Primary Angiosarcoma of the Breast: A Single-Center Retrospective Study in Korea
Abstract
:1. Introduction
2. Materials and Methods
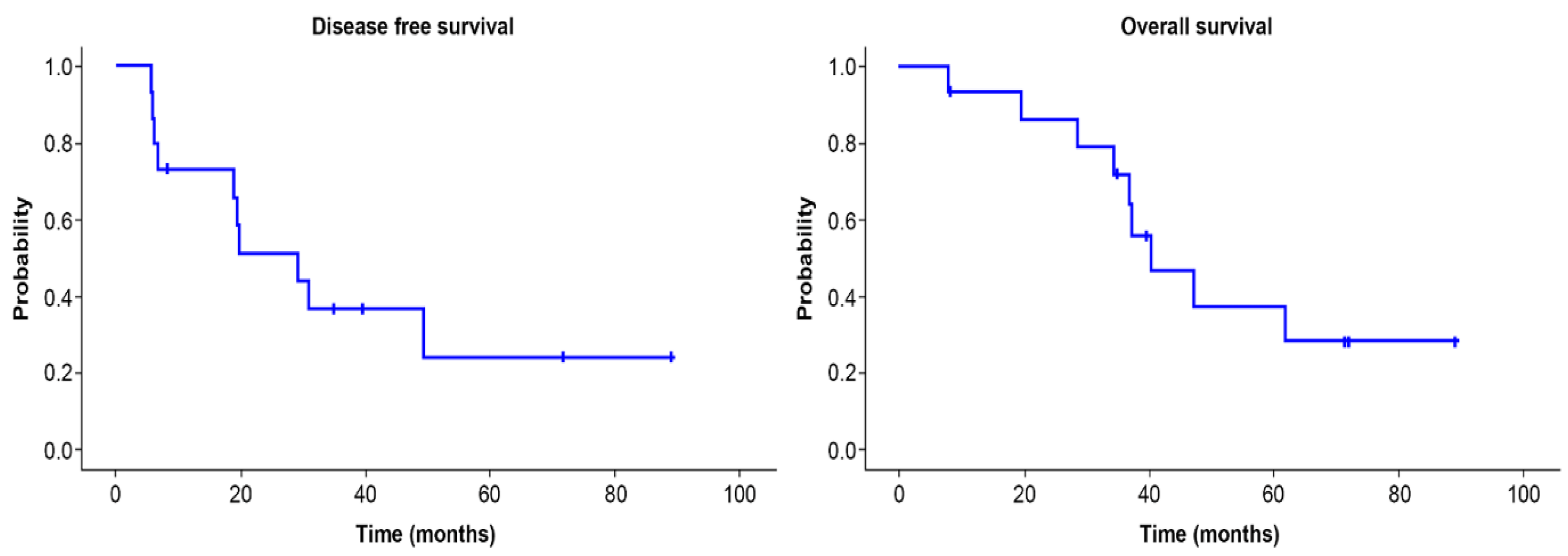
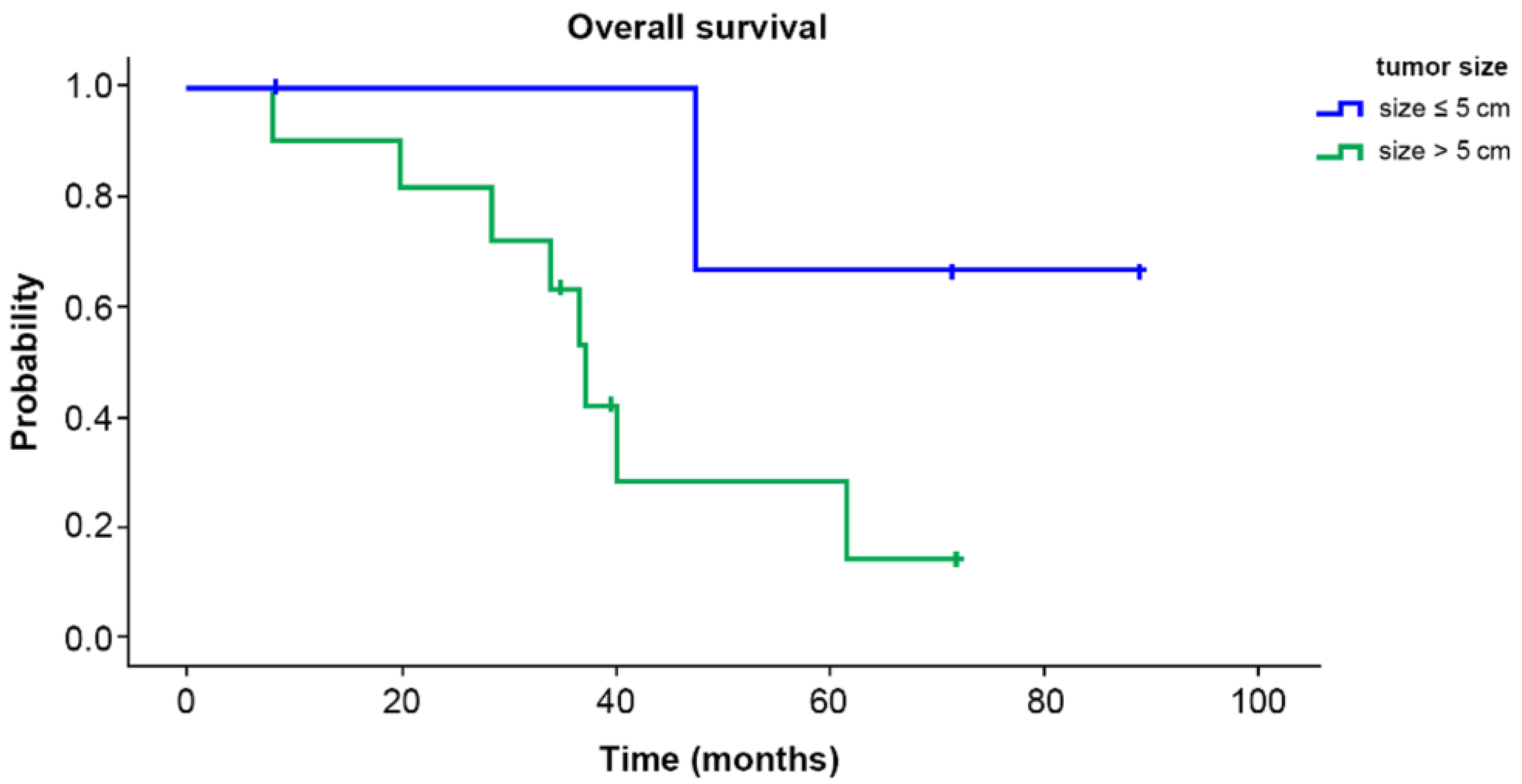
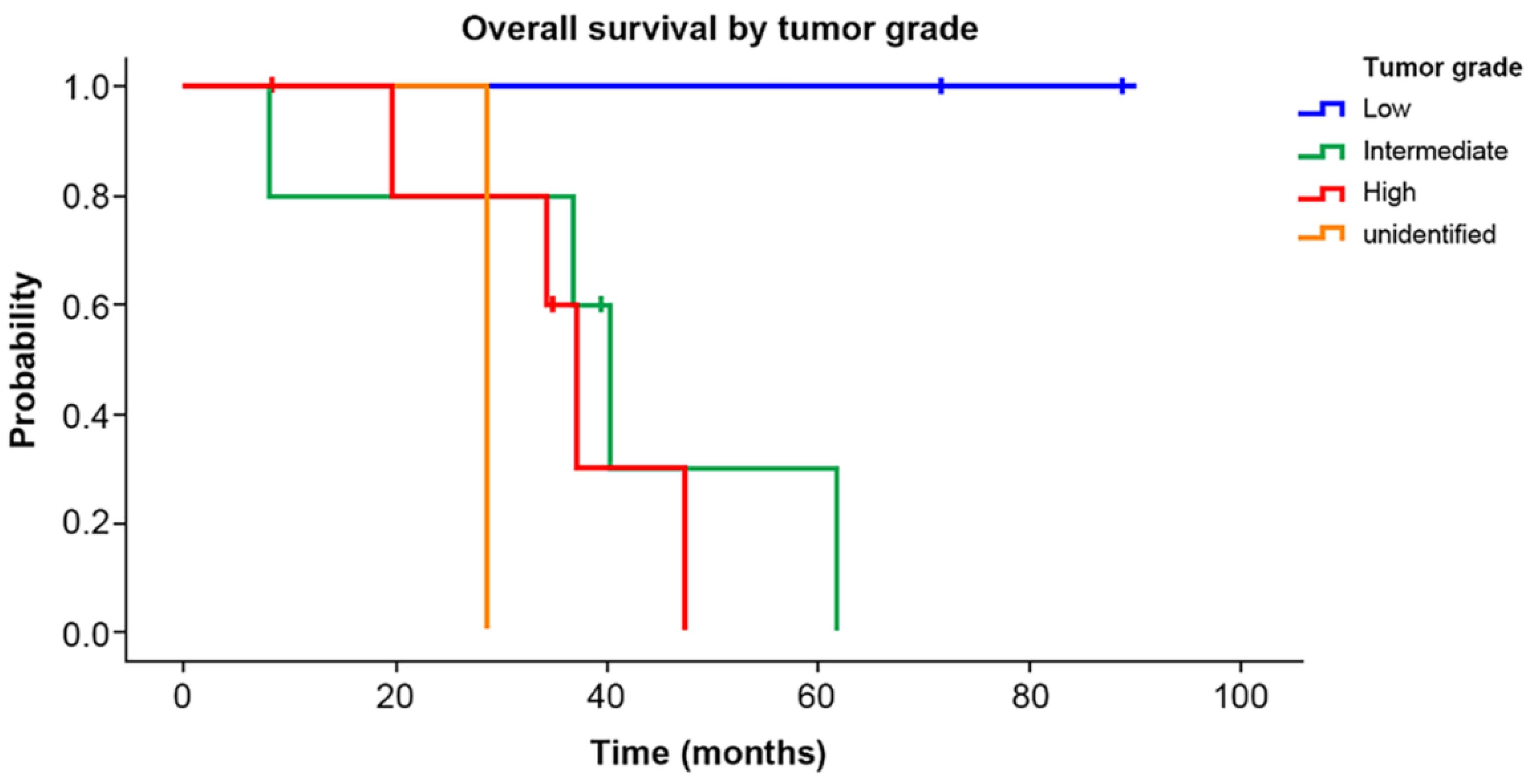
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hodgson, N.C.; Bowen-Wells, C.; Moffat, F.; Franceschi, D.; Avisar, E. Angiosarcomas of the breast: A review of 70 cases. Am. J. Clin. Oncol. 2007, 30, 570–573. [Google Scholar] [CrossRef] [PubMed]
- Abdou, Y.; Elkhanany, A.; Attwood, K.; Ji, W.; Takabe, K.; Opyrchal, M. Primary and secondary breast angiosarcoma: Single center report and a meta-analysis. Breast Cancer Res. Treat. 2019, 178, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Chae, B.J.; Kim, S.H.; Kang, B.J.; Song, B.J.; Lee, A. Primary angiosarcoma of the breast: A case report. Int. J. Clin. Exp. Pathol. 2019, 12, 664–668. [Google Scholar] [PubMed]
- Hui, A.; Henderson, M.; Speakman, D.; Skandarajah, A. Angiosarcoma of the breast: A difficult surgical challenge. Breast 2012, 21, 584–589. [Google Scholar] [CrossRef]
- Bae, S.Y.; Choi, M.Y.; Cho, D.H.; Lee, J.E.; Nam, S.J.; Yang, J.H. Large clinical experience of primary angiosarcoma of the breast in a single Korean medical institute. World J. Surg. 2011, 35, 2417–2421. [Google Scholar] [CrossRef]
- Biswas, T.; Tang, P.; Muhs, A.; Ling, M. Angiosarcoma of the breast: A rare clinicopathological entity. Am. J. Clin. Oncol. 2009, 32, 582–586. [Google Scholar] [CrossRef]
- Russo, D.; Campanino, M.R.; Cepurnaite, R.; Gencarelli, A.; De Rosa, F.; Corvino, A.; Menkulazi, M.; Tammaro, V.; Fuggi, M.; Insabato, L. Primary High-Grade Angiosarcoma of the Breast in a Young Woman with Breast Implants: A Rare Case and a Review of Literature. Int. J. Surg. Pathol. 2020, 28, 906–912. [Google Scholar] [CrossRef]
