabbinavar, F.F.; Hurwitz, H.I.; Rohr, U.P. Effect of bevacizumab in older patients with metastatic colorectal cancer: Pooled analysis of four randomized studies. J. Cancer Res. Clin. Oncol. 2010, 136, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Pfaendler, K.S.; Liu, M.C.; Tewari, K.S. Bevacizumab in Cervical Cancer: 5 Years After. Cancer J. 2018, 24, 187–192. [Google Scholar] [CrossRef]
- Ma, H.; Wu, X.; Tao, M.; Tang, N.; Li, Y.; Zhang, X.; Zhou, Q. Efficacy and safety of bevacizumab-based maintenance therapy in metastatic colorectal cancer: A meta-analysis. Medicine 2019, 98, e18227. [Google Scholar] [CrossRef] [PubMed]
- Bennouna, J.; Phelip, J.M.; André, T.; Asselain, B.; Koné, S.; Ducreux, M. Observational Cohort Study of Patients With Metastatic Colorectal Cancer Initiating Chemotherapy in Combination With Bevacizumab (CONCERT). Clin. Colorectal Cancer 2017, 16, 129–140.e4. [Google Scholar] [CrossRef] [PubMed]
- Khakoo, S.; Chau, I.; Pedley, I.; Ellis, R.; Steward, W.; Harrison, M.; Baijal, S.; Tahir, S.; Ross, P.; Raouf, S.; et al. ACORN investigators. ACORN: Observational Study of Bevacizumab in Combination With First-Line Chemotherapy for Treatment of Metastatic Colorectal Cancer in the UK. Clin. Colorectal Cancer 2019, 18, 280–291.e5. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kuang, M.; Gong, Y.; Cao, C.; Chen, J.; Tang, C. Survival benefit and safety of the combinations of FOLFOXIRI ± bevacizumab versus the combinations of FOLFIRI ± bevacizumab as first-line treatment for unresectable metastatic colorectal cancer: A meta-analysis. OncoTargets Ther. 2016, 9, 4833–4842. [Google Scholar]
- Botrel, T.E.A.; Clark, L.G.O.; Paladini, L.; Clark, O.A.C. Efficacy and safety of bevacizumab plus chemotherapy compared to chemotherapy alone in previously untreated advanced or metastatic colorectal cancer: A systematic review and meta-analysis. BMC Cancer 2016, 16, 677. [Google Scholar] [CrossRef] [Green Version]
- Welch, S.; Spithoff, K.; Rumble, R.B.; Maroun, J.; Gastrointestinal Cancer Disease Site Group. Bevacizumab combined with chemotherapy for patients with advanced colorectal cancer: A systematic review. Ann. Oncol. 2010, 21, 1152–1162. [Google Scholar] [CrossRef]
- Qu, C.Y.; Zheng, Y.; Zhou, M.; Zhang, Y.; Shen, F.; Cao, J.; Xu, L.M. Value of bevacizumab in treatment of colorectal cancer: A meta-analysis. World J. Gastroenterol. 2015, 21, 5072–5080. [Google Scholar] [CrossRef]
- Iwasa, S.; Nakajima, T.E.; Nagashima, K.; Honma, Y.; Kato, K.; Hamaguchi, T.; Yamada, Y.; Shimada, Y. Lack of association of proteinuria and clinical outcome in patients treated with bevacizumab for metastatic colorectal cancer. Anticancer. Res. 2013, 33, 309–316. [Google Scholar]
- Wu, S.; Kim, C.; Baer, L.; Zhu, X. Bevacizumab increases risk for severe proteinuria in cancer patients. J. Am. Soc. Nephrol. 2010, 21, 1381–1389. [Google Scholar] [CrossRef] [Green Version]
- Zhao, T.; Wang, X.; Xu, T.; Xu, X.; Liu, Z. Bevacizumab significantly increases the risks of hypertension and proteinuria in cancer patients: A systematic review and comprehensive meta-analysis. Oncotarget 2017, 8, 51492–51506. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Wu, S.; Dahut, W.L.; Parikh, C.R. Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am. J. Kidney Dis. 2007, 49, 186–193. [Google Scholar] [CrossRef]
