DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Population
2.2. MRI
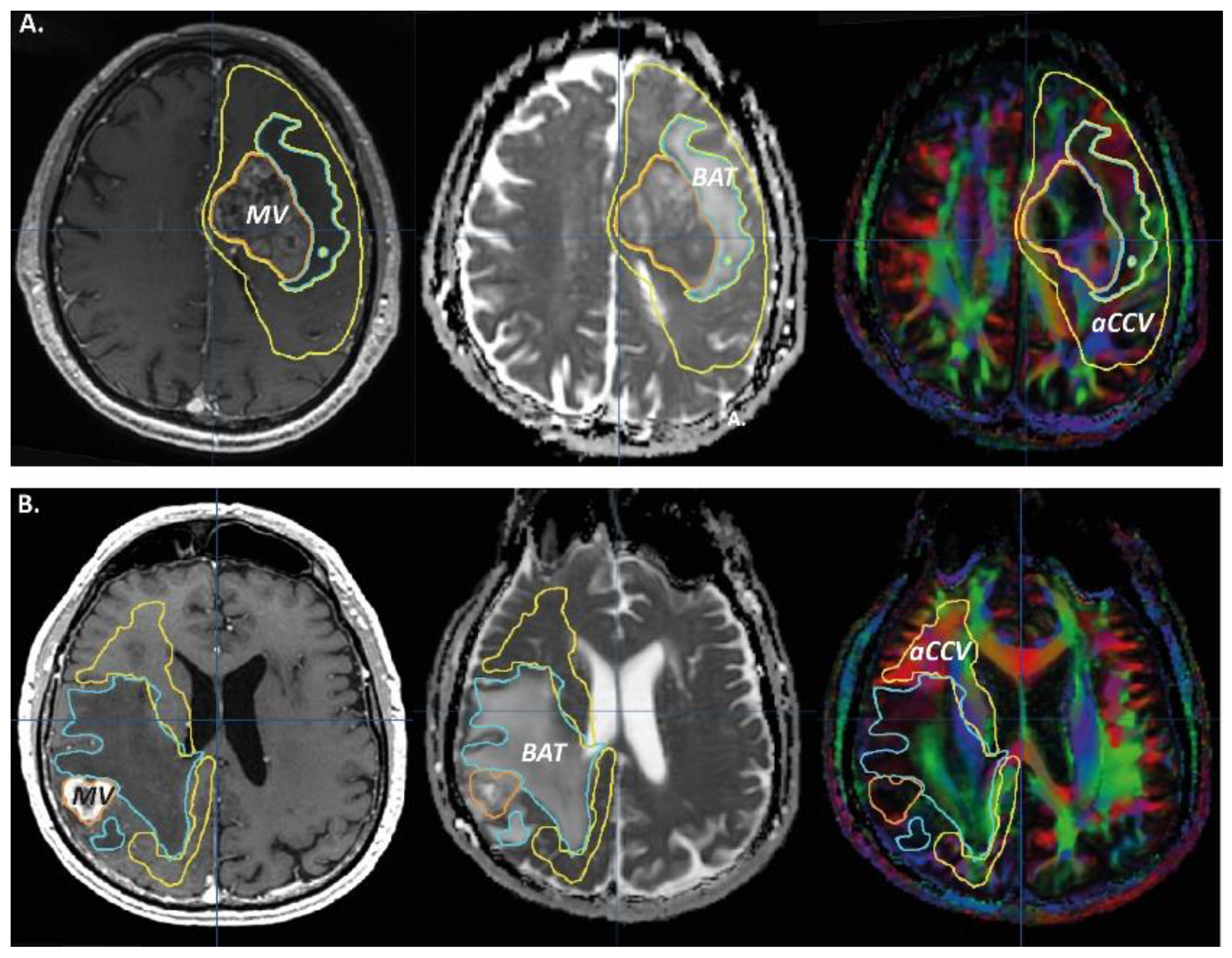
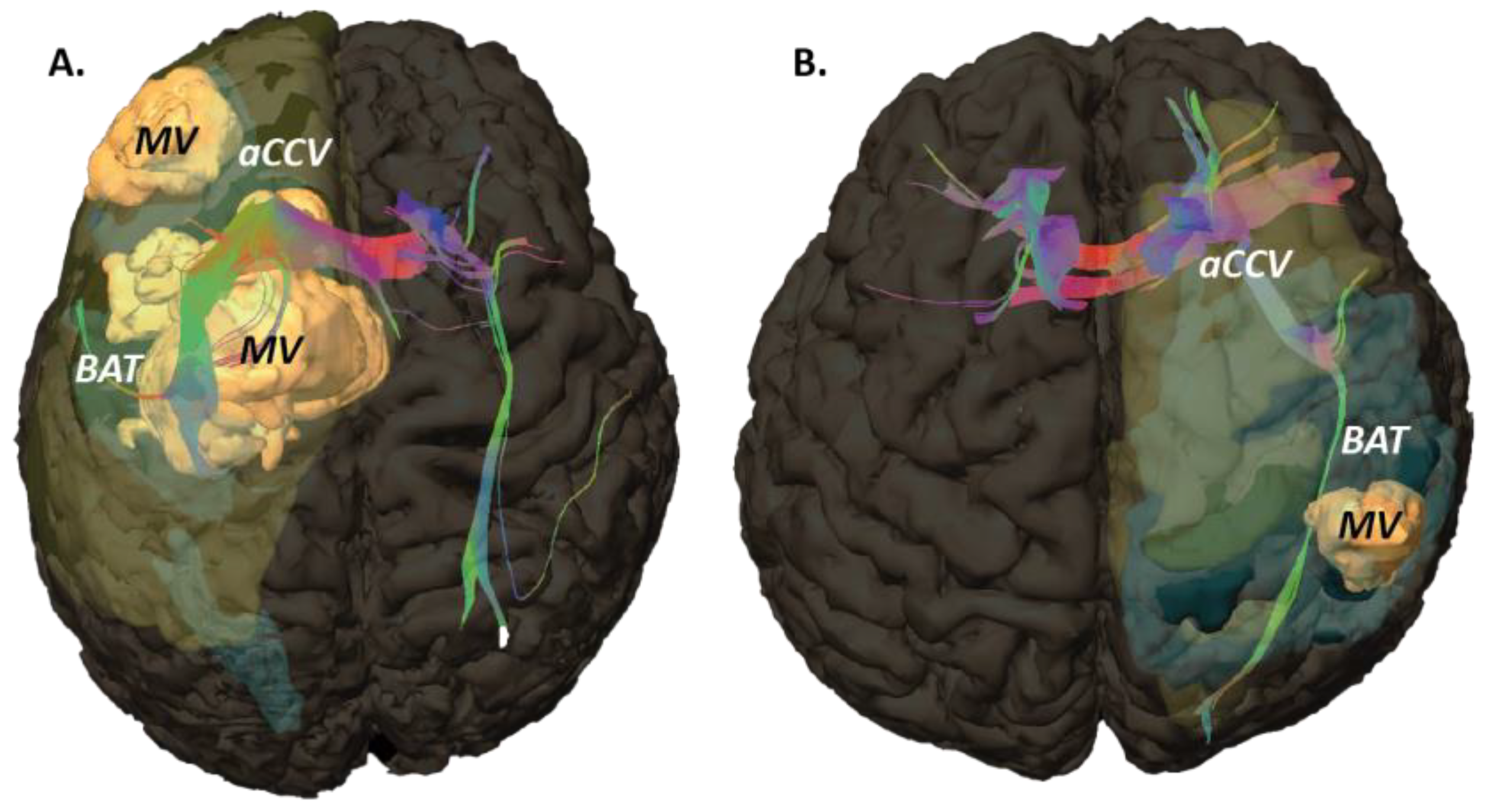
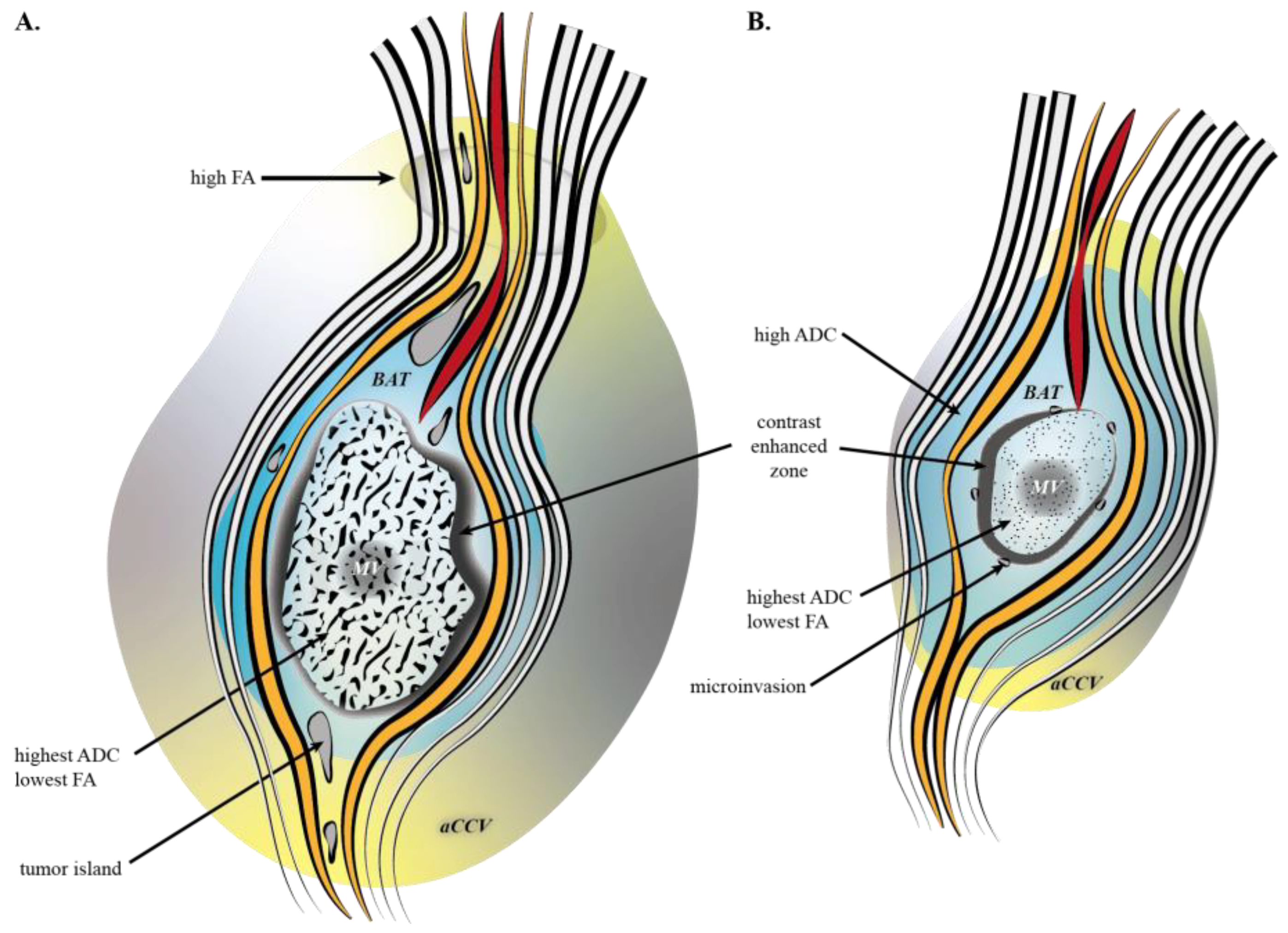
2.3. MRI Objects
2.4. Data Analysis
3. Results
3.1. Tumoral Related Volumes
3.2. ROIs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO Classification of Tumours of the Central Nervous System. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perry, A.; Wesseling, P. Histologic Classification of Gliomas. Handb. Clin. Neurol. 2016, 134, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Wesseling, P.; Capper, D. WHO 2016 Classification of Gliomas. Neuropathol. Appl. Neurobiol. 2018, 44, 139–150. [Google Scholar] [CrossRef]
- Kelly, P.J.; Daumas-Duport, C.; Kispert, D.B.; Kall, B.A.; Scheithauer, B.W.; Illig, J.J. Imaging-Based Stereotaxic Serial Biopsies in Untreated Intracranial Glial Neoplasms. J. Neurosurg. 1987, 66, 865–874. [Google Scholar] [CrossRef]
- Sahm, F.; Capper, D.; Jeibmann, A.; Habel, A.; Paulus, W.; Troost, D.; von Deimling, A. Addressing Diffuse Glioma as a Systemic Brain Disease with Single-Cell Analysis. Arch. Neurol. 2012, 69, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Osswald, M.; Jung, E.; Sahm, F.; Solecki, G.; Venkataramani, V.; Blaes, J.; Weil, S.; Horstmann, H.; Wiestler, B.; Syed, M.; et al. Brain Tumour Cells Interconnect to a Functional and Resistant Network. Nature 2015, 528, 93–98. [Google Scholar] [CrossRef]
- Scherer, H.J. A critical review: The pathology of cerebral gliomas. J. Neurol. Psychiatry 1940, 3, 147–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rees, J.H.; Smirniotopoulos, J.G.; Jones, R.V.; Wong, K. Glioblastoma Multiforme: Radiologic-Pathologic Correlation. Radiographics 1996, 16, 1413–1438; quiz 1462–1463. [Google Scholar] [CrossRef] [Green Version]
- Burnet, N.G.; Thomas, S.J.; Burton, K.E.; Jefferies, S.J. Defining the Tumour and Target Volumes for Radiotherapy. Cancer Imaging 2004, 4, 153–161. [Google Scholar] [CrossRef] [Green Version]
- Schiffer, D.; Annovazzi, L.; Caldera, V.; Mellai, M. On the Origin and Growth of Gliomas. Anticancer Res. 2010, 30, 1977–1998. [Google Scholar]
- Yan, J.-L.; Li, C.; Boonzaier, N.R.; Fountain, D.M.; Larkin, T.J.; Matys, T.; van der Hoorn, A.; Price, S.J. Multimodal MRI Characteristics of the Glioblastoma Infiltration beyond Contrast Enhancement. Ther. Adv. Neurol. Disord. 2019, 12, 1756286419844664. [Google Scholar] [CrossRef] [PubMed]
