Basosquamous Basal Cell Carcinoma with Bone Marrow Metastasis
Abstract
:1. Introduction
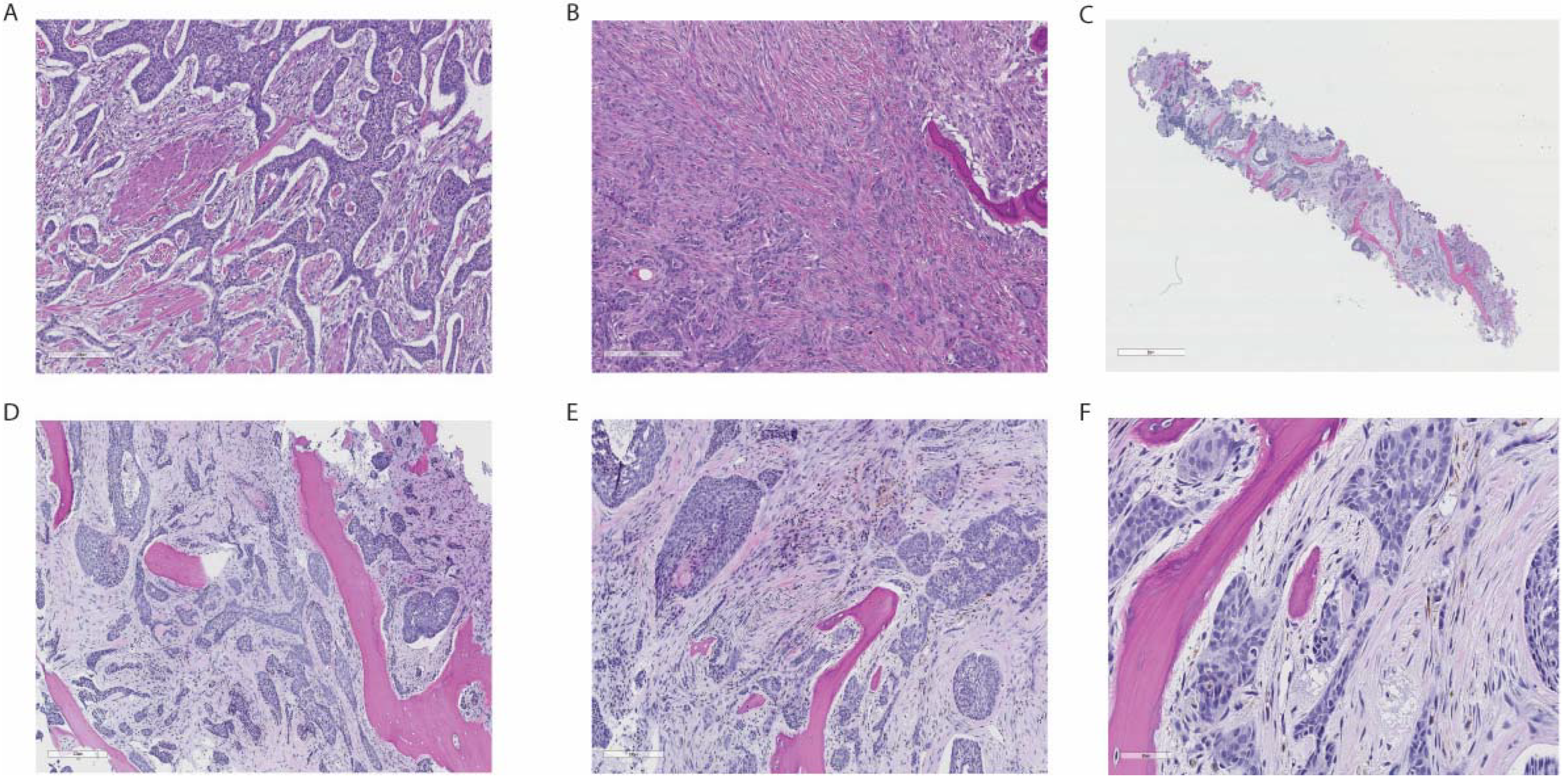
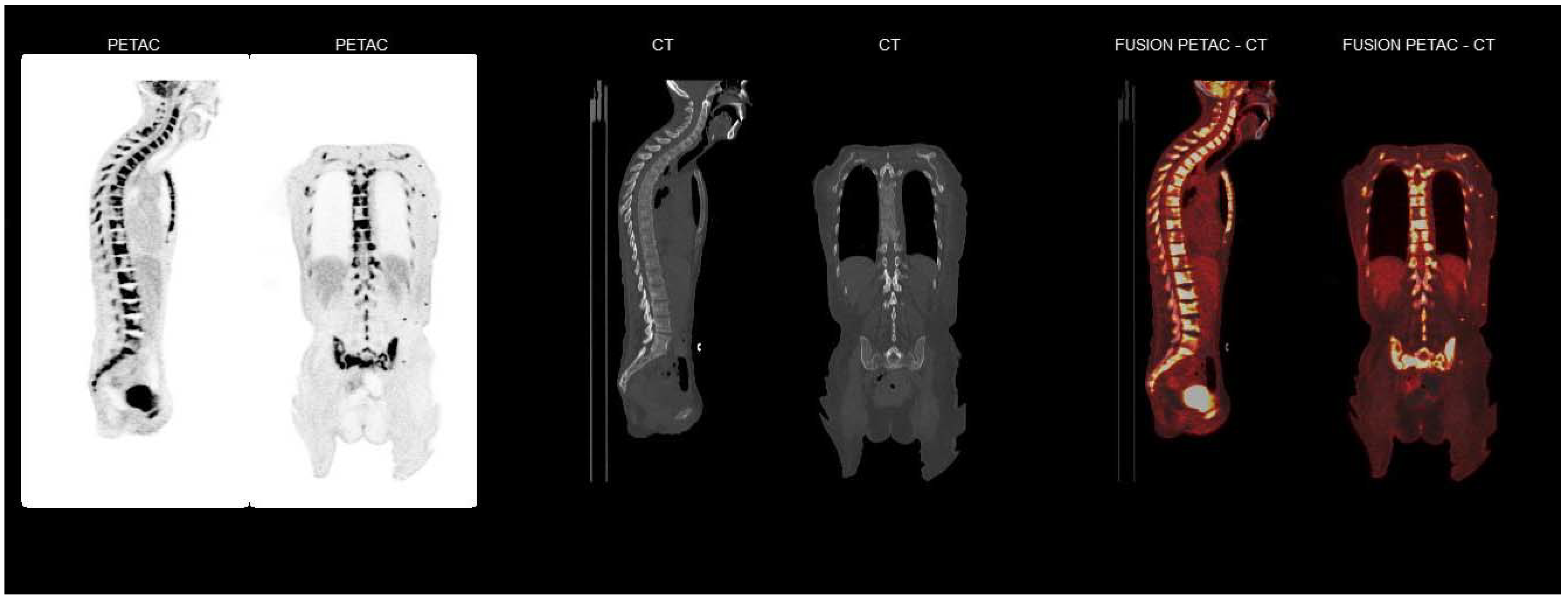
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Basset-Seguin, N.; Herms, F. Update in the Management of Basal Cell Carcinoma. Acta Derm. Venereol. 2020, 100, adv00140. [Google Scholar] [CrossRef] [PubMed]
- Dika, E.; Scarfi, F.; Ferracin, M.; Broseghini, E.; Marcelli, E.; Bortolani, B.; Campione, E.; Riefolo, M.; Ricci, C.; Lambertini, M. Basal Cell Carcinoma: A Comprehensive Review. Int. J. Mol. Sci. 2020, 21, 5572. [Google Scholar] [CrossRef] [PubMed]
- Rubin, A.I.; Chen, E.H.; Ratner, D. Basal-cell carcinoma. N. Engl. J. Med. 2005, 353, 2262–2269. [Google Scholar] [CrossRef] [PubMed]
- Kappelin, J.; Green, A.C.; Ingvar, A.; Ahnlide, I.; Nielsen, K. Incidence and trends of basal cell carcinoma in Sweden: A population-based registry study. Br. J. Dermatol. 2021, 20, 964. [Google Scholar] [CrossRef]
- Aydin, D.; Holmich, L.R.; Jakobsen, L.P. Metastatic basal cell carcinoma caused by carcinoma misdiagnosed as acne–case report and literature review. Clin. Case Rep. 2016, 4, 601–604. [Google Scholar] [CrossRef] [Green Version]
- Mehta, K.S.; Mahajan, V.K.; Chauhan, P.S.; Sharma, A.L.; Sharma, V.; Abhinav, C.; Khatri, G.; Prabha, N.; Sharma, S.; Negi, M. Metastatic Basal cell carcinoma: A biological continuum of Basal cell carcinoma? Case Rep. Dermatol. Med. 2012, 2012, 157187. [Google Scholar] [CrossRef] [PubMed]
- Di Lernia, V.; Ricci, C.; Zalaudek, I.; Argenziano, G. Metastasizing basal cell carcinoma. Cutis 2013, 92, 244–246. [Google Scholar] [PubMed]
