Mutation Profiles of Ovarian Seromucinous Borderline Tumors in Japanese Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Tumor Samples
2.2. Sample Processing and DNA Extraction
2.3. Mutation Analysis
2.4. Immunostaining of ARID1A, p53, and PTEN
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dubé, V.; Roy, M.; Plante, M.; Renaud, M.C.; Têtu, B. Mucinous Ovarian Tumors of Mullerian-Type: An Analysis of 17 Cases Including Borderline Tumors and Intraepithelial, Microinvasive, and Invasive Carcinomas. Int. J. Gynecol. Pathol. 2005, 24, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y. Endometriosis-related Ovarian Neoplasms: Pathogenesis and Histopathologic Features. Diagn. Histopathol. 2014, 20, 357–363. [Google Scholar] [CrossRef]
- Kurman, R.J.; International Agency for Research on Cancer; World Health Organization. WHO Classification of Tumors of Female Reproductive Organs; International Agency for Research on Cancer: Lyon, France, 2014; p. 307. [Google Scholar]
- Rutgers, J.K.L. Mullerian Mucinous/mixed Epithelial (Seromucinous) Ovarian Tumors. AJPS 2016, 21, 206–213. [Google Scholar]
- Rutgers, J.L.; Scully, R.E. Ovarian Mixed-Epithelial Papillary Cystadenomas of Borderline Malignancy of Mullerian Type. A Clinicopathologic Analysis. Cancer 1988, 61, 546–554. [Google Scholar] [CrossRef]
- Lee, K.R.; Nucci, M.R. Ovarian Mucinous and Mixed Epithelial Carcinomas of Mullerian (endocervical-like) Type: A Clinicopathologic Analysis of Four Cases of an Uncommon Variant Associated with Endometriosis. Int. J. Gynecol. Pathol. 2003, 22, 42–51. [Google Scholar] [CrossRef]
- Nagamine, M.; Mikami, Y. Ovarian Seromucinous Tumors: Pathogenesis, Morphologic Spectrum, and Clinical Issues. Diagnostics 2020, 10, 77. [Google Scholar] [CrossRef]
- Nagai, Y.; Kishimoto, T.; Nikaido, T.; Nishihara, K.; Matsumoto, T.; Suzuki, C.; Ogishima, T.; Kuwahara, Y.; Hurukata, Y.; Mizunuma, M.; et al. Squamous Predominance in Mixed-Epithelial Papillary Cystadenomas of Borderline Malignancy of Mullerian Type Arising in Endometriotic Cysts: A Study of Four Cases. Am. J. Surg. Pathol. 2003, 27, 242–247. [Google Scholar] [CrossRef]