- Yamahara, T.; Numa, Y.; Oishi, T.; Kawaguchi, T.; Seno, T.; Asai, A.; Kawamoto, K. Morphological and Flow Cytometric Analysis of Cell Infiltration in Glioblastoma: A Comparison of Autopsy Brain and Neuroimaging. Brain Tumor. Pathol. 2010, 27, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Price, S.J.; Gillard, J.H. Imaging Biomarkers of Brain Tumour Margin and Tumour Invasion. Br. J. Radiol. 2011, 84, S159–S167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Tanaka, R.; Takeda, N. Magnetic Resonance Imaging and Histopathology of Cerebral Gliomas. Neuroradiology 1992, 34, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Jiang, R.; Du, F.-Z.; He, C.; Gu, M.; Ke, Z.-W.; Li, J.-H. The Value of Diffusion Tensor Imaging in Differentiating High-Grade Gliomas from Brain Metastases: A Systematic Review and Meta-Analysis. PLoS ONE 2014, 9, e112550. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.; Yan, Y.; Zhong, D.; Yang, G.; Tang, W.; Lü, F.; Xie, B.; Liu, B. Quantitative Analysis of Glioma Cell Invasion by Diffusion Tensor Imaging. J. Clin. Neurosci. 2010, 17, 1530–1536. [Google Scholar] [CrossRef] [PubMed]
- Kolakshyapati, M.; Adhikari, R.B.; Karlowee, V.; Takayasu, T.; Nosaka, R.; Amatya, V.J.; Takeshima, Y.; Akiyama, Y.; Sugiyama, K.; Kurisu, K.; et al. Nonenhancing Peritumoral Hyperintense Lesion on Diffusion-Weighted Imaging in Glioblastoma: A Novel Diagnostic and Specific Prognostic Indicator. J. Neurosurg. 2017, 128, 667–678. [Google Scholar] [CrossRef] [Green Version]
- Price, S.J.; Burnet, N.G.; Donovan, T.; Green, H.A.L.; Peña, A.; Antoun, N.M.; Pickard, J.D.; Carpenter, T.A.; Gillard, J.H. Diffusion Tensor Imaging of Brain Tumours at 3T: A Potential Tool for Assessing White Matter Tract Invasion? Clin. Radiol. 2003, 58, 455–462. [Google Scholar] [CrossRef]
- Won, Y.I.; Chung, C.K.; Kim, C.H.; Park, C.-K.; Koo, B.-B.; Lee, J.-M.; Jung, H.-W. White Matter Change Revealed by Diffusion Tensor Imaging in Gliomas. Brain Tumor Res. Treat. 2016, 4, 100–106. [Google Scholar] [CrossRef]
- Kallenberg, K.; Goldmann, T.; Menke, J.; Strik, H.; Bock, H.C.; Stockhammer, F.; Buhk, J.H.; Frahm, J.; Dechent, P.; Knauth, M. Glioma Infiltration of the Corpus Callosum: Early Signs Detected by DTI. J. Neurooncol. 2013, 112, 217–222. [Google Scholar] [CrossRef] [Green Version]
- Stadlbauer, A.; Hammen, T.; Grummich, P.; Buchfelder, M.; Kuwert, T.; Dörfler, A.; Nimsky, C.; Ganslandt, O. Classification of Peritumoral Fiber Tract Alterations in Gliomas Using Metabolic and Structural Neuroimaging. J. Nucl. Med. 2011, 52, 1227–1234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, T.P.L.; Liu, F.; Kassner, A.; Mori, S.; Guha, A. Fiber Density Index Correlates with Reduced Fractional Anisotropy in White Matter of Patients with Glioblastoma. AJNR Am. J. Neuroradiol. 2005, 26, 2183–2186. [Google Scholar] [PubMed]