- Walling, H.W.; Fosko, S.W.; Geraminejad, P.A.; Whitaker, D.C.; Arpey, C.J. Aggressive basal cell carcinoma: Presentation, pathogenesis, and management. Cancer Metastasis Rev. 2004, 23, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Wysong, A.; Aasi, S.Z.; Tang, J.Y. Update on metastatic basal cell carcinoma: A summary of published cases from 1981 through 2011. JAMA Dermatol. 2013, 149, 615–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCusker, M.; Basset-Seguin, N.; Dummer, R.; Lewis, K.; Schadendorf, D.; Sekulic, A.; Hou, J.; Wang, L.; Yue, H.; Hauschild, A. Metastatic basal cell carcinoma: Prognosis dependent on anatomic site and spread of disease. Eur. J. Cancer 2014, 50, 774–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beer, T.W.; Shepherd, P.; Theaker, J.M. Ber EP4 and epithelial membrane antigen aid distinction of basal cell, squamous cell and basosquamous carcinomas of the skin. Histopathology 2000, 37, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Peris, K.; Fargnoli, M.C.; Garbe, C.; Kaufmann, R.; Bastholt, L.; Seguin, N.B.; Bataille, V.; Marmol, V.D.; Dummer, R.; Harwood, C.A.; et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer 2019, 118, 10–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fecher, L.A.; Sharfman, W.H. Advanced basal cell carcinoma, the hedgehog pathway, and treatment options–role of smoothened inhibitors. Biologics 2015, 9, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stratigos, A.J.; Sekulic, A.; Peris, K.; Bechter, O.; Prey, S.; Kaatz, M.; Lewis, K.D.; Basset-Seguin, N.; Chang, A.L.S.; Dalle, S.; et al. Cemiplimab in locally advanced basal cell carcinoma after hedgehog inhibitor therapy: An open-label, multi-centre, single-arm, phase 2 trial. Lancet Oncol. 2021, 22, 848–857. [Google Scholar] [CrossRef]
- Laga, A.C.; Schaefer, I.M.; Sholl, L.M.; French, C.A.; Hanna, J. Metastatic Basal Cell Carcinoma. Am. J. Clin. Pathol. 2019, 152, 706–717. [Google Scholar] [CrossRef] [PubMed]
- Nasr, I.; McGrath, E.J.; Harwood, C.A.; Botting, J.; Buckley, P.; Budny, P.G.; Fairbrother, P.; Fife, K.; Gupta, G.; Hashme, M.; et al. British Association of Dermatologists guidelines for the management of adults with basal cell carcinoma 2021. Br. J. Dermatol. 2021, 185, 899–920. [Google Scholar] [CrossRef] [PubMed]