- Johnson, C.M.; Garguilo, G.A. Angiosarcoma of the breast: A case report and literature review. Curr. Surg. 2002, 59, 490–494. [Google Scholar] [CrossRef]
- Johnstone, P.A.; Pierce, L.J.; Merino, M.J.; Yang, J.C.; Epstein, A.H.; DeLaney, T.F. Primary soft tissue sarcomas of the breast: Local-regional control with post-operative radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 1993, 27, 671–675. [Google Scholar] [CrossRef]
- Pandey, M.; Mathew, A.; Abraham, E.K.; Rajan, B. Primary sarcoma of the breast. J. Surg. Oncol. 2004, 87, 121–125. [Google Scholar] [CrossRef]
- Torres, K.E.; Ravi, V.; Kin, K.; Yi, M.; Guadagnolo, B.A.; May, C.D.; Arun, B.K.; Hunt, K.K.; Lam, R.; Lahat, G.; et al. Long-term outcomes in patients with radiation-associated angiosarcomas of the breast following surgery and radiotherapy for breast cancer. Ann. Surg. Oncol. 2013, 20, 1267–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Depla, A.L.; Scharloo-Karels, C.H.; de Jong, M.A.A.; Oldenborg, S.; Kolff, M.W.; Oei, S.B.; van Coevorden, F.; van Rhoon, G.C.; Baartman, E.A.; Scholten, R.J.; et al. Treatment and prognostic factors of radiation-associated angiosarcoma (RAAS) after primary breast cancer: A systematic review. Eur. J. Cancer 2014, 50, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Sher, T.; Hennessy, B.T.; Valero, V.; Broglio, K.; Woodward, W.A.; Trent, J.; Hunt, K.K.; Hortobagyi, G.N.; Gonzalez-Angulo, A.M. Primary angiosarcomas of the breast. Cancer 2007, 110, 