- Del Bene, M.; Osti, D.; Faletti, S.; Beznousenko, G.V.; DiMeco, F.; Pelicci, G. Extracellular Vesicles: The Key for Precision Medicine in Glioblastoma. Neuro-Oncology 2021, 24, 184–196. [Google Scholar] [CrossRef] [PubMed]
- Lao, J.; Chen, Y.; Li, Z.-C.; Li, Q.; Zhang, J.; Liu, J.; Zhai, G. A Deep Learning-Based Radiomics Model for Prediction of Survival in Glioblastoma Multiforme. Sci. Rep. 2017, 7, 10353. [Google Scholar] [CrossRef] [PubMed]
- Roesler, R.; Dini, S.A.; Isolan, G.R. Neuroinflammation and Immunoregulation in Glioblastoma and Brain Metastases: Recent Developments in Imaging Approaches. Clin. Exp. Immunol. 2021, 206, 314–324. [Google Scholar] [CrossRef]
- Wen, P.Y.; Reardon, D.A. Neuro-Oncology in 2015: Progress in Glioma Diagnosis, Classification and Treatment. Nat. Rev. Neurol. 2016, 12, 69–70. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Rajky, O.; Winkler, F.; Bartsch, R.; Furtner, J.; Hainfellner, J.A.; Goodman, S.L.; Weller, M.; Schittenhelm, J.; Preusser, M. Invasion Patterns in Brain Metastases of Solid Cancers. Neuro-Oncology 2013, 15, 1664–1672. [Google Scholar] [CrossRef] [Green Version]
- Siam, L.; Bleckmann, A.; Chaung, H.-N.; Mohr, A.; Klemm, F.; Barrantes-Freer, A.; Blazquez, R.; Wolff, H.A.; Lüke, F.; Rohde, V.; et al. The Metastatic Infiltration at the Metastasis/Brain Parenchyma-Interface Is Very Heterogeneous and Has a Significant Impact on Survival in a Prospective Study. Oncotarget 2015, 6, 29254–29267. [Google Scholar] [CrossRef] [Green Version]
- Belli, G.; Busoni, S.; Ciccarone, A.; Coniglio, A.; Esposito, M.; Giannelli, M.; Mazzoni, L.N.; Nocetti, L.; Sghedoni, R.; Tarducci, R.; et al. Quality Assurance Multicenter Comparison of Different MR Scanners for Quantitative Diffusion-Weighted Imaging. J. Magn. Reson. Imaging 2016, 43, 213–219. [Google Scholar] [CrossRef]
- Nestler, U.; Lutz, K.; Pichlmeier, U.; Stummer, W.; Franz, K.; Reulen, H.-J.; Bink, A.; on behalf of the 5-ALA Glioma Study Group. Anatomic Features of Glioblastoma and Their Potential Impact on Survival. Acta Neurochir. 2015, 157, 179–186. [Google Scholar] [CrossRef]
- Barz, M.; Gerhardt, J.; Bette, S.; Aftahy, A.K.; Huber, T.; Combs, S.E.; Ryang, Y.-M.; Wiestler, B.; Skardelly, M.; Gepfner-Tuma, I.; et al. Prognostic Value of Tumour Volume in Patients with a Poor Karnofsky Performance Status Scale – a Bicentric Retrospective Study. BMC Neurol. 2021, 21, 446. [Google Scholar] [CrossRef]
- Wijetunga, A.R.; Jayamanne, D.T.; Adams, J.; Back, M.F. Volumetric Response of Limited Brain Metastatic Disease to Focal Hypofractionated Radiation Therapy. Brain Sci. 2021, 11, 1457. [Google Scholar] [CrossRef] [PubMed]
