Cost-Effectiveness of Brexucabtagene Autoleucel versus Best Supportive Care for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma following Treatment with a Bruton’s Tyrosine Kinase Inhibitor in Canada
Abstract
:1. Introduction
2. Materials and Methods
2.1. Target Population
2.2. Comparators
2.3. Model Perspective
2.4. Time Horizon, Discounting, and Cycle Length
2.5. Model Structure and Approach
2.5.1. Partitioned Survival Model
2.5.2. Partitioned Survival Mixture Cure Model
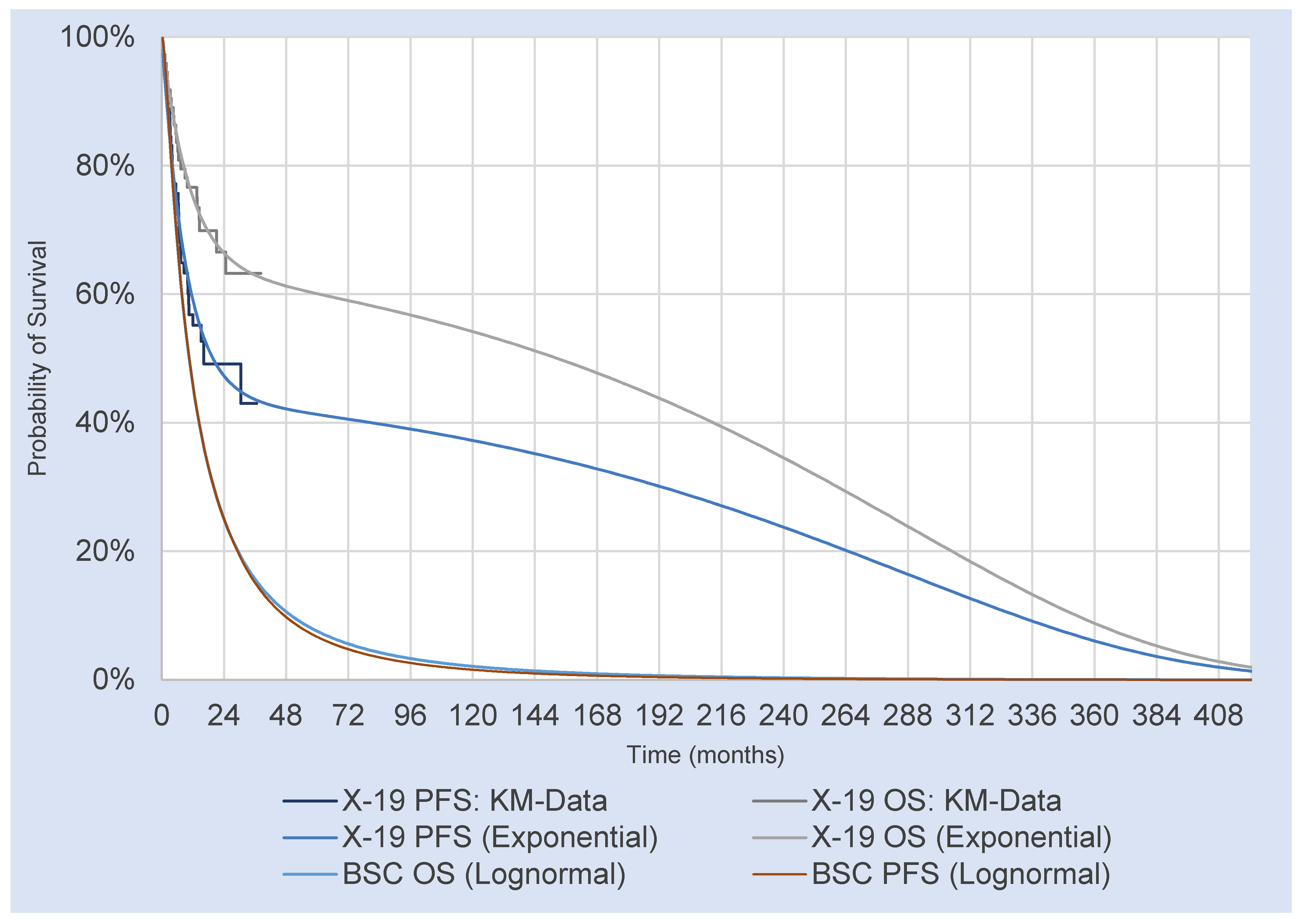
2.6. Survival Estimates for Brexu-Cel
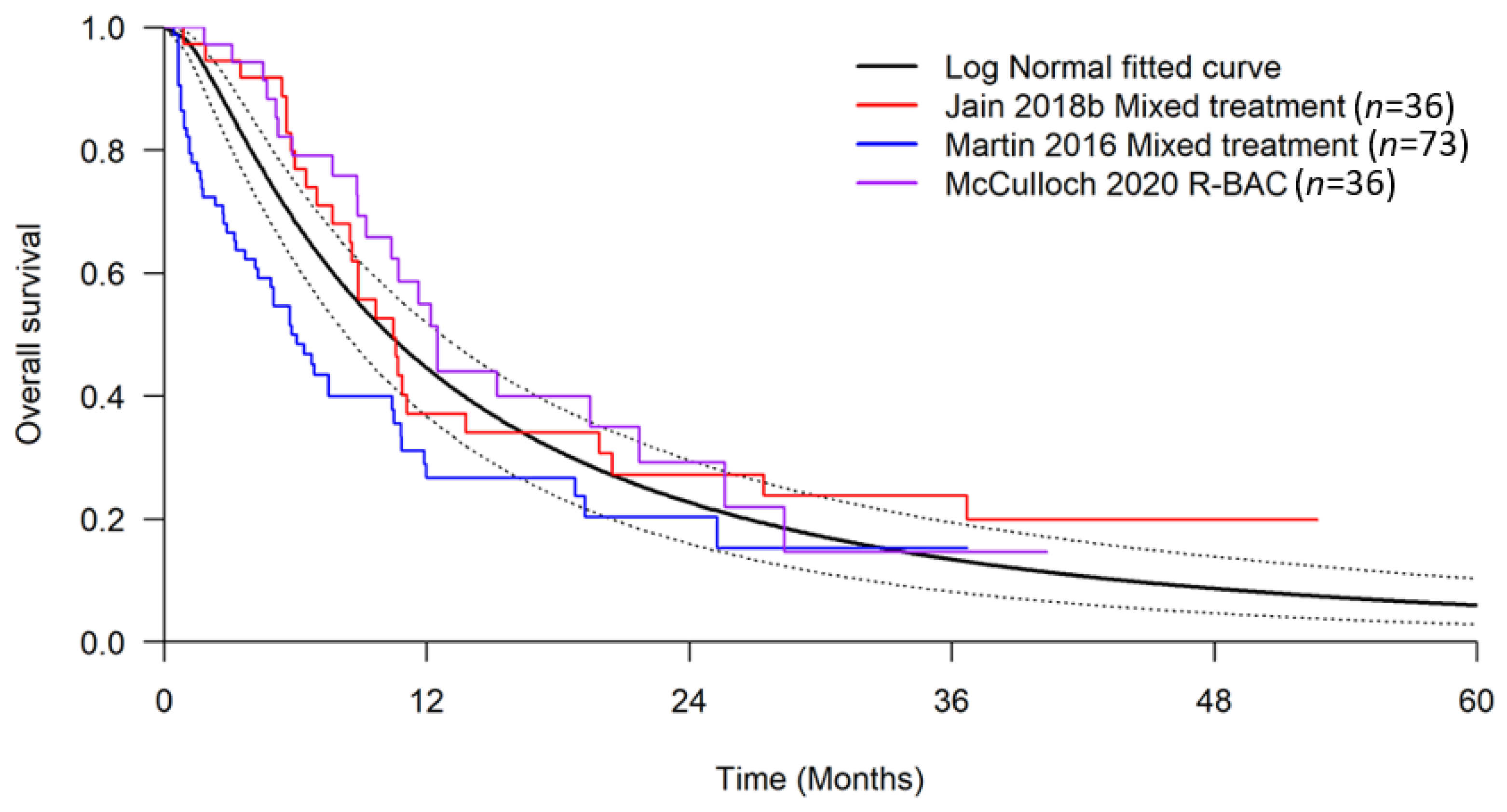
2.7. Survival Estimates for Best Supportive Care
2.8. Cost and Resource Use
2.9. Drug Administration
2.10. End-of-Life Costs
2.11. Brexu-Cel Specific Treatment Costs
2.12. BSC Specific Treatment Costs
2.13. Drug Acquisition
Health State Resource Use and Costs
2.14. Adverse Events
2.15. Utility Values
3. Results
3.1. Deterministic Analysis
3.2. Probabilistic Sensitivity Analysis
3.3. Univariate Sensitivity Analysis
3.4. Scenario Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Resource | Progression-Free | Progression-Free Post-5 Years | Progressed | ||||
|---|---|---|---|---|---|---|---|
| % of Patients | Frequency per Cycle | % of Patients | Frequency per Cycle | % of Patients | Frequency per Cycle | ||
| Physician visits | Specialist visit | 100% | 0.33 | 100% | 0.17 | 100% | 1.00 |
| Laboratory tests | Complete Blood Count | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 |
| Lactate Dehydrogenase | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 | |
| Blood glucose | 100% | 0.33 | 0% | 0.00 | 100% | 1.00 | |
| Radiology | CT scan | 100% | 0.33 | 0% | 0.00 | 100% | 0.33 |
| X-ray | 100% | 0.17 | 0% | 0.00 | 100% | 0.33 | |
| Hospitalization | 0% | 0.00 | 0% | 0.00 | 100% | 0.08 | |
| Resource | Unit Cost | Reference |
|---|---|---|
| Specialist visit | CAD 157.00 | Ontario MoHLTC Schedule of Benefits—Physician Services, Haematology Consultation [43] |
| Complete blood count | CAD 3.98 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, Hematology—CBC [55] |
| Lactate dehydrogenase | CAD 1.28 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, Lactate Dehydrogenase [55] |
| Blood glucose | CAD 1.28 | Ontario MoHLTC Schedule of Benefits—Laboratory Services, C-reactive Protein [55] |
| CT scan (abdominal, thorax) | CAD 195.00 | Ontario MoHLTC Schedule of Benefits—Physician Services, CT abdomen and thorax, with and without IV contrast. [43] |