173–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.Y.; Kim, Y.S.; Kim, Z.; Kim, H.Y.; Kim, H.J.; Park, S.; Bae, S.Y.; Yoon, K.H.; Lee, S.B.; Lee, S.K.; et al. Breast Cancer Statistics in Korea in 2017: Data from a Breast Cancer Registry. J. Breast Cancer 2020, 23, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.Y.; Jakowski, J.; Tawfik, O.W.; Thomas, P.A.; Fan, F. Angiosarcoma of the breast: A clinicopathologic analysis of cases from the last 10 years. Ann. Diagn. Pathol. 2009, 13, 147–150. [Google Scholar] [CrossRef]
- Yin, M.; Wang, W.; Drabick, J.J.; Harold, H.A. Prognosis and treatment of non-metastatic primary and secondary breast angiosarcoma: A comparative study. BMC Cancer 2017, 17, 295. [Google Scholar] [CrossRef] [Green Version]
- Donnell, R.M.; Rosen, P.P.; Lieberman, P.H.; Kaufman, R.J.; Kay, S.; Braun, D.W., Jr.; Kinne, D.W. Angiosarcoma and other vascular tumors of the breast. Am. J. Surg. Pathol. 1981, 5, 629–642. [Google Scholar] [CrossRef]
- Kunkiel, M.; Maczkiewicz, M.; Jagiello-Gruszfeld, A.; Nowecki, Z. Primary angiosarcoma of the breast-series of 11 consecutive cases-a single-centre experience. Curr. Oncol. 2018, 25, e50–e53. [Google Scholar] [CrossRef] [Green Version]
- Arora, T.K.; Terracina, K.P.; Soong, J.; Idowu, M.O.; Takabe, K. Primary and secondary angiosarcoma of the breast. Gland Surg. 2014, 3, 28–34. [Google Scholar] [CrossRef]
- Bousquet, G.; Confavreux, C.; Magné, N.; de Lara, C.T.; Poortmans, P.; Senkus, E.; de Lafontan, B.; Bolla, M.; Largillier, R.; Lagneau, E.; et al. Outcome and prognostic factors in breast sarcoma: A multicenter study from the rare cancer network. Radiother. Oncol. 2007, 85, 355–361. [Google Scholar] [CrossRef]