- Tanaka, H.; Takahashi, K.; Yamaguchi, K.; Kontani, K.; Motoki, T.; Asakura, M.; Kosaka, S.; Yokomise, H.; Houchi, H. Hypertension and Proteinuria as Predictive Factors of Effects of Bevacizumab on Advanced Breast Cancer in Japan. Biol. Pharm. Bull. 2018, 41, 644–648. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, B.; Lopes, R.G.; Linhares, P.; Costa, A.; Caeiro, C.; Fernandes, A.C.; Tavares, N.; Osório, L.; Vaz, R. Hypertension and proteinuria as clinical biomarkers of response to bevacizumab in glioblastoma patients. J. Neuro-Oncol. 2020, 147, 109–116. [Google Scholar] [CrossRef]
- Feliu, J.; Salud, A.; Safont, M.J.; García-Girón, C.; Aparicio, J.; Losa, F.; Bosch, C.; Escudero, P.; Casado, E.; Jorge, M.; et al. Correlation of hypertension and proteinuria with outcome in elderly bevacizumab-treated patients with metastatic colorectal cancer. PLoS ONE 2015, 10, e0116527. [Google Scholar]
- Lalami, Y.; Klastersky, J. Impact of chemotherapy-induced neutropenia (CIN) and febrile neutropenia (FN) on cancer treatment outcomes: An overview about well-established and recently emerging clinical data. Crit. Rev. Oncol. Hematol. 2017, 120, 163–179. [Google Scholar] [CrossRef]
- Crawford, J.; Dale, D.C.; Lyman, G.H. Chemotherapy-induced neutropenia: Risks, consequences, and new directions for its management. Cancer 2004, 100, 228–237. [Google Scholar] [CrossRef]
- Oblak, I.; Cesnjevar, M.; Anzic, M.; Hadzic, J.B.; Ermenc, A.S.; Anderluh, F.; Velenik, V.; Jeromen, A.; Korosec, P. The impact of anaemia on treatment outcome in patients with squamous cell carcinoma of anal canal and anal margin. Radiol. Oncol. 2016, 50, 113–120. [Google Scholar] [CrossRef] [Green Version]
- Wilson, M.J.; van Haaren, M.; Harlaar, J.J.; Park, H.C.; Bonjer, H.J.; Jeekel, J.; Zwaginga, J.J.; Schipperus, M. Long-term prognostic value of preoperative anemia in patients with colorectal cancer: A systematic review and meta-analysis. Surg. Oncol. 2017, 26, 96–104. [Google Scholar] [CrossRef]
- An, M.S.; Yoo, J.H.; Kim, K.H.; Bae, K.B.; Choi, C.S.; Hwang, J.W.; Kim, J.H.; Kim, B.M.; Kang, M.S.; Oh, M.K.; et al. T4 stage and preoperative anemia as prognostic factors for the patients with colon cancer treated with adjuvant FOLFOX chemotherapy. World J. Surg. Oncol. 2015, 13, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Demircan, N.C.; Dane, F.; Ozturk, M.A.; Babacan, N.A.; Besiroglu, M.; Kaya, S.; Ercelep, O.; Tanrikulu, E.; Halil, S.; Koca, S.; et al. Assessment of survival and prognostic factors in metastatic colorectal cancer patients treated with first-line bevacizumab-based therapy. JBUON 2019, 24, 1494–1500. [Google Scholar] [PubMed]
- Shitara, K.; Matsuo, K.; Yokota, T.; Takahari, D.; Shibata, T.; Ura, T.; Inaba, Y.; Yamaura, H.; Sato, Y.; Najima, M.; et al. Prognostic factors for metastatic colorectal cancer patients undergoing irinotecan-based second-line chemotherapy. Gastrointest. Cancer Res. 2011, 4, 168–172. [Google Scholar] [PubMed]
- Fendler, W.P.; Ilhan, H.; Paprottka, P.M.; Jakobs, T.F.; Heinemann, V.; Bartenstein, P.; Khalaf, F.; Ezziddin, S.; Hacker, M.; Haug, A.R. Nomogram including pretherapeutic parameters for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Eur. Radiol. 2015, 25, 2693–2700. [Google Scholar] [CrossRef] [PubMed]
- Jubb, A.M.; Hurwitz, H.I.; Bai, W.; Holmgren, E.B.; Tobin, P.; Guerrero, A.S.; Kabbinavar, F.; Holden, S.N.; Novotny, W.F.; Frantz, G.D.; et al. Impact of vascular endothelial growth factor-A expression, thrombospondin-2 expression, and microvessel density on the treatment effect of bevacizumab in metastatic colorectal cancer. J. Clin. Oncol. 2006, 24, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Bernaards, C.; Hegde, P.; Chen, D.; Holmgren, E.; Zheng, M.; Jubb, A.M.; Koeppen, H.; Scherer, S.J.; Chen, D.S. Circulating vascular endothelial growth factor (VEGF) as a biomarker for bevacizumab-based therapy in metastatic colorectal, non-small cell lung, and renal cell cancers: Analysis of phase III studies. J. Clin. Oncol. 2010, 28, 10519. [Google Scholar] [CrossRef]