| X-ray | CAD 32.25 | Ontario MoHLTC Schedule of Benefits—Physician Services, Skeletal survey studies; assumed 3 views. [43] |
| Hospitalization | CAD 12,756.57 | CIHI Patient Cost Estimator [45] |
| IV administration | CAD 54.25 | Ontario Schedule of Benefits for Physician Services. [43] |
| Conditional chemotherapy administration | CAD 105.15 | Ontario Schedule of Benefits for Physician Services. [43] |
| Palliative care (one-off) | CAD 34,037 | Walker et al. 2011. [41] |
| Office visit | CAD 157.00 | Ontario Schedule of Benefits for Physician Services. [43] |
| Brexucabtagene autoleucel one-time treatment cost | CAD 533,523.10 | Kite list price |
| Brexucabtagene autoleucel administration | CAD 185.00 | Ontario Schedule of Benefits for Physician Services. [43] |
| Apheresis | CAD 1343.98 | Holbro et al. 2013. [44]. |
| Adverse event: cytokine release syndrome | CAD 18,366.96 | Cost of 6 days of tocilizumab treatment and 11 days hospitalized. Fifty-nine percent of patients were treated with tocilizumab. Cost per hospital day is weighted average of cost per inpatient day from CIHI patient cost estimator. [45] |
| Resource | Unit Cost | Reference |
|---|---|---|
| Rituximab 100 mg | CAD 482.31 | Ontario Exceptional Access Program [47] |
| Bendamustine 25 mg | CAD 312.50 | pCODR Economic Guidance Report for Bendamustine [48] |
| Lenalidomide 25 mg | CAD 424.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 20 mg | CAD 403.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 15 mg | CAD 382.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 10 mg | CAD 361.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 5 mg | CAD 340.00 | Ontario Exceptional Access Program [47] |
| Lenalidomide 2.5 mg | CAD 329.00 | Ontario Exceptional Access Program [47] |
| Bortezomib 3.5 mg | CAD 1402.42 | pCODR Economic Guidance Report for Daratumumab [49] |
| Anthracycline 1 mg | CAD 5.05 | pCODR Economic Guidance Report for Pertuzumab-Trastuzumab [50] |
| Fludarabine 50 mg | CAD 255.00 | pCODR Economic Guidance Report for Ibrutinib [5] |
| Cyclophosphamide 1g | CAD 52.06 | pCODR Economic Guidance Report for Ibrutinib [5] |
| Ibrutinib 140 mg | CAD 97.60 | Ontario Exceptional Access Program [47] |
| Dexamethasone 4 mg | CAD 0.30 | Ontario Drug Benefit Formulary [66] |
| Tocilizumab 20 mg | CAD 182.80 | Ontario Exceptional Access Program [47] |