- Vorburger, S.A.; Xing, Y.; Hunt, K.K.; Lakin, G.E.; Benjamin, R.S.; Feig, B.W.; Pisters, P.W.T.; Ballo, M.T.; Chen, L.; Trent, J., III; et al. Angiosarcoma of the breast. Cancer 2005, 104, 2682–2688. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.C.; Aft, R.L.; Gillanders, W.E.; Eberlein, T.J.; Margenthaler, J.A. Treatment and outcomes of patients with primary breast sarcoma. Am. J. Surg. 2008, 196, 559–561. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, E.R.; Bhargava, R.; Vargo, J.A.; Florea, A.V.; Beriwal, S. Primary and Radiation-induced Breast Angiosarcoma: Clinicopathologic Predictors of Outcomes and the Impact of Adjuvant Radiation Therapy. Am. J. Clin. Oncol. 2016, 39, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Toesca, A.; Spitaleri, G.; De Pas, T.; Botteri, E.; Gentilini, O.; Bottiglieri, L.; Rotmentsz, N.; Sangalli, C.; Marrazzo, E.; Cassano, E.; et al. Sarcoma of the breast: Outcome and reconstructive options. Clin. Breast Cancer 2012, 12, 438–444. [Google Scholar] [CrossRef]
- Ragavan, S.; Lim, H.J.; Tan, J.W.; Hendrikson, J.; Chan, J.Y.; Farid, M.; Chia, C.S.; Tan, G.H.C.; Soo, K.C.; Teo, M.C.C.; et al. Axillary Lymph Node Dissection in Angiosarcomas of the Breast: An Asian Institutional Perspective. Sarcoma 2020, 2020, 4890803. [Google Scholar] [CrossRef]

| Clinicopathological Features | No. of Patients (%) | |
|---|---|---|
| Age | ||
| Median (range) | 33 years (range 14–63 years) | |
| Grade | Low | 3 (20.0) |
| Intermediate | 5 (33.3) | |
| High | 6 (40.0) | |
| Unknown | 1 (6.7) | |
| Tumor size (cm) | >5 cm | 11 (73.3) |
| ≤5 cm | 4 (26.7) | |
| Operation | Mastectomy | 13 (86.7) |
| Wide excision | 2 (13.3) | |
| Adjuvant chemotherapy | Yes | 4 (26.7) |
| No | 11 (73.3) | |