- Klimas, A.; Drzazga, Z.; Kluczewska, E.; Hartel, M. Regional ADC Measurements during Normal Brain Aging in the Clinical Range of b Values: A DWI Study. Clin. Imaging 2013, 37, 637–644. [Google Scholar] [CrossRef] [PubMed]
- Löbel, U.; Sedlacik, J.; Reddick, W.E.; Kocak, M.; Ji, Q.; Broniscer, A.; Hillenbrand, C.M.; Patay, Z. Quantitative Diffusion-Weighted and Dynamic Susceptibility-Weighted Contrast-Enhanced Perfusion MR Imaging Analysis of T2 Hypointense Lesion Components in Pediatric Diffuse Intrinsic Pontine Glioma. AJNR Am. J. Neuroradiol. 2011, 32, 315–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naganawa, S.; Sato, K.; Katagiri, T.; Mimura, T.; Ishigaki, T. Regional ADC Values of the Normal Brain: Differences Due to Age, Gender, and Laterality. Eur. Radiol. 2003, 13, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Sener, R.N. Diffusion MRI: Apparent Diffusion Coefficient (ADC) Values in the Normal Brain and a Classification of Brain Disorders Based on ADC Values. Comput. Med. Imaging Graph. 2001, 25, 299–326. [Google Scholar] [CrossRef]
- Mori, S.; van Zijl, P.C.M. Fiber Tracking: Principles and Strategies—A Technical Review. NMR Biomed. 2002, 15, 468–480. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Zilles, K.; Amunts, K.; Faria, A.; Jiang, H.; Li, X.; Akhter, K.; Hua, K.; Woods, R.; Toga, A.W.; et al. Human Brain White Matter Atlas: Identification and Assignment of Common Anatomical Structures in Superficial White Matter. NeuroImage 2008, 43, 447–457. [Google Scholar] [CrossRef] [Green Version]
- Vassal, F.; Schneider, F.; Boutet, C.; Jean, B.; Sontheimer, A.; Lemaire, J.-J. Combined DTI Tractography and Functional MRI Study of the Language Connectome in Healthy Volunteers: Extensive Mapping of White Matter Fascicles and Cortical Activations. PLoS ONE 2016, 11, e0152614. [Google Scholar] [CrossRef]
- Löbel, U.; Sedlacik, J.; Güllmar, D.; Kaiser, W.; Reichenbach, J.; Mentzel, H.-J. Diffusion Tensor Imaging: The Normal Evolution of ADC, RA, FA, and Eigenvalues Studied in Multiple Anatomical Regions of the Brain. Neuroradiology 2009, 51, 253–263. [Google Scholar] [CrossRef]
- Lawrenz, M.; Brassen, S.; Finsterbusch, J. Microscopic Diffusion Anisotropy in the Human Brain: Reproducibility, Normal Values, and Comparison with the Fractional Anisotropy. NeuroImage 2015, 109, 283–297. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.J.; Westin, C.-F. An Introduction to Diffusion Tensor Image Analysis. Neurosurg. Clin. N. Am. 2011, 22, 185–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortin, J.-P.; Parker, D.; Tunç, B.; Watanabe, T.; Elliott, M.A.; Ruparel, K.; Roalf, D.R.; Satterthwaite, T.D.; Gur, R.C.; Gur, R.E.; et al. Harmonization of Multi-Site Diffusion Tensor Imaging Data. NeuroImage 2017, 161, 149–170. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The Multifactorial Roles of Microglia and Macrophages in the Maintenance and Progression of Glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef]