| Chemotherapy | Admin Route | mg/m2/day | Frequency | mg/Unit | Cost/Unit | Source |
|---|---|---|---|---|---|---|
| Rituximab | IV | 375 | Q4W for 6 cycles [54] | 100 | 482.31 | Ontario EAP [47] |
| Bendamustine | IV | 70 | Q4W 2 days for 6 cycles [54] | 25 | 312.50 | pCODR economic review of Bendamustine [48] |
| Lenalidomide | Oral | 25 | 21 days on, 7 off [53] | 25 | 424.00 | Ontario EAP [47] |
| Bortezomib | IV | 1.3 | Q3W 4 days, 9 cycles [51] | 3.5 | 1402.42 | pCODR economic review of Daratumumab [49] |
| Anthracycline | IV | 50 | Q3W [52] | 1 | 5.05 | pCODR economic review of Pertuzumab-Trastuzumab [50] |
| Item | Value | S.E. | Source |
|---|---|---|---|
| Proportion of brexu-cel patients who visit ICU | 22.7% | ZUMA-2 Clinical Study Report [38] | |
| % of BSC patients who visit ICU | 0 | 0 | ~ |
| Average duration in ICU | 21.2 days | 1.80 | ZUMA-2 Clinical Study report [38] |
| Total hospitalization cost | CAD 63,758.81 | 0 | ~ |
| Incidence | ||
|---|---|---|
| Adverse Events | Brexucabtagene Autoleucel (%) (se) | BSC (%) (se) |
| Cytokine release syndrome (CRS) Grade ≥2 | 62 (6) | 0 (0) |
| Pyrexia | 13 (4) | 0 (0) |
| Anemia | 50 (6) | 0 (0) |
| Platelet count decreased | 38 (6) | 0 (0) |
| Hypotension | 22 (5) | 0 (0) |
| Neutrophil count decreased | 50 (6) | 0 (0) |
| White blood cell count decreased | 40 (6) | 0 (0) |
| Hypoxia | 21 (5) | 0 (0) |
| Hypophosphatemia | 22 (5) | 0 (0) |
| Neutropenia | 34 (6) | 0 (0) |
| Hyponatremia | 10 (4) | 0 (0) |
| ALT increased | 9 (3) | 0 (0) |
| Encephalopathy | 19 (5) | 0 (0) |
| Hypokalemia | 7 (3) | 0 (0) |
| Hypocalcemia | 6 (3) | 0 (0) |
| Thrombocytopenia | 16 (4) | 0 (0) |
| AST increased | 10 (4) | 0 (0) |
| Confusional state | 12 (4) | 0 (0) |
| Hyperglycemia | 6 (3) | 0 (0) |
| Hypertension | 13 (4) | 0 (0) |
| Acute Kidney Injury | 7 (3) | 0 (0) |
| Leukopenia | 13 (4) | 0 (0) |
| Lymphocyte count decreased | 9 (3) | 0 (0) |
| Pneumonia | 9 (3) | 0 (0) |
| Respiratory Failure | 6 (3) | 0 (0) |
| Sepsis | 6 (3) | 0 (0) |
| Brexucabtagene Autoleucel | BSC | Incremental | |
|---|---|---|---|
| Total discounted years | 11.21 | 1.72 | 9.49 |
| Pre-progression | 8.01 | 1.50 | 6.51 |
| Post-progression | 3.20 | 0.22 | 2.98 |
| Total discounted QALYs | 8.31 | 1.31 | 7.00 |
| Pre-progression | 6.28 | 1.17 | 5.11 |
| Pre-progression, pre-cure point | 1.99 | 1.03 | 0.95 |
| Pre-progression, post-cure point | 4.29 | 0.14 | 4.15 |
| Post-progression | 2.07 | 0.14 | 1.92 |