| Adjuvant Radiotherapy | Yes | 8 (53.3) |
| No | 7 (46.7) | |
| Patient | Age | Grade | Tumor Size (cm) | Surgery (Date, Type) | Adjuvant Chemotherapy | Adjuvant Radiotherapy | Recurrence | Treatment of 1st Recurrence |
|---|---|---|---|---|---|---|---|---|
| 1 | 35 | 3 | 6.0 | 27 November 1997 Lt. Total mastectomy | No | Yes | Local (Lt. chest skin) | Wide excision |
| 2 | 31 | 2 | 10.0 | 27 October 1999 Rt. Total mastectomy | No | Yes | Distant (Bone) | Palliative chemoTx. |
| 3 | 29 | 3 | 4.2 | 02 December 1999 Lt. Total mastectomy | No | Yes | Local (Lt. chest skin) | Palliative chemoTx. |
| 4 | 19 | 2 | 11.2 | 27 February 2001 Rt. Total mastectomy | No | Yes | Local (Rt. chest skin) | Wide excision |
| 5 | 21 | 2 | 10.0 | 02 April 2004 Lt. Total mastectomy +ALND | No | No | Distant (Bone) | Palliative chemoTx. |
| 6 | 44 | 1 | 3.5 | 24 December 2009 Lt. wide excision | No | No | No | |
| 7 | 28 | 2 | 8.0 | 30 March 2010 Rt. Total mastectomy +ALND | Yes AI # 4 + paclitaxel # 4 | Yes | Local contralateral breast (Lt. chest skin) | Wide excision |
| 8 | 14 | unidentified | 25.0 | 17 February 2011 Rt. Total mastectomy | Yes EI (# 45) | No | Distant (Bone) | Palliative chemoTx. |
| 9 | 47 | 3 | 5.5 | 22 April 2011 Rt. Total mastectomy +ALND | No | No | Local contralateral breast (Lt. chest skin) | Wide excision |
| 10 | 63 | 1 | 1.0 | 20 August 2013 Rt. wide excision | No | No | No | |
| 11 | 14 | 3 | 9.0 | 28 February 2014 Lt. Total mastectomy + SLNBx, Rt. Wide excision | No | No | Local +Distant (Lt.chest skin, Lung) | Palliative chemoTx. |
| 12 | 47 | 1 | 5.5 | 25 August 2015 Lt. Total mastectomy +ALND | Neoadjuvant Tx. AC # 4+ D # 4 | No | Local (Lt. chest skin) | Wide excision |
