The Clinical Impact of Neoadjuvant Endocrine Treatment on Luminal-like Breast Cancers and Its Prognostic Significance: Results from a Single-Institution Prospective Cohort Study
Abstract
1. Introduction
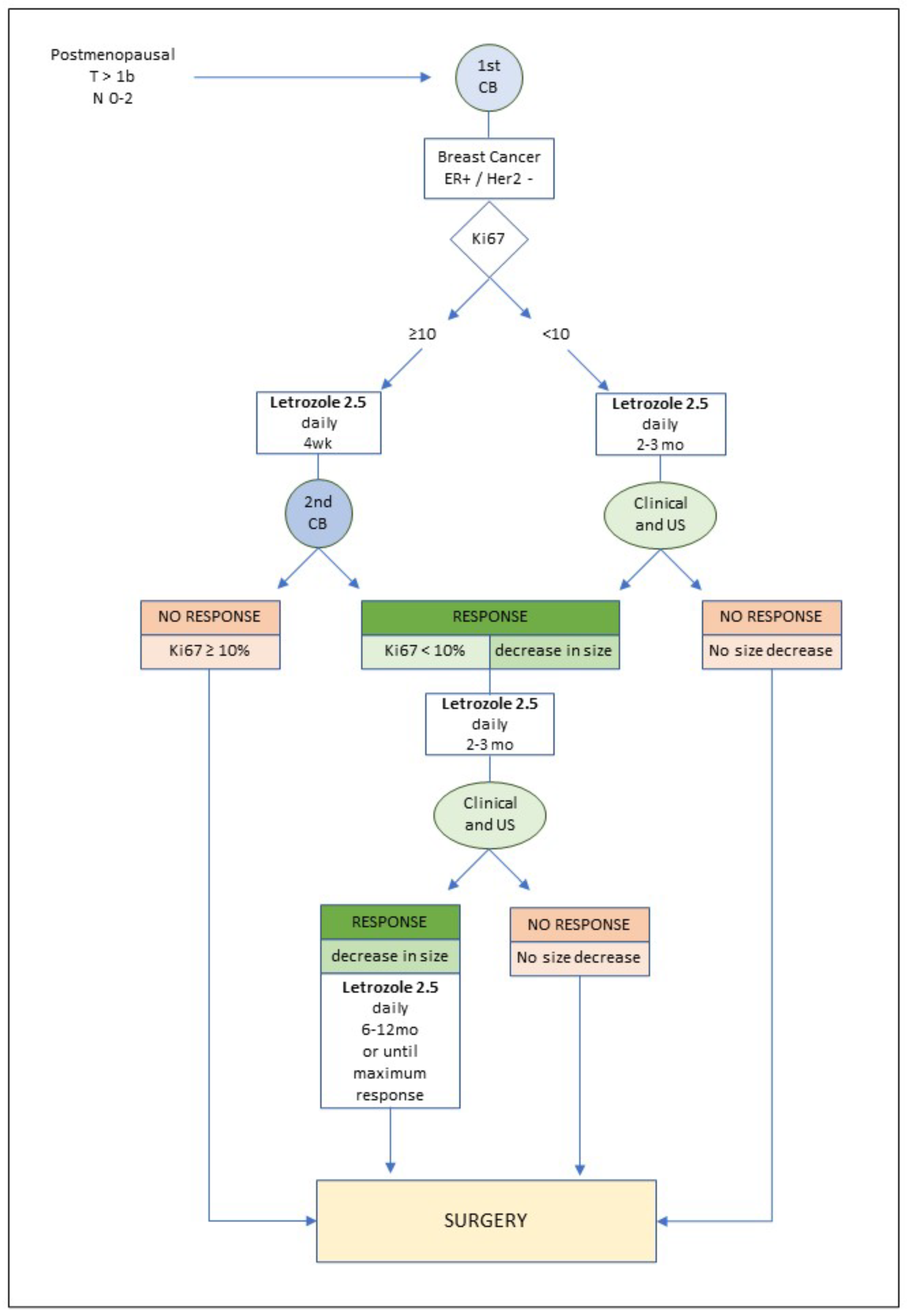
2. Methods
3. Results
3.1. Clinical and Tumor Characteristics
3.2. Treatment Characteristics
3.3. Pathological Changes after NET
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Morgan, J.L.; Reed, M.W.; Wyld, L. Primary endocrine therapy as a treatment for older women with operable breast cancer—A comparison of randomised controlled trial and cohort study findings. Eur. J. Surg. Oncol. 2014, 40, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Sella, T.; Weiss, A.; Mittendorf, E.A.; King, T.A.; Pilewskie, M.; Giuliano, A.E.; Metzger-Filho, O. Neoadjuvant Endocrine Therapy in Clinical Practice: A Review. JAMA Oncol. 2021, 7, 1700–1708. [Google Scholar] [CrossRef] [PubMed]
- Martí, C.; Sánchez-Méndez, J.I. The Present and Future of Neoadjuvant Endocrine Therapy for Breast Cancer Treatment. Cancers 2021, 13, 2538. [Google Scholar] [CrossRef]
- Eiermann, W.; Paepke, S.; Appfelstaedt, J.; Llombart-Cussac, A.; Eremin, J.; Vinholes, J.; Mauriac, L.; Ellis, M.; Lassus, M.; Chaudri-Ross, H.A.; et al. Preoperative treatment of postmenopausal breast cancer patients with letrozole: A randomized double-blind multicenter study. Ann. Oncol. 2001, 12, 1527–1532. [Google Scholar] [CrossRef] [PubMed]
- Ellis, M.J.; Tao, Y.; Luo, J.; A’Hern, R.; Evans, D.B.; Bhatnagar, A.S.; Chaudri Ross, H.A.; von Kameke, A.; Miller, W.R.; Smith, I.; et al. Outcome prediction for estrogen receptor-positive breast cancer based on postneoadjuvant endocrine therapy tumor characteristics. J. Natl. Cancer Inst. 2008, 100, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Smith, I.E.; Dowsett, M.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Blohmer, J.U.; Ashley, S.E.; Francis, S.; Boeddinghaus, I.; Walsh, G.; et al. Neoadjuvant treatment of postmenopausal breast cancer with anastrozole, tamoxifen, or both in combination: The Immediate Preoperative Anastrozole, Tamoxifen, or Combined With Tamoxifen (IMPACT) multicenter double-blind randomized trial. J. Clin. Oncol. 2005, 23, 5108–5116. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Love, C.D.B.; Bellamy, C.O.C.; Cameron, D.A.; Leonard, R.C.F.; Smith, H.; Miller, W.R. Letrozole as primary medical therapy for locally advanced and large operable breast cancer. Breast Cancer Res. Treat. 2001, 66, 191–199. [Google Scholar] [CrossRef]