| Adverse events | −0.04 | −0.01 | −0.03 |
| Total discounted costs | CAD 689,636 | CAD 68,066 | CAD 621,571 |
| Total treatment-related costs | CAD 592,182 | CAD 27,701 | CAD 564,480 |
| Total drug acquisition | CAD 533,523 | CAD 26,989 | CAD 506,534 |
| Total apheresis | CAD 1374 | CAD 0 | CAD 1374 |
| Total drug administration | CAD 211 | CAD 713 | CAD −502 |
| Total lymphodepletion chemotherapy | CAD 646 | CAD 0 | CAD 646 |
| Total bridging therapy | CAD 222 | CAD 0 | CAD 222 |
| Total hospitalization | CAD 56,206 | CAD 0 | CAD 56,206 |
| Total disease management | CAD 56,500 | CAD 4692 | CAD 51,808 |
| Pre-progression | CAD 3996 | CAD 1145 | CAD 2851 |
| Post-progression | CAD 52,504 | CAD 3547 | CAD 48,957 |
| Other costs | CAD 40,954 | CAD 35,672 | CAD 5282 |
| End-of-life care | CAD 29,603 | CAD 34,591 | CAD −4988 |
| Adverse events | CAD 11,351 | CAD 1081 | CAD 10,270 |
| Cost/QALY | CAD 88,814 |
| Parameter | Original Value | Lower Limit | Upper Limit |
|---|---|---|---|
| Patient Characteristics | |||
| Bodyweight | 81.8000 | 77.9723 | 85.6277 |
| BSA | 1.9780 | 1.9251 | 2.0309 |
| Resource Use | |||
| Pre-Progression Resource Use: Full blood count | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: X-ray | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Resource Use: Blood glucose | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: Lactate dehydrogenase | 0.3333 | 0.2157 | 0.4761 |
| Pre-Progression Resource Use: CT Scan | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Resource Use: Office visit | 0.1667 | 0.1079 | 0.2381 |
| Pre-Progression Cured: Resource Use: Office visit | 0.1667 | 0.1079 | 0.2381 |
| Post-Progression Resource Use: Full blood count | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: X-ray | 0.3333 | 0.2157 | 0.4761 |
| Post-Progression Resource Use: Blood glucose | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: Lactate dehydrogenase | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: Office visit | 1.0000 | 0.6471 | 1.4284 |
| Post-Progression Resource Use: CT Scan | 0.3333 | 0.2157 | 0.4761 |
| Post-Progression Resource Use: Hospitalization | 0.0833 | 0.0539 | 0.1190 |
| End-of-life Resource Use: Palliative care (one-off) | 1.0000 | 0.6471 | 1.4284 |
| Brexucabtagene autoleucel—Proportion ICU Stay | 0.2200 | 0.1402 | 0.3119 |
| Brexucabtagene autoleucel—Proportion Non-ICU—Hospital days | 16.5000 | −4.5696 | 37.5696 |
| Bridging Therapy Proportion | 0.3676 | 0.3133 | 0.4237 |