- Duda, D.G. Molecular Biomarkers of Response to Antiangiogenic Therapy for Cancer. Int. Sch. Res. Not. 2012, 2012, 587259. [Google Scholar] [CrossRef] [Green Version]
- Zee, Y.K.; Murukesh, N.; Kumaran, G.; Swindell, R.; Saunders, M.P.; Clamp, A.R.; Valle, J.W.; Wilson, G.; Jayson, G.C.; Hasan, J. Hypertension (HTN) and proteinuria (PTN) as biomarkers of efficacy in antiangiogenic therapy for metastatic colorectalcancer (mCRC). J. Clin. Oncol. 2010, 28, e13580. [Google Scholar] [CrossRef]
- Lee, C.S.; Alwan, L.M.; Sun, X.; McLean, K.A.; Urban, R.R. Routine proteinuria monitoring for bevacizumab in patients with gynecologic malignancies. J. Oncol. Pharm. Pract. 2016, 22, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Teramachi, H.; Shiga, H.; Komada, N.; Tamura, K.; Yasuda, M.; Umeda, M.; Tachi, T.; Goto, C.; Tsuchiya, T. Risk factors contributing to urinary protein expression resulting from bevacizumab combination chemotherapy. Pharmazie 2013, 68, 217–220. [Google Scholar]
- Lafayette, R.A.; McCall, B.; Li, N.; Chu, L.; Werner, P.; Das, A.; Glassock, R. Incidence and relevance of proteinuria in bevacizumab-treated patients: Pooled analysis from randomized controlled trials. Am. J. Nephrol. 2014, 40, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Izzedine, H.; Massard, C.; Spano, J.P.; Goldwasser, F.; Khayat, D.; Soria, J.C. VEGF signalling inhibition-induced proteinuria: Mechanisms, significance and management. Eur. J. Cancer 2010, 46, 439–448. [Google Scholar] [CrossRef] [Green Version]
- Eremina, V.; Jefferson, J.A.; Kowalewska, J.; Hochster, H.; Haas, M.; Weisstuch, J.; Richardson, C.; Kopp, J.B.; Kabir, M.G.; Backx, P.H.; et al. VEGF inhibition and renal thrombotic microangiopathy. N. Engl. J. Med. 2008, 358, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, D.; Claes, B.; Delmar, P.; Reumers, J.; Mazzone, M.; Yesilyurt, B.T.; Devlieger, R.; Verslype, C.; Tejpar, S.; Wildiers, H.; et al. VEGF pathway genetic variants as biomarkers of treatment outcome with bevacizumab: An analysis of data from the AViTA and AVOREN randomised trials. Lancet Oncol. 2012, 13, 724–733. [Google Scholar] [CrossRef]
- Garcia-Donas, J.; Esteban, E.; Leandro-García, L.J.; Castellano, D.E.; González del Alba, A.; Climent, M.A.; Arranz, J.A.; Gallardo, E.; Puente, J.; Bellmunt, J.; et al. Single nucleotide polymorphism associations with response and toxic effects in patients with advanced renal-cell carcinoma treated with first-line sunitinib: A multicentre, observational, prospective study. Lancet Oncol. 2011, 12, 1143–1150. [Google Scholar] [CrossRef]
- Hansen, T.F.; dePont Christensen, R.; Andersen, R.F.; Garm Spindler, K.L.; Johnsson, A.; Jakobsen, A. The predictive value of single nucleotide polymorphisms in the VEGF system to the efficacy of first-line treatment with bevacizumab plus chemotherapy in patients with metastatic colorectal cancer. Int. J. Colorectal Dis. 2011, 27, 715–720. [Google Scholar] [CrossRef]