- Wu, R.C.; Chen, S.J.; Chen, H.C.; Tan, K.T.; Jung, S.M.; Lin, C.Y.; Chao, A.S.; Huang, K.G.; Chou, H.H.; Chang, T.C.; et al. Comprehensive Genomic Profiling Reveals Ubiquitous KRAS Mutations and Frequent PIK3CA Mutations in Ovarian Seromucinous Borderline Tumor. Mod. Pathol. 2020, 33, 2534–2543. [Google Scholar] [CrossRef]
- Wu, C.H.; Mao, T.L.; Vang, R.; Ayhan, A.; Wang, T.L.; Kurman, R.J.; Shih, I.E.M. Endocervical-Type Mucinous Borderline Tumors are Related to Endometrioid Tumors Based on Mutation and Loss of Expression of ARID1A. Int. J. Gynecol. Pathol. 2012, 31, 297–303. [Google Scholar] [CrossRef]
- Kim, K.R.; Choi, J.; Hwang, J.E.; Baik, Y.A.; Shim, J.Y.; Kim, Y.M.; Robboy, S.J. Endocervical-Like (Müllerian) Mucinous Borderline Tumours of the Ovary are Frequently Associated with the KRAS Mutation. Histopathology 2010, 57, 587–596. [Google Scholar] [CrossRef]
- Nakayama, K.; Takebayashi, Y.; Namiki, T.; Tamahashi, N.; Nakayama, S.; Uchida, T.; Miyazaki, K.; Fukumoto, M. Comprehensive Allelotype Study of Ovarian Tumors of Low Malignant Potential: Potential Differences in Pathways Between Tumors with and without Genetic Predisposition to Invasive Carcinoma. Int. J. Cancer 2001, 94, 605–609. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nonomura, Y.; Nakayama, K.; Nakamura, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; Sato, S.; Nakayama, S.; Otsuki, Y.; et al. Ovarian Endometrioid and Clear Cell Carcinomas with Low Prevalence of Microsatellite Instability: A Unique Subset of Ovarian Carcinomas Could Benefit from Combination Therapy with Immune Checkpoint Inhibitors and Other Anticancer Agents. Healthcare 2022, 10, 694. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, K.; Takebayashi, Y.; Nakayama, S.; Hata, K.; Fujiwaki, R.; Fukumoto, M.; Miyazaki, K. Prognostic Value of Overexpression of p53 in Human Ovarian Carcinoma Patients Receiving Cisplatin. Cancer Lett. 2003, 192, 227–235. [Google Scholar] [CrossRef]
- Katagiri, A.; Nakayama, K.; Rahman, M.T.; Rahman, M.; Katagiri, H.; Nakayama, N.; Ishikawa, M.; Ishibashi, T.; Iida, K.; Kobayashi, H.; et al. Loss of ARID1A Expression is Related to Shorter Progression-free Survival and Chemoresistance in Ovarian Clear Cell Carcinoma. Mod. Pathol. 2012, 25, 282–288. [Google Scholar] [CrossRef]