- Miles, K.A. Tumour Angiogenesis and Its Relation to Contrast Enhancement on Computed Tomography: A Review. Eur. J. Radiol. 1999, 30, 198–205. [Google Scholar] [CrossRef]
- Prada, F.; Vitale, V.; Del Bene, M.; Boffano, C.; Sconfienza, L.M.; Pinzi, V.; Mauri, G.; Solbiati, L.; Sakas, G.; Kolev, V.; et al. Contrast-Enhanced MR Imaging versus Contrast-Enhanced US: A Comparison in Glioblastoma Surgery by Using Intraoperative Fusion Imaging. Radiology 2017, 285, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Chiang, I.C.; Kuo, Y.-T.; Lu, C.-Y.; Yeung, K.-W.; Lin, W.-C.; Sheu, F.-O.; Liu, G.-C. Distinction between High-Grade Gliomas and Solitary Metastases Using Peritumoral 3-T Magnetic Resonance Spectroscopy, Diffusion, and Perfusion Imagings. Neuroradiology 2004, 46, 619–627. [Google Scholar] [CrossRef]
- Toh, C.H.; Siow, T.Y.; Castillo, M. Peritumoral Brain Edema in Metastases May Be Related to Glymphatic Dysfunction. Front. Oncol. 2021, 11, 725354. [Google Scholar] [CrossRef]
- Martín-Noguerol, T.; Mohan, S.; Santos-Armentia, E.; Cabrera-Zubizarreta, A.; Luna, A. Advanced MRI Assessment of Non-Enhancing Peritumoral Signal Abnormality in Brain Lesions. Eur. J. Radiol. 2021, 143, 109900. [Google Scholar] [CrossRef]
- Zenonos, G.; Friedlander, R.M. Diffusion Weighted Imaging: What Are We Really Seeing? Neurosurgery 2010, 67, N26–N29. [Google Scholar] [CrossRef] [Green Version]
- Noell, S.; Ritz, R.; Wolburg-Buchholz, K.; Wolburg, H.; Fallier-Becker, P. An Allograft Glioma Model Reveals the Dependence of Aquaporin-4 Expression on the Brain Microenvironment. PLoS ONE 2012, 7, e36555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Field, A.S.; Alexander, A.L.; Wu, Y.-C.; Hasan, K.M.; Witwer, B.; Badie, B. Diffusion Tensor Eigenvector Directional Color Imaging Patterns in the Evaluation of Cerebral White Matter Tracts Altered by Tumor. J. Magn. Reson. Imaging 2004, 20, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Jellison, B.J.; Field, A.S.; Medow, J.; Lazar, M.; Salamat, M.S.; Alexander, A.L. Diffusion Tensor Imaging of Cerebral White Matter: A Pictorial Review of Physics, Fiber Tract Anatomy, and Tumor Imaging Patterns. Am. J. Neuroradiol. 2004, 25, 356–369. [Google Scholar] [PubMed]