- Cataliotti, L.; Buzdar, A.U.; Noguchi, S.; Bines, J.; Takatsuka, Y.; Petrakova, K.; Dube, P.; Tosello de Oliveira, C. Comparison of anastrozole versus tamoxifen as preoperative therapy in postmenopausal women with hormone receptor-positive breast cancer: The Pre-Operative “Arimidex” Compared to Tamoxifen (PROACT) trial. Cancer 2006, 106, 2095–2103. [Google Scholar] [CrossRef]
- Cardoso, F.; Senkus, E.; Costa, A.; Papadopoulos, E.; Aapro, M.; André, F.; Harbeck, N.; Aguilar Lopez, B.; Barrios, C.H.; Bergh, J.; et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4). Ann. Oncol. 2018, 29, 1634–1657. [Google Scholar] [CrossRef]
- Burstein, H.J.; Curigliano, G.; Loibl, S.; Dubsky, P.; Gnant, M.; Poortmans, P.; Colleoni, M.; Denkert, C.; Piccart-Gebhart, M.; Regan, M.; et al. Estimating the benefits of therapy for early-stage breast cancer: The St. Gallen International Consensus Guidelines for the primary therapy of early breast cancer 2019. Ann. Oncol. 2019, 30, 1541–1557. [Google Scholar] [CrossRef]
- Korde, L.A.; Somerfield, M.R.; Carey, L.A.; Crews, J.R.; Denduluri, N.; Hwang, E.S.; Khan, S.A.; Loibl, S.; Morris, E.A.; Perez, A.; et al. Neoadjuvant Chemotherapy, Endocrine Therapy, and Targeted Therapy for Breast Cancer: ASCO Guideline. J. Clin. Oncol. 2021, 39, 1485–1505. [Google Scholar] [CrossRef] [PubMed]
- Fowler, A.M.; Mankoff, D.A.; Joe, B.N. Imaging neoadjuvant therapy response in breast cancer. Radiology 2017, 285, 358–375. [Google Scholar] [CrossRef]
- Hilal, T.; Covington, M.; Kosiorek, H.E.; Zwart, C.; Ocal, I.T.; Pockaj, B.A.; Northfelt, D.W.; Patel, B.K. Breast MRI phenotype and background parenchymal enhancement may predict tumor response to neoadjuvant endocrine therapy. Breast J. 2018, 24, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, J.; Zhu, Q.; Chang, C. Prediction of pathologic complete response by ultrasonography and magnetic resonance imaging after neoadjuvant chemotherapy in patients with breast cancer. Cancer Manag. Res. 2020, 12, 2603–2612. [Google Scholar] [CrossRef] [PubMed]
- Takeda, K.; Kanao, S.; Okada, T.; Ueno, T.; Toi, M.; Ishiguro, H.; Mikami, Y.; Tanaka, S.; Togashi, K. MRI evaluation of residual tumor size after neoadjuvant endocrine therapy vs. neoadjuvant chemotherapy. Eur. J. Radiol. 2012, 81, 2148–2153. [Google Scholar] [CrossRef][Green Version]
- Urruticoechea, A.; Smith, I.E.; Dowsett, M. Proliferation marker Ki-67 in early breast cancer. J. Clin. Oncol. 2005, 23, 7212–7220. [Google Scholar] [CrossRef] [PubMed]