| Cost: Initial Hospitalization: Intensive Care Unit Day | CAD 8,343.7300 | CAD 5399.6221 | 1 CAD 1918.2165 |
| Cost: Initial Hospitalization: Inpatient Day (Non-ICU) | CAD 1580.5300 | CAD 1022.8357 | CAD 2257.6352 |
| Cost: Stem cell transplant | CAD 166,855.5300 | CAD 10,7980.1014 | CAD 23,8337.0904 |
| Cost: Office visit | CAD 174.6400 | CAD 113.0178 | CAD 249.4565 |
| Cost: Palliative care (one-off) | CAD 35,262.4800 | CAD 22,820.0178 | CAD 50,369.0641 |
| Cost: Full blood count | CAD 4.1200 | CAD 2.6662 | CAD 5.8850 |
| Cost: X-ray | CAD 23.1500 | CAD 14.9815 | CAD 33.0676 |
| Cost: Blood glucose | CAD 1.3300 | CAD 0.8607 | CAD 1.8998 |
| Cost: Lactate dehydrogenase | CAD 1.3300 | CAD 0.8607 | CAD 1.8998 |
| Cost: Inpatient stay | CAD 1580.5300 | CAD 1022.8357 | CAD 2257.6352 |
| Cost: CT Scan | CAD 195.0000 | CAD 126.1937 | CAD 278.5388 |
| Cost: Hospitalization | CAD 13,215.8400 | CAD 8552.5948 | CAD 18,877.5574 |
| Utility | |||
| Utility: Pre-progression (up to 60 months) | 0.7800 | 0.7601 | 0.7993 |
| Utility: Pre-progression, cured (beyond 60 months) | 0.7852 | 0.7653 | 0.8045 |
| Utility: Post-progression | 0.6800 | 0.6321 | 0.7261 |
| Adverse Events | |||
| Brexucabtagene autoleucel AE incidence: Hypotension | 0.2206 | 0.1305 | 0.3265 |
| Brexucabtagene autoleucel AE incidence: Neutrophil count decreased | 0.5294 | 0.4103 | 0.6468 |
| Brexucabtagene autoleucel AE incidence: White blood cell count decreased | 0.4118 | 0.2977 | 0.5308 |
| Brexucabtagene autoleucel AE incidence: Hypoxia | 0.2059 | 0.1186 | 0.3097 |
| Brexucabtagene autoleucel AE incidence: Hypophosphataemia | 0.2206 | 0.1305 | 0.3265 |
| Brexucabtagene autoleucel AE incidence: Neutropenia | 0.3382 | 0.2308 | 0.4548 |
| Brexucabtagene autoleucel AE incidence: Hyponatraemia | 0.1029 | 0.0427 | 0.1855 |
| Brexucabtagene autoleucel AE incidence: Alanine aminotransferase increased | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Encephalopathy | 0.1765 | 0.0956 | 0.2756 |
| Brexucabtagene autoleucel AE incidence: Hypokalaemia | 0.1471 | 0.0735 | 0.2405 |
| Brexucabtagene autoleucel AE incidence: Hypocalcaemia | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Thrombocytopenia | 0.1618 | 0.0844 | 0.2582 |
| Brexucabtagene autoleucel AE incidence: Aspartate aminotransferase increased | 0.1029 | 0.0427 | 0.1855 |
| Brexucabtagene autoleucel AE incidence: Confusional state | 0.1176 | 0.0526 | 0.2042 |