- Chong, S.T.; Liu, X.; Kao, H.-W.; Lin, C.-Y.E.; Hsu, C.-C.H.; Kung, Y.-C.; Kuo, K.-T.; Huang, C.-C.; Lo, C.-Y.Z.; Li, Y.; et al. Exploring Peritumoral Neural Tracts by Using Neurite Orientation Dispersion and Density Imaging. Front. Neurosci. 2021, 15, 702353. [Google Scholar] [CrossRef]
- Prabhu, S.P.; Ng, S.; Vajapeyam, S.; Kieran, M.W.; Pollack, I.F.; Geyer, R.; Haas-Kogan, D.; Boyett, J.M.; Kun, L.; Poussaint, T.Y. DTI Assessment of the Brainstem White Matter Tracts in Pediatric BSG before and after Therapy. Childs Nerv. Syst. 2011, 27, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Jütten, K.; Mainz, V.; Gauggel, S.; Patel, H.J.; Binkofski, F.; Wiesmann, M.; Clusmann, H.; Na, C.-H. Diffusion Tensor Imaging Reveals Microstructural Heterogeneity of Normal-Appearing White Matter and Related Cognitive Dysfunction in Glioma Patients. Front. Oncol. 2019, 9, 536. [Google Scholar] [CrossRef] [Green Version]
- Sternberg, E.J.; Lipton, M.L.; Burns, J. Utility of Diffusion Tensor Imaging in Evaluation of the Peritumoral Region in Patients with Primary and Metastatic Brain Tumors. AJNR Am. J. Neuroradiol. 2014, 35, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Jones, D.K.; Knösche, T.R.; Turner, R. White Matter Integrity, Fiber Count, and Other Fallacies: The Do’s and Don’ts of Diffusion MRI. Neuroimage 2013, 73, 239–254. [Google Scholar] [CrossRef]
- Monga, V.; Jones, K.; Chang, S. Clinical relevance of molecular markers in gliomas. Rev. Médica Clínica Condes 2017, 28, 343–351. [Google Scholar] [CrossRef]
- Geraghty, B.J.; Dasgupta, A.; Sandhu, M.; Malik, N.; Maralani, P.J.; Detsky, J.; Tseng, C.-L.; Soliman, H.; Myrehaug, S.; Husain, Z.; et al. Predicting Survival in Patients with Glioblastoma Using MRI Radiomic Features Extracted from Radiation Planning Volumes. J. Neurooncol. 2022, 156, 579–588. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.G.; Furtak, J.; Kowalewski, J.; Lewandowska, M.A. Prognostic and Predictive Biomarkers in Gliomas. Int. J. Mol. Sci. 2021, 22, 10373. [Google Scholar] [CrossRef] [PubMed]
- de Causans, A.; Carré, A.; Roux, A.; Tauziède-Espariat, A.; Ammari, S.; Dezamis, E.; Dhermain, F.; Reuzé, S.; Deutsch, E.; Oppenheim, C.; et al. Development of a Machine Learning Classifier Based on Radiomic Features Extracted from Post-Contrast 3D T1-Weighted MR Images to Distinguish Glioblastoma From Solitary Brain Metastasis. Front. Oncol. 2021, 11, 2577. [Google Scholar] [CrossRef] [PubMed]
- Carré, A.; Klausner, G.; Edjlali, M.; Lerousseau, M.; Briend-Diop, J.; Sun, R.; Ammari, S.; Reuzé, S.; Alvarez Andres, E.; Estienne, T.; et al. Standardization of Brain MR Images across Machines and Protocols: Bridging the Gap for MRI-Based Radiomics. Sci. Rep. 2020, 10, 12340. [Google Scholar] [CrossRef] [PubMed]