| 13 | 43 | 2 | 5.5 | 21 December 2017 Rt. Total mastectomy + SLNBx | No | Yes | No | |
| 14 | 25 | 3 | 7.5 | 24 August 2018 Rt. Skin sparing mastectomy +SLNBx | Yes AC # 4 | Yes | No | |
| 15 | 41 | 3 | 3.0 | 22 May 2020 Lt. Total mastectomy + SLNBx | Yes AC # 4 | Yes | No |
| Outcomes | No. of Patients (%) | |
|---|---|---|
| Recurrence | Local | 4 (26.7) |
| Local contralateral breast | 2 (13.3) | |
| Distant | 3 (20.0) | |
| Local + Distant | 1 (6.7) | |
| No recurrence | 5 (33.3) | |
| Survival | Alive | 6 (40.0) |
| Death | 9 (60.0) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, Y.-J.; Ryu, J.-M.; Lee, S.-K.; Chae, B.-J.; Kim, S.-W.; Nam, S.-J.; Yu, J.-H.; Lee, J.-E. Primary Angiosarcoma of the Breast: A Single-Center Retrospective Study in Korea. Curr. Oncol. 2022, 29, 3272-3281. https://doi.org/10.3390/curroncol29050267
Kim Y-J, Ryu J-M, Lee S-K, Chae B-J, Kim S-W, Nam S-J, Yu J-H, Lee J-E. Primary Angiosarcoma of the Breast: A Single-Center Retrospective Study in Korea. Current Oncology. 2022; 29(5):3272-3281. https://doi.org/10.3390/curroncol29050267
Chicago/Turabian StyleKim, Yeon-Jin, Jai-Min Ryu, Se-Kyung Lee, Byung-Joo Chae, Seok-Won Kim, Seok-Jin Nam, Jong-Han Yu, and Jeong-Eon Lee. 2022. "Primary Angiosarcoma of the Breast: A Single-Center Retrospective Study in Korea" Current Oncology 29, no. 5: 3272-3281. https://doi.org/10.3390/curroncol29050267
APA StyleKim, Y.-J., Ryu, J.-M., Lee, S.-K., Chae, B.-J., Kim, S.-W., Nam, S.-J., Yu, J.-H., & Lee, J.-E. (2022). Primary Angiosarcoma of the Breast: A Single-Center Retrospective Study in Korea. Current Oncology, 29(5), 3272-3281. https://doi.org/10.3390/curroncol29050267