- Pham, C.M.; Syed, A.A.; Siddiqui, H.A.; Keller, R.A.; Kowalewski, C. Case of metastatic basal cell carcinoma to bone marrow, resulting in myelophthisic anemia. Am. J. Dermatopathol. 2013, 35, e34–e36. [Google Scholar] [CrossRef] [PubMed]

| Blood Tests | Values | References |
|---|---|---|
| Leukocytes (×109/L) | 8.6 | 3.5–11.0 |
| Haemoglobin (g/dL) | 9.4 | 11.7–15.3 |
| MCV (fL) | 82 | 82–98 |
| Reticulocytes (×1012/L) | 0.046 | 0.02–0.08 |
| Thrombocytes (×109/L) | 288 | 165–387 |
| CRP (mg/L) | 152 | <5 |
| Procalcitonin (µg/L) | <0.10 | <0.10 |
| SR (mm) | 77 | <20 |
| Creatinine (µmol/L) | 41 | 45–90 |
| ALP (U/L) | 211 | 35–105 |
| Ferritin (µg/L) | 1919 | 15–200 |
| LDH (U/L) | 582 | 105–205 |
| Albumin (g/L) | 28 | 39–48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thunestvedt, L.M.Ø.; Helgeland, L.; Bachmann, I.M.; Karlsdottir, Å.; Haslerud, T.M.; Reikvam, H. Basosquamous Basal Cell Carcinoma with Bone Marrow Metastasis. Curr. Oncol. 2022, 29, 2193-2198. https://doi.org/10.3390/curroncol29040178
Thunestvedt LMØ, Helgeland L, Bachmann IM, Karlsdottir Å, Haslerud TM, Reikvam H. Basosquamous Basal Cell Carcinoma with Bone Marrow Metastasis. Current Oncology. 2022; 29(4):2193-2198. https://doi.org/10.3390/curroncol29040178
Chicago/Turabian StyleThunestvedt, Lise Mayrin Økland, Lars Helgeland, Ingeborg Margrethe Bachmann, Åsa Karlsdottir, Torjan Magne Haslerud, and Håkon Reikvam. 2022. "Basosquamous Basal Cell Carcinoma with Bone Marrow Metastasis" Current Oncology 29, no. 4: 2193-2198. https://doi.org/10.3390/curroncol29040178
APA StyleThunestvedt, L. M. Ø., Helgeland, L., Bachmann, I. M., Karlsdottir, Å., Haslerud, T. M., & Reikvam, H. (2022). Basosquamous Basal Cell Carcinoma with Bone Marrow Metastasis. Current Oncology, 29(4), 2193-2198. https://doi.org/10.3390/curroncol29040178