- Lerma-Usabiaga, G.; Mukherjee, P.; Ren, Z.; Perry, M.L.; Wandell, B.A. Replication and Generalization in Applied Neuroimaging. NeuroImage 2019, 202, 116048. [Google Scholar] [CrossRef]

| GBM | ||||
| Age | Gender | Location | Symptoms | Tumor Sample |
| 70 | M | occipital (left) | memory disorder | biopsy |
| 70 | M | anterior cingulate (left) | cognitive disorder, contralateral motor paresis | biopsy |
| 67 | M | fronto-temporo-insular (left) | frontal syndrome, aphasia, contralateral motor paresis | biopsy |
| 71 | M | fronto-callosal (right) | frontal syndrome | resection |
| 63 | M | temporal (left) | headache, aphasia, contralateral motor paresis | resection |
| 67 | F | occipital (right) | headache, lateral homonym hemianopsia | biopsy |
| 66 | F | temporo-insular (right) | contralateral motor paresis | resection |
| 55 | M | temporo-insular (left) | behavior disorder, aphasia, contralateral facial paresis | biopsy |
| 61 | F | anterior cingulate (right) | contralateral motor paresis | biopsy |
| 65 | M | temporal (right) | headache, contralateral facial paresis | resection |
| 78 | M | fronto-temporo-insular (left) | frontal syndrome, aphasia, contralateral motor paresis | biopsy |
| 75 | F | occipital (right) | headache, lateral homonym hemianopsia | resection |
| 49 | F | frontal (left) | aphasia | resection |
| 85 | M | temporo-insular (left) | frontal syndrome, aphasia | biopsy |
| 57 | F | frontal (right) | memory disorder, contralateral facial paresis | resection |
| Metastasis | ||||
| Age | Gender | Location | Symptoms (Cancer) | Tumor Sample |
| 78 | F | frontal (left) | inaugural (lung) | biopsy |
| 55 | M | paraventricular trigone (left) | headache (neuroendocrine) | resection |
| 71 | M | parietal (right) | contralateral motor paresis (kidney) | resection |
| 70 | F | temporal and parietal (right) | contralateral motor paresis (lung) | resection |
| 64 | F | precentral (left) | contralateral motor paresis (lung) | resection |
| 43 | M | precentral (right) | contralateral motor paresis (lung) | resection |
| 59 | M | temporal (left) | inaugural seizure (lung) | resection |
| 75 | M | frontal (left) | headache, contralateral motor paresis (lung) | resection |
| 59 | F | frontal (left) | aphasia (anal) | resection |
| MRI Objects | GBM (GBM5 *) | Metastasis | HS | Difference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | p Value | |||
| Volumes | volume (cm3) | MV | 47.12 | 28.44 | 12.96 | 17.61 | n.a. | n.a. | 0.0032 |
| BAT | 70.65 | 42.01 | 75.98 | 73.93 | n.a. | n.a. | 0.6983 | ||
| aCCV | 176,49 | 74,21 | 44.80 | 44.73 | n.a. | n.a. | 0.0006 | ||