- Dowsett, M.; Smith, I.E.; Ebbs, S.R.; Dixon, J.M.; Skene, A.; Griffith, C.; Boeddinghaus, I.; Salter, J.; Detre, S.; Margaret Hills, M.; et al. Short-term changes in Ki-67 during neoadjuvant treatment of primary breast cancer with anastrozole or tamoxifen alone or combined correlate with recurrence-free survival. Clin. Cancer Res. 2005, 11, 951–959. [Google Scholar]
- Ellis, M.J. Lessons in precision oncology from neoadjuvant endocrine therapy trials in ER+ breast cancer. Breast 2017, 34, S104–S107. [Google Scholar] [CrossRef]
- Smith, I.; Robertson, J.; Kilburn, L.; Wilcox, M.; Evans, A.; Holcombe, C.; Horgan, K.; Kirwan, C.; Mallon, E.; Sibbering, M.; et al. Long-term outcome and prognostic value of Ki67 after perioperative endocrine therapy in postmenopausal women with hormone-sensitive early breast cancer (POETIC): An open-label, multicentre, parallel-group, randomised, phase 3 trial. Lancet Oncol. 2020, 21, 1443–1454. [Google Scholar] [CrossRef]
- Ellis, M.J.; Suman, V.J.; Hoog, J.; Goncalves, R.; Sanati, S.; Creighton, C.J.; DeSchryver, K.; Crouch, E.; Brink, A.; Watson, M.; et al. Ki67 proliferation index as a tool for chemotherapy decisions during and after neoadjuvant aromatase inhibitor treatment of breast cancer: Results from the American college of surgeons oncology group Z1031 trial (alliance). J. Clin. Oncol. 2017, 35, 1061–1069. [Google Scholar] [CrossRef]
- Martí, C.; Sánchez-Méndez, J.I. Neoadjuvant endocrine therapy for luminal breast cancer treatment: A first-choice alternative in times of crisis such as the COVID-19 pandemic. Ecancermedicalscience 2020, 14, 1027. [Google Scholar] [CrossRef] [PubMed]
- Cortazar, P.; Zhang, L.; Untch, M.; Mehta, K.; Costantino, J.P.; Wolmark, N.; Bonnefoi, H.; Cameron, D.; Gianni, L.; Valagussa, P.; et al. Pathological complete response and long-term clinical benefit in breast cancer: The CTNeoBC pooled analysis. Lancet 2014, 384, 164–172. [Google Scholar] [CrossRef]
- Da Silva, L.R.; de Andrade, C.A.; Brenelli, F.; Ramalho, S.; Reinert, T.; de Souza, A.B.A.; da Silva, A.E.R.; de Paula Leite Kraft, M.B.; de Vasconcelos, V.C.A.; Frasson, A.L.; et al. Real-world data on neoadjuvant endocrine therapy in ER-positive/HER2-negative breast cancer. Breast Cancer Res. Treat. 2021, 186, 753–760. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R.; Sullivan, D.C.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: New York, NY, USA; American Joint Comission on Cancer: New York, NY, USA, 2017; Part 11. [Google Scholar]