- Nakamura, H.; Saji, H.; Ogata, A.; Hosaka, M.; Hagiwara, M.; Kawasaki, N.; Kato, H. Correlation between Encoded Protein Overexpression and Copy Number of the HER2 Gene with Survival in non-small Cell Lung Cancer. Int. J. Cancer 2003, 103, 61–66. [Google Scholar] [CrossRef]
- Fishman, D.A.; Cohen, L.; Blank, S.V.; Shulman, L.; Singh, D.; Bozorgi, K.; Tamura, R.; Timor-Tritsch, I.; Schwartz, P.E. The Role of Ultrasound Evaluation in the Detection of Early-stage Epithelial Ovarian Cancer. Am. J. Obstet. Gynecol. 2005, 192, 1214–1221. [Google Scholar] [CrossRef] [PubMed]
- Rasool, N.; Rose, P.G. Fertility-preserving Surgical Procedures for Patients with Gynecologic Malignancies. Clin. Obstet. Gynecol. 2010, 53, 804–814. [Google Scholar] [CrossRef]
- DiSaia, P.J. Conservative Management of the Patient with Early Gynecologic Cancer. CA Cancer J. Clin. 1989, 39, 135–154. [Google Scholar] [CrossRef]
- Huber, D.; Cimorelli, V.; Usel, M.; Bouchardy, C.; Rapiti, E.; Petignat, P. How many Ovarian Cancer Patients are Eligible for Fertility-sparing Surgery. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 170, 270–274. [Google Scholar] [CrossRef]
- Franasiak, J.M.; Scott, R.T. Demographics of Cancer in the Reproductive Age Female. In Cancer and Fertility; Sabanegh, J.E., Ed.; Humana Press: Cham, Switzerland, 2016; pp. 11–19. [Google Scholar] [CrossRef]
- Gerstl, B.; Sullivan, E.; Vallejo, M.; Koch, J.; Johnson, M.; Wand, H.; Webber, K.; Ives, A.; Anazodo, A. Reproductive Outcomes Following Treatment for a Gynecological Cancer Diagnosis: A Systematic Review. J. Cancer Surviv. 2019, 13, 269–281. [Google Scholar] [CrossRef]
- La Rosa, V.L.; Garzon, S.; Gullo, G.; Fichera, M.; Sisti, G.; Gallo, P.; Riemma, G.; Schiattarella, A. Fertility Preservation in Women Affected by Gynaecological Cancer: The Importance of an Integrated Gynaecological and Psychological Approach. Ecancermedicalscience 2020, 14, 1035. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.K.; Tsang, Y.T.; Deavers, M.T.; Mok, S.C.; Zu, Z.; Sun, C.; Malpica, A.; Wolf, J.K.; Lu, K.H.; Gershenson, D.M. BRAF Mutation is Rare in Advanced-stage Low-grade Ovarian Serous Carcinomas. Am. J. Pathol. 