- Schneider, B.P.; Wang, M.; Radovich, M.; Sledge, G.W.; Badve, S.; Thor, A.; Flockhart, D.A.; Hancock, B.; Davidson, N.; Gralow, J.; et al. ECOG 2100. Association of vascular endothelial growth factor and vascular endothelial growth factor receptor-2 genetic polymorphisms with paclitaxel plus bevacizumab in advanced breast cancer: ECOG 2100. J. Clin. Oncol. 2008, 26, 4672–4678. [Google Scholar] [CrossRef]
- Nikzamir, A.; Esteghamati, A.; Hammedian, A.A.; Mahmoudi, T. The role of vascular endothelial growth factor +405 G/C polymorphism and albuminuria in patients with type 2 diabetes mellitus. Mol. Biol. Rep. 2002, 39, 881–886. [Google Scholar] [CrossRef]
- Caro, J.J.; Salas, M.; Ward, A.; Goss, G. Anemia as an independent prognostic factor for survival in patients with cancer: A systemic, quantitative review. Cancer 2001, 91, 2214–2221. [Google Scholar] [CrossRef]
- Valentini, V.; van Stiphout, R.G.; Lammering, G.; Gambacorta, M.A.; Barba, M.C.; Bebenek, M.; Bonnetain, F.; Bosset, J.F.; Bujko, K.; Cionini, L.; et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 2011, 29, 3163–3172. [Google Scholar] [CrossRef]
- Peng, J.; Ding, Y.; Tu, S.; Shi, D.; Sun, L.; Li, X.; Wu, H.; Cai, S. Prognostic nomograms for predicting survival and distant metastases in locally advanced rectal cancers. PLoS ONE 2014, 9, e106344. [Google Scholar] [CrossRef] [Green Version]
- Köhne, C.H.; Cunningham, D.; Di Costanzo, F.; Glimelius, B.; Blijham, G.; Aranda, E.; Scheithauer, W.; Rougier, P.; Palmer, M.; Wils, J.; et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: Results of a multivariate analysis of 3825 patients. Ann. Oncol. 2002, 13, 308–317. [Google Scholar] [CrossRef]
- Franko, J.; Shi, Q.; Meyers, J.P.; Maughan, T.S.; Adams, R.A.; Seymour, M.T.; Saltz, L.; Punt, C.J.A.; Koopman, M.; Tournigand, C.; et al. Analysis and Research in Cancers of the Digestive System (ARCAD) Group. Prognosis of patients with peritoneal metastatic colorectal cancer given systemic therapy: An analysis of individual patient data from prospective randomised trials from the Analysis and Research in Cancers of the Digestive System (ARCAD) database. Lancet Oncol. 2016, 17, 1709–1719. [Google Scholar]
- Oh, Y.; Taylor, S.; Bekele, B.N.; Debnam, J.M.; Allen, P.K.; Suki, D.; Sawaya, R.; Komaki, R.; Stewart, D.J.; Karp, D.D. Number of metastatic sites is a strong predictor of survival in patients with nonsmall cell lung cancer with or without brain metastases. Cancer 2009, 115, 2930–2938. [Google Scholar] [CrossRef]
- Chen, K.; Deng, X.; Yang, Z.; Yu, D.; Zhang, X.; Li, W.; Xie, D.; He, Z.; Cheng, D. Sites of distant metastases and the cancer-specific survival of metastatic Siewert type II esophagogastric junction adenocarcinoma: A population-based study. Expert Rev. Gastroenterol. Hepatol. 2020, 14, 491–497. [Google Scholar] [CrossRef]
- Jobsen, J.J.; ten Cate, L.N.; Lybeert, M.L.; van der Steen-Banasik, E.M.; Scholten, A.; van der Palen, J.; Slot, A.; Kroese, M.C.; Schutter, E.M.; Siesling, S. The number of metastatic sites for stage IIIA endometrial carcinoma, endometrioid cell type, is a strong negative prognostic factor. Gynecol. Oncol. 2010, 117, 32–36. [Google Scholar] [CrossRef]
- Hegewisch-Becker, S.; Nöpel-Dünnebacke, S.; Hinke, A.; Graeven, U.; Reinacher-Schick, A.; Hertel, J.; Lerchenmüller, C.A.; Killing, B.; Depenbusch, R.; Al-Batran, S.E.; et al. Impact of primary tumour location and RAS/BRAF mutational status in metastatic colorectal cancer treated with first-line regimens containing oxaliplatin and bevacizumab: Prognostic factors from the AIO KRK0207 first-line and maintenance therapy trial. Eur. J. Cancer 2018, 101, 105–113. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).