- Dowsett, M.; Nielsen, T.O.; A’Hern, R.; Bartlett, J.; Coombes, R.C.; Cuzick, J.; Ellis, M.; Henry, N.L.; Hugh, J.C.; Lively, T.; et al. Assessment of Ki67 in Breast Cancer: Recommendations from the international Ki67 in breast cancer working Group. J. Natl. Cancer Inst. 2011, 103, 1656–1664. [Google Scholar] [CrossRef]
- Goldhirsch, A.; Winer, E.P.; Coates, A.S.; Gelber, R.D.; Piccart-Gebhart, M.; Thürlimann, B.; Senn, H.J. Personalizing the treatment of women with early breast cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2013. Ann. Oncol. 2013, 24, 2206–2223. [Google Scholar] [CrossRef]
- Von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- Krauss, K.; Stickeler, E. Endocrine Therapy in Early Breast Cancer. Breast Care 2020, 15, 337–346. [Google Scholar] [CrossRef]
- Arthur, L.M.; Turnbull, A.K.; Khan, L.R.; Dixon, J.M. Pre-operative Endocrine Therapy. Curr. Breast Cancer Rep. 2017, 9, 202–209. [Google Scholar] [CrossRef]
- Cui, X.; Schiff, R.; Arpino, G.; Osborne, C.K.; Lee, A.V. Biology of progesterone receptor loss in breast cancer and its implications for endocrine therapy. J. Clin. Oncol. 2005, 23, 7721–7735. [Google Scholar] [CrossRef]
- Rastelli, F.; Crispino, S. Factors predictive of response to hormone therapy in breast cancer. Tumori 2008, 94, 370–383. [Google Scholar] [CrossRef]
- Van Dam, P.A.; van Dam, V.C.N.; Altintas, S.; Papadimitriou, K.; Rolfo, C.; Trinh, X.B. Neoadjuvant endocrine treatment in early breast cancer: An overlooked alternative? Eur. J. Surg. Oncol. 2016, 42, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.M.; Renshaw, L.; MacAskill, E.J.; Young, O.; Murray, J.; Cameron, D.; Kerr, G.R.; Evans, D.B.; Miller, W.R. Increase in response rate by prolonged treatment with neoadjuvant letrozole. Breast Cancer Res. Treat. 2009, 113, 145–151. [Google Scholar] [CrossRef]
- Allevi, G.; Strina, C.; Andreis, D.; Zanoni, V.; Bazzola, L.; Bonardi, S.; Milani, M.; Cappelletti, M.R.; Gussago, F.; Aguggini, S.; et al. Increased pathological complete response rate after a long-term neoadjuvant letrozole treatment in postmenopausal oestrogen and/or progesterone receptor-positive breast cancer. Br. J. Cancer 2013, 108, 1587–1592. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, R.; Doughty, J.C.; Cordiner, C.; Moss, N.; Gandhi, A.; Wilson, C.; Andrews, C.; Ellis, G.; Gui, G.; Skene, A.I. Optimum duration of neoadjuvant letrozole to permit breast conserving surgery. Breast Cancer Res. Treat. 2014, 144, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Montagna, G.; Sevilimedu, V.; Fornier, M.; Jhaveri, K.; Morrow, M.; Pilewskie, M.L. How Effective is Neoadjuvant Endocrine Therapy (NET) in Downstaging the Axilla and Achieving Breast-Conserving Surgery? Ann. Surg. Oncol. 2020, 27, 4702–4710. [Google Scholar] [CrossRef] [PubMed]