2010, 177, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.; Wang, T.L.; Kurman, R.J.; Nakayama, K.; Velculescu, V.E.; Vogelstein, B.; Kinzler, K.W.; Papadopoulos, N.; Shih, I.E.M. Low-grade Serous Carcinomas of the Ovary Contain Very Few Point Mutations. J. Pathol. 2012, 226, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Singer, G.; Kurman, R.J.; Chang, H.W.; Cho, S.K.; Shih, I.E.M. Diverse Tumorigenic Pathways in Ovarian Serous Carcinoma. Am. J. Pathol. 2002, 160, 1223–1228. [Google Scholar] [CrossRef]
- Ishibashi, T.; Nakayama, K.; Razia, S.; Ishikawa, M.; Nakamura, K.; Yamashita, H.; Dey, P.; Iida, K.; Kurioka, H.; Nakayama, S.; et al. High Frequency of PIK3CA Mutations in Low-Grade Serous Ovarian Carcinomas of Japanese Patients. Diagnostics 2019, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Ghafoor, A.; Jemal, A.; Ward, E.; Cokkinides, V.; Smith, R.; Thun, M. Trends in Breast Cancer by Race and Ethnicity. CA Cancer J. Clin. 2003, 53, 342–355. [Google Scholar] [CrossRef]
- Li, C.I.; Malone, K.E.; Daling, J.R. Differences in Breast Cancer Hormone Receptor Status and Histology by Race and Ethnicity Among Women 50 years of Age and Older. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 601–607. [Google Scholar]
- Phan, V.H.; Moore, M.M.; McLachlan, A.J.; Piquette-Miller, M.; Xu, H.; Clarke, S.J. Ethnic Differences in Drug Metabolism and Toxicity from Chemotherapy. Expert Opin. Drug Metab. Toxicol. 2009, 5, 243–257. [Google Scholar] [CrossRef]
- Hamada, T.; Kiyokawa, T.; Nomura, K.; Hano, H. Immunohistochemical Analysis of Reserve Cell-like Cells of Ovarian Müllerian Mucinous/Mixed Epithelial Borderline Tumor. Int. J. Gynecol. Pathol. 2008, 27, 199–206. [Google Scholar] [CrossRef]
- Rambau, P.F.; McIntyre, J.B.; Taylor, J.; Lee, S.; Ogilvie, T.; Sienko, A.; Morris, D.; Duggan, M.A.; McCluggage, W.G.; Koöbel, M. Morphologic reproducibility, genotyping, and immunohistochemical profiling do not support a category of seromucinous carcinoma of the ovary. Am. J. Surg. Pathol. 2017, 41, 685–695. [Google Scholar] [CrossRef]
- Kobel, M.; Kim, K.-R.; McCluggage, W.L.; Shih, I.; Single, N. Seromucinous Carcinoma. Tumors of Ovary. In WHO Classification of Female Genital Tumors, 5th ed.; Cheung, A.N., Ellenson, L.H., Gilks, C.B., Kim, K.-R., Kong, C.S., Lax, S.F., Longacre, T.A., Malpica, A., McCluggage, W.G., Olive, E., et al., Eds.; WHO Classification of Tumors Editorial Board, International Agency for Research on Cancer (IARC): Lyon, France, 2020; Volume 70. [Google Scholar]