| mean ADC value (10−3 × mm²/s) | MV | 1.726 * | 0.527 * | 1.539 | 0.287 | n.a. | n.a. | 0.4634 | |
| BAT | 1.093 * | 0.527 * | 1.504 | 0.158 | n.a. | n.a. | 0.0196 | ||
| aCCV | 0.910 * | 0.088 * | 0.920 | 0.103 | n.a. | n.a. | 0.9469 | ||
| mean FA value | MV | 0.135 | 0.046 | 0.089 | 0.017 | n.a. | n.a. | 0.0026 | |
| BAT | 0.206 | 0.056 | 0.154 | 0.026 | n.a. | n.a. | 0.0157 | ||
| aCCV | 0.315 | 0.052 | 0.324 | 0.062 | n.a. | n.a. | 0.6123 | ||
| ROIs | mean ADC value (10−3 × mm²/s) | CR-contra | 0.708 * | 0.343 * | 0.722 | 0.220 | n.a. | n.a. | 0.2527 (vs. CR-R_L) |
| WMf-ipsi | 0.840 * | 0.071 * | 0.817 | 0.056 | n.a. | n.a. | 0.5485 | ||
| WMf-contra | 0.849 * | 0.059 * | 0.797 | 0.046 | n.a. | n.a. | 0.1615 | ||
| CR-right (CR-R) | n.a. | n.a. | n.a. | n.a. | 0.801 | 0.032 | n.a. | ||
| CR-left (CR-L) | n.a. | n.a. | n.a. | n.a. | 0.790 | 0.038 | |||
| mean FA value | CR-contra | 0.396 | 0.119 | 0.308 | 0.065 | n.a. | n.a. | 0.0764 (vs. CR-R_L) | |
| WMf-ipsi | 0.434 | 0.083 | 0.376 | 0.090 | n.a. | n.a. | 0.0786 | ||
| WMf-contra | 0.388 | 0.103 | 0.641 | 0.841 | n.a. | n.a. | 0.7884 | ||
| CR-right (CR-R) | n.a. | n.a. | n.a. | n.a. | 0.333 | 0.048 | n.a. | ||
| CR-left (CR-L) | n.a. | n.a. | n.a. | n.a. | 0.333 | 0.052 | |||
| number of fibers | WMf-ipsi | 20,392.6 | 11,720.29 | 19,629.22 | 7223.04 | n.a. | n.a. | 0.6123 | |
| WMf-contra | 14,915.27 | 11,345.83 | 14,927.22 | 6190.56 | n.a. | n.a. | 0.8815 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ouadih, Y.; Pereira, B.; Biau, J.; Claise, B.; Chaix, R.; Verrelle, P.; Khalil, T.; Durando, X.; Lemaire, J.-J. DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects. Curr. Oncol. 2022, 29, 2823-2834. https://doi.org/10.3390/curroncol29040230
El Ouadih Y, Pereira B, Biau J, Claise B, Chaix R, Verrelle P, Khalil T, Durando X, Lemaire J-J. DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects. Current Oncology. 2022; 29(4):2823-2834. https://doi.org/10.3390/curroncol29040230
Chicago/Turabian StyleEl Ouadih, Youssef, Bruno Pereira, Julian Biau, Béatrice Claise, Rémi Chaix, Pierre Verrelle, Toufik Khalil, Xavier Durando, and Jean-Jacques Lemaire. 2022. "DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects" Current Oncology 29, no. 4: 2823-2834. https://doi.org/10.3390/curroncol29040230
APA StyleEl Ouadih, Y., Pereira, B., Biau, J., Claise, B., Chaix, R., Verrelle, P., Khalil, T., Durando, X., & Lemaire, J.-J. (2022). DTI Abnormalities Related to Glioblastoma: A Prospective Comparative Study with Metastasis and Healthy Subjects. Current Oncology, 29(4), 2823-2834. https://doi.org/10.3390/curroncol29040230