- Rusz, O.; Vörös, A.; Varga, Z.; Kelemen, G.; Uhercsák, G.; Nikolényi, A.; Ormándi, K.; Simonka, Z.; Kahán, Z. One-Year Neoadjuvant Endocrine Therapy in Breast Cancer. Pathol. Oncol. Res. 2015, 21, 977–984. [Google Scholar] [CrossRef]
- Ma, C.X.; Suman, V.J.; Leitch, A.M.; Sanati, S.; Vij, K.R.; Unzeitig, G.W.; Hoog, J.; Watson, M.; Hahn, O.M.; Guenther, J.M.; et al. ALTERNATE: Neoadjuvant endocrine treatment (NET) approaches for clinical stage II or III estrogen receptor-positive HER2-negative breast cancer (ER+ HER2− BC) in postmenopausal (PM) women: Alliance A011106. J. Clin. Oncol. 2020, 38, 504. [Google Scholar] [CrossRef]
- Hofmann, D.; Nitz, U.; Gluz, O.; Kates, R.E.; Schinkoethe, T.; Staib, P.; Harbeck, N. WSG ADAPT—Adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: Adjuvant dynamic marker-adjusted personalized therapy trial optimizing risk assessment and therapy response prediction in early breast cancer: Study protocol for a prospective, multi-center, controlled, non-blinded, randomized, investigator initiated phase II/III trials. Trials 2013, 14, 261. [Google Scholar]
- Chen, K.; Li, S.; Li, Q.; Zhu, L.; Liu, Y.; Song, E.; Su, F. Breast-conserving surgery rates in breast cancer patients with different molecular subtypes an observational study based on surveillance, epidemiology, and end results (SEER) database. Medicine 2016, 95, e2593. [Google Scholar] [CrossRef]
- Li, J.J.; Shao, Z.M. Endocrine therapy as adjuvant or neoadjuvant therapy for breast cancer: Selecting the best agents, the timing and duration of treatment. Chin. Clin. Oncol. 2016, 5, 40. [Google Scholar] [CrossRef]
- Hammond, J.B.; Scott, D.W.; Kosiorek, H.E.; Parnall, T.H.; Gray, R.J.; Ernst, B.J.; Northfelt, D.W.; McCullough, A.E.; Ocal, I.T.; Pockaj, B.A.; et al. Characterizing Occult Nodal Disease Within a Clinically Node-Negative, Neoadjuvant Breast Cancer Population. Clin. Breast Cancer 2022, 22, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Kurosumi, M.; Yoshida, T.; Hayashi, Y.; Higuchi, T.; Uchida, S.; Oba, H.; Inoue, K.; Nagai, S.; Tabei, T.; et al. Neoadjuvant endocrine therapy of breast cancer: Which patients