- Kobel, M.; Huntsman, D.G.; Lim, D.; McCluggage, W.G.; Rabban, T.T.; Shih, I. Endometrioid carcinoma of the ovary. Tumors of Ovary. In WHO Classification of Female Genital Tumors, 5th ed.; Cheung, A.N., Ellenson, L.K., Gillks, C.B., Kim, K.-R., Kong, C.S., Lax, S.F., Longacre, T.A., Malpica, A., McCluggage, W.G., Olive, E., et al., Eds.; WHO Classification of Tumors Editorial Board, International Agency for Research on Cancer (IARC): Lyon, France, 2020; Volume 70. [Google Scholar]
- Jones, J.M.; Cui, X.S.; Medina, D.; Donehower, L.A. Heterozygosity of p21WAF1/CIP1 Enhances Tumor Cell Proliferation and Cyclin D1-associated Kinase Activity in a Murine Mammary Cancer Model. Cell Growth Differ. 1999, 10, 213–222. [Google Scholar]
- Wiegand, K.C.; Shah, S.P.; Al-Agha, O.M.; Zhao, Y.; Tse, K.; Zeng, T.; Senz, J.; McConechy, M.K.; Anglesio, M.S.; Kalloger, S.E.; et al. ARID1A Mutations in Endometriosis-associated Ovarian Carcinomas. N. Engl. J. Med. 2010, 363, 1532–1543. [Google Scholar] [CrossRef]
- Guan, B.; Mao, T.L.; Panuganti, P.K.; Kuhn, E.; Kurman, R.J.; Maeda, D.; Chen, E.; Jeng, Y.M.; Wang, T.L.; Shih, I.E.M. Mutation and Loss of Expression of ARID1A in Uterine Low-grade Endometrioid Carcinoma. Am. J. Surg. Pathol. 2011, 35, 625–632. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Nakayama, K.; Nakamura, K.; Ono, R.; Sanuki, K.; Yamashita, H.; Ishibashi, T.; Minamoto, T.; Iida, K.; Razia, S.; et al. Affinity-purified DNA-based mutation profiles of endometriosis-related ovarian neoplasms in Japanese patients. Oncotarget 2018, 9, 14754–14763. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, K.; Nakayama, K.; Ishikawa, M.; Ishibashi, T.; Yamashita, H.; Nakamura, K.; Minamoto, T.; Iida, K.; Razia, S.; Ishikawa, N.; et al. Mucinous Borderline Ovarian Tumors with BRAFV600E Mutation may have Low Risk for Progression to Invasive Carcinomas. Arch. Gynecol. Obstet. 2020, 302, 487–495. [Google Scholar] [CrossRef] [PubMed]
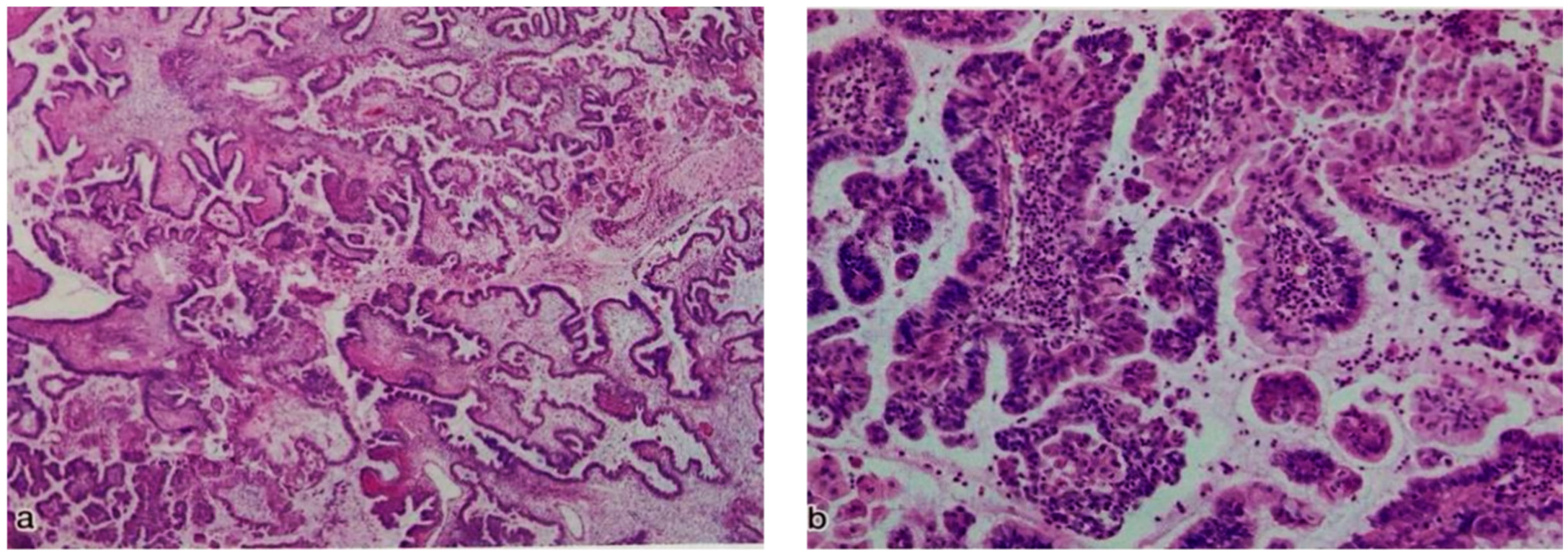
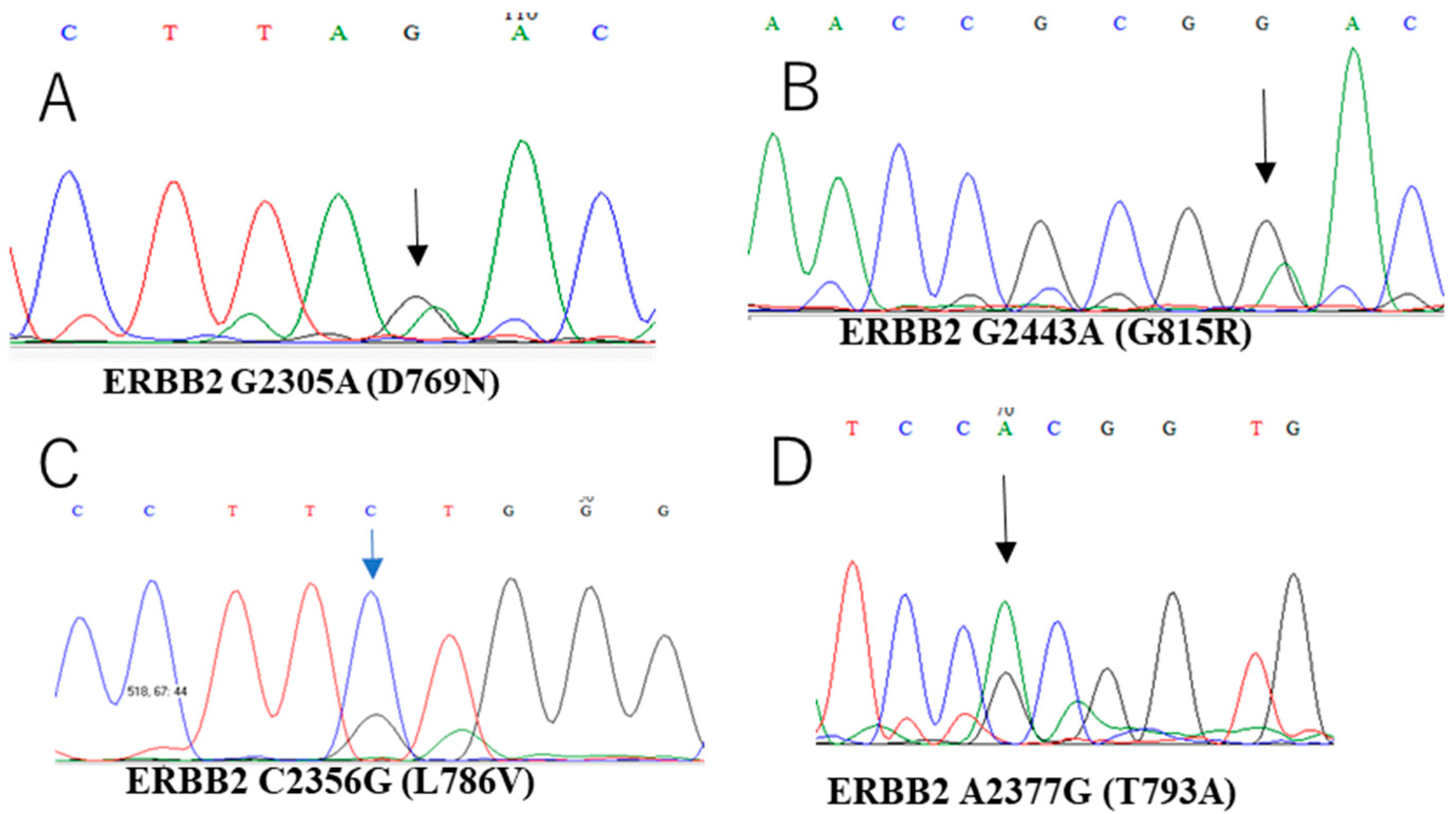

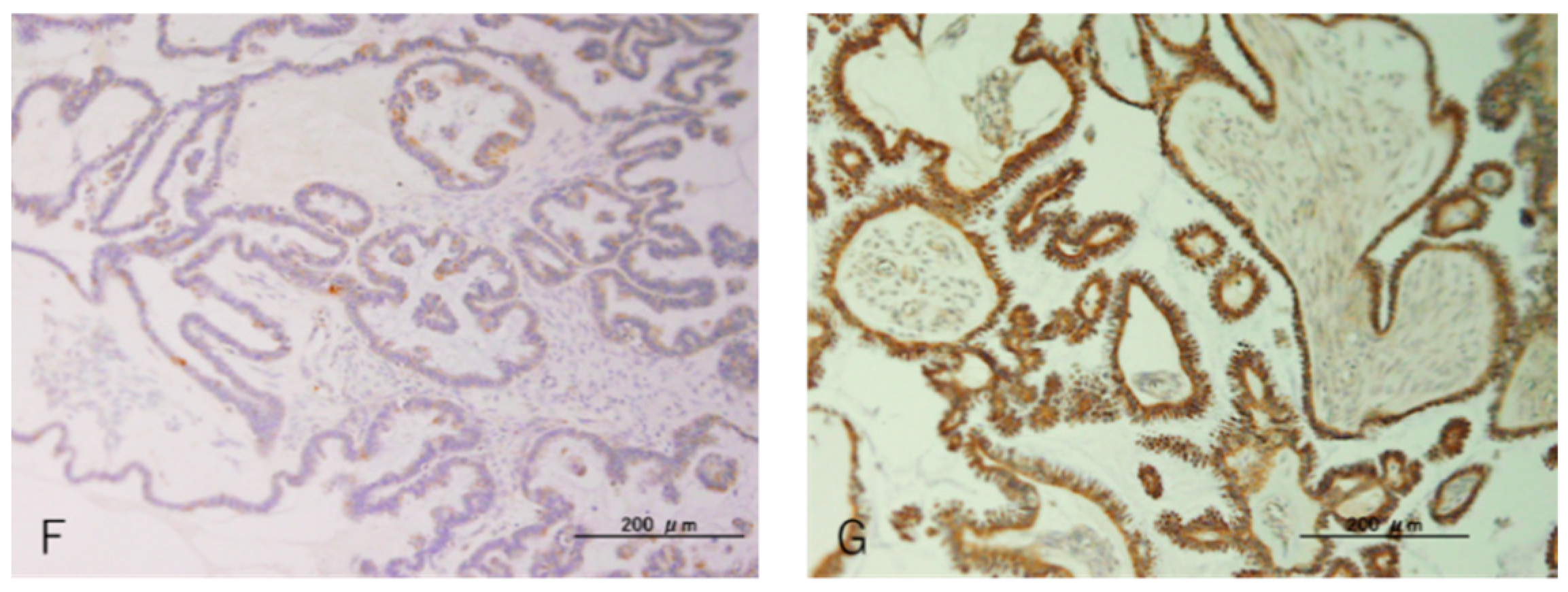
| Case NO. | Age | Stage | Site of Tumor | Tumor Size (CM) | Surgical Type | Endometriosis |
|---|---|---|---|---|---|---|
| 1 | 57 | IA | Right | 17 | BSO+TAH+omentechtomy+pelviclymphadenectomy | No |
| 2 | 66 | IA | Right | 15 | BSO+omentechtomy | No |
| 3 | 18 | IA | Left | 10 | LSO | No |
| 4 | 49 | IA | Left | 10 | BSO+TAH+omentechtomy | No |
| 5 | 28 | IA | Right | 17 | RSO+omentechtomy | No |
| 6 | 77 | IA | Right | 6 | BSO+TLH | No |
| 7 | 37 | IA | Left | 6.5 | BSO+TAH+omentechtomy | No |
| 8 | 41 | IA | Right | 9 | BSO+TAH+omentechtomy | Yes |
| 9 | 37 | IA | Right | 19 | BSO+TAH+omentechtomy+pelviclymphadenectomy | No |
| 10 | 47 | IA | Left | 22 | BSO+TAH+omentechtomy | No |
| 11 | 76 | IA | Right | ND | RSO | No |
| 12 | 54 | IA | Left | ND | LSO | No |
| 13 | 45 | IA | Right | ND | BSO | Yes |
| 14 | 58 | IA | Left | ND | BSO+TAH | Yes |
| 15 | 31 | IA | Right | 21 | RSO | No |
| 16 | 67 | IIA | Left | 9.5 | BSO+TAH+pelviclymphadenectomy | No |
| 17 | 47 | IA | Right | 12 | RSO | No |
| 18 | 26 | IA | Right | 31 | RSO | No |
| 19 | 83 | IA | Left | 3.7 | BSO+TAH | No |
| 20 | 72 | IA | Left | 9.2 | BSO | No |
| 21 | 76 | IA | Right | 5 | BSO+TAH+omentechtomy | No |
| 22 | 40 | IA | Right | 30 | RSO | No |
| 23 | 60 | IA | Left | 8.8 | LSO | No |
| Case NO. | p53 (IHC) | ARID1A (IHC) | PTEN (IHC) | KRAS | BRAF | PIK3CA-E9 | PIK3CA-E20 | ERBB2 | ERBB2 (IHC) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Normal | Normal | Normal | - | - | - | - | D769N. c.2305G>A | High |
| 2 | Loss | Normal | Normal | - | - | - | - | - | High |
| 3 | Loss | Normal | Normal | - | - | - | - | - | High |
| 4 | Overexpression | Normal | Normal | G12V, c.35G>T | - | - | - | - | Low |
| 5 | Overexpression | Normal | Normal | - | V600E, c.1799T>A | - | - | - | High |
| 6 | Normal | Normal | Normal | - | - | - | - | - | High |
| 7 | Normal | Normal | Normal | - | - | - | - | - | ND |
| 8 | Normal | Normal | Normal | - | - | - | - | - | Low |
| 9 | Normal | Normal | Normal | - | - | - | - | - | Low |
| 10 | Normal | Normal | Normal | - | - | - | - | - | Low |
| 11 | Normal | Normal | Normal | - | - | - | - | - | High |
| 12 | Normal | Loss | Normal | - | - | - | - | - | Low |
| 13 | Normal | Normal | Normal | - | - | - | - | - | ND |