would benefit and what are the advantages? Breast Cancer 2011, 18, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Kane, G.; Fleming, C.; Heneghan, H.; McCartan, D.; James, P.; Trueick, R.; Harrington, L.; Nally, F.; Quinn, C.; O’Doherty, A.; et al. False-negative rate of ultrasound-guided fine-needle aspiration cytology for identifying axillary lymph node metastasis in breast cancer patients. Breast J. 2019, 25, 848–852. [Google Scholar] [CrossRef] [PubMed]
- Hotton, J.; Salleron, J.; Henrot, P.; Buhler, J.; Leufflen, L.; Rauch, P.; Marchal, F. Pre-operative axillary ultrasound with fine-needle aspiration cytology performance and predictive factors of false negatives in axillary lymph node involvement in early breast cancer. Breast Cancer Res. Treat. 2020, 183, 639–647. [Google Scholar] [CrossRef]
- Helfgott, R.; Mittlböck, M.; Miesbauer, M.; Moinfar, F.; Haim, S.; Mascherbauer, M.; Schlagnitweit, P.; Heck, D.; Knauer, M.; Fitzal, F. The influence of breast cancer subtypes on axillary ultrasound accuracy: A retrospective single center analysis of 583 women. Eur. J. Surg. Oncol. 2019, 45, 538–543. [Google Scholar] [CrossRef]
- Schipper, R.; de Bruijn, A.; Voogd, A.C.; Bloemen, J.G.; Van Riet, Y.V.; Vriens, B.E.P.; Smidt, M.L.; Siesling, S.; van der Sangen, M.J.C.; Nieuwenhuijzen, G.A.P. Rate and predictors of nodal pathological complete response following neoadjuvant endocrine treatment in clinically biopsy-proven node-positive breast cancer patients. Eur. J. Surg. Oncol. 2021, 47, 1928–1933. [Google Scholar] [CrossRef]
- Hammond, J.B.; Parnall, T.H.; Scott, D.W.; Kosiorek, H.E.; Pockaj, B.; Ernst, B.J.; Northfelt, D.W.; McCullough, A.E.; Ocal, I.T.; Cronin, P.A. Gauging the efficacy of neoadjuvant endocrine therapy in breast cancer patients with known axillary disease. J. Surg. Oncol. 2020, 122, 619–622. [Google Scholar] [CrossRef]
- Kantor, O.; Wong, S.; Weiss, A.; Metzger, O.; Mittendorf, E.A.; King, T.A. Prognostic significance of residual nodal disease after neoadjuvant endocrine therapy for hormone receptor-positive breast cancer. Breast Cancer. 2020, 6, 5–10. [Google Scholar] [CrossRef]
- Kantor, O.; Wakeman, M.; Weiss, A.; Wong, S.; Laws, A.; Grossmith, S.; Mittendorf, E.A.; King, T.A. Axillary Management After Neoadjuvant Endocrine Therapy for Hormone Receptor-Positive Breast Cancer. Ann. Surg. Oncol. 2021, 28, 1358–1367. [Google Scholar] [CrossRef]
- Weiss, A.; Wong, S.; Golshan, M.; Freedman, R.A.; Metzger, O.; Bellon, J.; Mittendorf, E.A.; King, T.A. Patterns of Axillary Management in Stages 2 and 3 Hormone Receptor-Positive Breast Cancer by Initial Treatment Approach. Ann. Surg. Oncol. 2019, 26, 4326–4336. [Google Scholar] [CrossRef]
- Spring, L.M.; Gupta, A.; Reynolds, K.L.; Gadd, M.A.; Ellisen, L.W.; Isakoff, S.J.; Moy, B.; Bardia, A. Neoadjuvant endocrine therapy for estrogen receptor-positive breast cancer a systematic review and meta-Analysis. JAMA Oncol. 2016, 2, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Kalinsky, K.; Barlow, W.E.; Gralow, J.R.; Meric-Bernstam, F.; Albain, K.S.; Hayes, D.F.; Lin, N.U.; Perez, E.A.; Goldstein, L.J.; Chia, S.K.; et al. 21-Gene Assay to Inform Chemotherapy Benefit in Node-Positive Breast Cancer. N.Engl. J. Med. 2021, 16, 2336–2347. [Google Scholar] [CrossRef] [PubMed]

| Characteristics of the Cohort | n | (%) |
|---|---|---|
| 115 | (100.0) | |
| Clinical Stage | ||
| IA | 46 | (40.0) |
| IIA | 45 | (39.1) |
| IB | 0 | (0.0) |
| IIB | 19 | (16.6) |
| IIIA | 5 | (4.3) |
| Clinical node evaluation-cN | ||
| Negative | 94 | (81.7) |
| Positive | 21 | (18.3) |
| Histological type | ||
| Ductal | 85 | (73.9) |
| Lobular | 23 | (20.0) |
| Others | 7 | (6.1) |
| Histological grade | ||
| 1 | 24 | (20.9) |
| 2 | 77 | (66.9) |
| 3 | 14 | (12.2) |
| Immunophenotype | ||
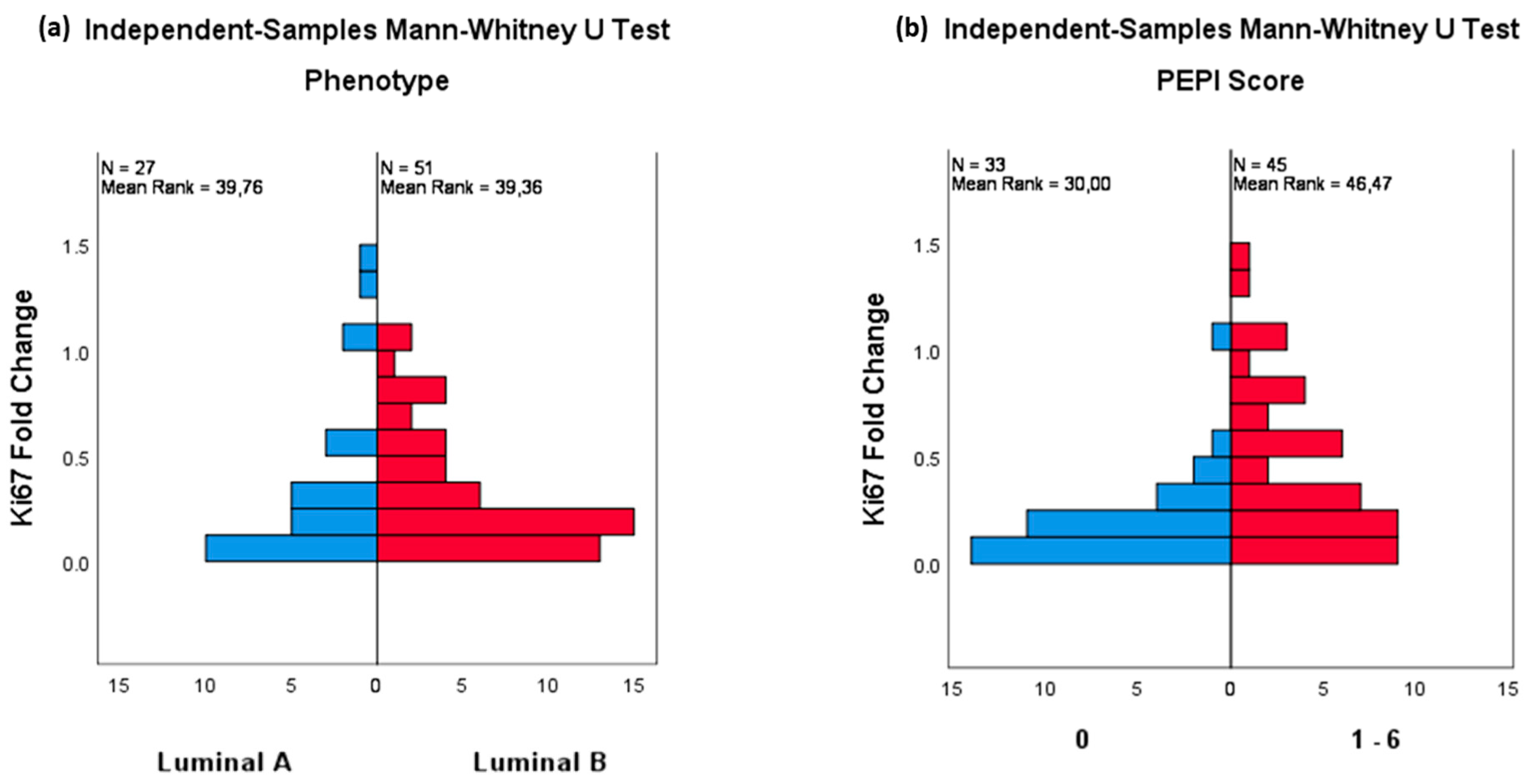
| Luminal A-like | 48 | (41.7) |
| Luminal B-like | 67 | (58.3) |
| Median | (IQR) | |
| Age (years) | 69.0 | (62.0–78.0) |
| Pre-NET Size (mm) | 25.0 | (17.0–40.0) |
| Pre-NET ER expression (%) | 100.0 | (100.0–100.0) |
| Pre-NET PR expression (%) | 70.0 | (20.0–100.0) |
| Pre-NET Ki67 (%) | 20.0 | (12.0–30.0) |
| NET duration (months) | 5.0 | (2.0–6.0) |
| n | % | |
|---|---|---|
| 115 | (100%) | |
| Pathological complete response | 0 | (0.0) |
| Pathological node status | ||
| ypN0 | 71 | (61.7) |
| ypN1 | 44 | (38.3) |
| PEPI score | ||
| 0 | 53 | (46.1) |
| 1 | 17 | (14.8) |
| 2 | 8 | (7.0) |
| 3 | 23 | (20.0) |
| 4 | 10 | (8.7) |
| 5 | 2 | (1.7) |
| 6 | 2 | (1.7) |
| Pre-NET | Intermedial | Surgical Sample | |||||
|---|---|---|---|---|---|---|---|
| 115 | 78 | 115 | |||||
| median | I.Q.R | median | I.Q.R | median | I.Q.R | ||
| Size (mm) | 25.0 | (17.0–40.0) | 19.5 | (13.0–30.0) | 15.0 | (10.0–20.0) | |
| p | Pre-NET/Surgical | <0.0001 | |||||
| Pre-NET/Intermed | <0.0001 | ||||||
| Intermed/Surgical | <0.0001 | ||||||
| ER Expression (%) | 100.0 | (100.0–100.0) | 100.0 | (100.0–100.0) | 100.0 | (100.0–100.0) | |
| p | Pre-NET/Surgical | <0.05 | |||||