| 14 | Normal | Normal | Normal | - | V600E, c.1799T>A | - | - | T793A, c.2377A>G | High |
| 15 | Normal | Normal | Normal | - | - | - | - | - | High |
| 16 | Overexpression | Normal | Normal | - | - | - | - | - | Low |
| 17 | Normal | Normal | Normal | - | - | - | - | - | Low |
| 18 | Normal | Normal | Normal | - | - | - | - | G815R, c.2443G>A | High |
| 19 | Normal | Normal | Normal | - | - | - | - | L786V, c.2356 C>G | High |
| 20 | Normal | Normal | Normal | - | - | - | - | - | High |
| 21 | Normal | Normal | Normal | - | - | T544P, c.1630A>C | - | - | High |
| 22 | Overexpression | Normal | Normal | - | - | T544P, c.1630A>C | - | - | High |
| 23 | Normal | Normal | Normal | - | - | - | - | - | High |
| Gene. | Frequency of Genetic Alteration |
|---|---|
| KRAS | 4.3% (1/23) |
| BRAF | 8.6% (2/23) |
| PIK3CA | 8.6% (2/23) |
| ERBB2 | 17.3% (4/23) |
| ARID1A | 4.3% (1/23) |
| p53 | 26% (6/23) |
| PTEN | 0% (0/23) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasamori, H.; Nakayama, K.; Razia, S.; Yamashita, H.; Ishibashi, T.; Ishikawa, M.; Sato, S.; Nakayama, S.; Otsuki, Y.; Fujiwaki, R.; et al. Mutation Profiles of Ovarian Seromucinous Borderline Tumors in Japanese Patients. Curr. Oncol. 2022, 29, 3658-3667. https://doi.org/10.3390/curroncol29050294
Sasamori H, Nakayama K, Razia S, Yamashita H, Ishibashi T, Ishikawa M, Sato S, Nakayama S, Otsuki Y, Fujiwaki R, et al. Mutation Profiles of Ovarian Seromucinous Borderline Tumors in Japanese Patients. Current Oncology. 2022; 29(5):3658-3667. https://doi.org/10.3390/curroncol29050294
Chicago/Turabian StyleSasamori, Hiroki, Kentaro Nakayama, Sultana Razia, Hitomi Yamashita, Tomoka Ishibashi, Masako Ishikawa, Seiya Sato, Satoru Nakayama, Yoshiro Otsuki, Ritsuto Fujiwaki, and et al. 2022. "Mutation Profiles of Ovarian Seromucinous Borderline Tumors in Japanese Patients" Current Oncology 29, no. 5: 3658-3667. https://doi.org/10.3390/curroncol29050294
APA StyleSasamori, H., Nakayama, K., Razia, S., Yamashita, H., Ishibashi, T., Ishikawa, M., Sato, S., Nakayama, S., Otsuki, Y., Fujiwaki, R., Ishikawa, N., & Kyo, S. (2022). Mutation Profiles of Ovarian Seromucinous Borderline Tumors in Japanese Patients. Current Oncology, 29(5), 3658-3667. https://doi.org/10.3390/curroncol29050294