| Pre-NET/Intermed | ns | ||||||
| Intermed/Surgical | ns | ||||||
| PR Expression (%) | 70.0 | (20.0–100.0) | 4.0 | (0.0–35.0) | 1.0 | (0.0–20.0) | |
| p | Pre-NET/Surgical | <0.0001 | |||||
| Pre-NET/Intermed | <0.0001 | ||||||
| Intermed/Surgical | <0.005 | ||||||
| Ki67 (%) | 20.0 | (12.0–30.0) | 5.0 | (1.8–10.0) | 2.0 | (1.0–8.0) | |
| p | Pre-NET/Surgical | <0.0001 | |||||
| Pre-NET/Intermed | <0.0001 | ||||||
| Intermed/Surgical | ns | ||||||
| Histological Grade | n | % | n | % | n | % | |
| G1 | 24 | (20.9) | 26 | (33.3) | 55 | (47.8) | |
| G2 | 77 | (67.0) | 49 | (62.7) | 53 | (46.1) | |
| G3 | 14 | (12.2) | 3 | (4.0) | 7 | (6.1) | |
| p | Pre-NET/Surgical | <0.0001 | |||||
| Pre-NET/Intermed | <0.0001 | ||||||
| Intermed/Surgical | <0.0001 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martí, C.; Yébenes, L.; Oliver, J.M.; Moreno, E.; Frías, L.; Berjón, A.; Loayza, A.; Meléndez, M.; Roca, M.J.; Córdoba, V.; et al. The Clinical Impact of Neoadjuvant Endocrine Treatment on Luminal-like Breast Cancers and Its Prognostic Significance: Results from a Single-Institution Prospective Cohort Study. Curr. Oncol. 2022, 29, 2199-2210. https://doi.org/10.3390/curroncol29040179
Martí C, Yébenes L, Oliver JM, Moreno E, Frías L, Berjón A, Loayza A, Meléndez M, Roca MJ, Córdoba V, et al. The Clinical Impact of Neoadjuvant Endocrine Treatment on Luminal-like Breast Cancers and Its Prognostic Significance: Results from a Single-Institution Prospective Cohort Study. Current Oncology. 2022; 29(4):2199-2210. https://doi.org/10.3390/curroncol29040179
Chicago/Turabian StyleMartí, Covadonga, Laura Yébenes, José María Oliver, Elisa Moreno, Laura Frías, Alberto Berjón, Adolfo Loayza, Marcos Meléndez, María José Roca, Vicenta Córdoba, and et al. 2022. "The Clinical Impact of Neoadjuvant Endocrine Treatment on Luminal-like Breast Cancers and Its Prognostic Significance: Results from a Single-Institution Prospective Cohort Study" Current Oncology 29, no. 4: 2199-2210. https://doi.org/10.3390/curroncol29040179
APA StyleMartí, C., Yébenes, L., Oliver, J. M., Moreno, E., Frías, L., Berjón, A., Loayza, A., Meléndez, M., Roca, M. J., Córdoba, V., Hardisson, D., Rodríguez, M. Á., & Sánchez-Méndez, J. I. (2022). The Clinical Impact of Neoadjuvant Endocrine Treatment on Luminal-like Breast Cancers and Its Prognostic Significance: Results from a Single-Institution Prospective Cohort Study. Current Oncology, 29(4), 2199-2210. https://doi.org/10.3390/curroncol29040179