| Brexucabtagene autoleucel AE incidence: Hypertension | 0.1324 | 0.0629 | 0.2225 |
| Brexucabtagene autoleucel AE incidence: Acute Kidney Injury | 0.0735 | 0.0244 | 0.1465 |
| Brexucabtagene autoleucel AE incidence: Leukopenia | 0.1471 | 0.0735 | 0.2405 |
| Brexucabtagene autoleucel AE incidence: Lymphocyte count decreased | 0.0882 | 0.0333 | 0.1663 |
| Brexucabtagene autoleucel AE incidence: Pneumonia | 0.1324 | 0.0629 | 0.2225 |
| Brexucabtagene autoleucel AE incidence: Respiratory Failure | 0.0588 | 0.0163 | 0.1259 |
| Brexucabtagene autoleucel AE incidence: Sepsis | 0.0588 | 0.0163 | 0.1259 |
| Disutility Cytokine release syndrome | 0.7800 | 0.4087 | 0.9850 |
| Disutility Pyrexia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Anaemia | 0.1200 | 0.0771 | 0.1708 |
| Disutility Platelet Count decreased | 0.1100 | 0.0707 | 0.1566 |
| Disutility Hypotension | 0.1500 | 0.0961 | 0.2133 |
| Disutility Neutrophil count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility White blood cell count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypoxia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Hypophosphataemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Neutropenia | 0.0900 | 0.0579 | 0.1282 |
| Disutility Hyponatraemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Alanine aminotransferase increased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Encephalopathy | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypokalaemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypocalcaemia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Thrombocytopenia | 0.1100 | 0.0707 | 0.1566 |
| Disutility Aspartate aminotransferase increased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Confusional state | 0.1500 | 0.0961 | 0.2133 |
| Disutility Hypertension | 0.1500 | 0.0961 | 0.2133 |
| Disutility Acute Kidney Injury | 0.1500 | 0.0961 | 0.2133 |
| Disutility Leukopenia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Lymphocyte count decreased | 0.1500 | 0.0961 | 0.2133 |
| Disutility Pneumonia | 0.1500 | 0.0961 | 0.2133 |
| Disutility Respiratory Failure | 0.1500 | 0.0961 | 0.2133 |
| Disutility Sepsis | 0.1500 | 0.0961 | 0.2133 |
| Duration Cytokine release syndrome | 4.0000 | 2.5886 | 5.7136 |
| Duration Pyrexia | 2.0000 | 1.2943 | 2.8568 |
| Duration Anaemia | 14.0000 | 9.0601 | 19.9977 |
| Duration Platelet Count decreased | 50.0000 | 32.3574 | 71.4202 |
| Duration Hypotension | 5.0000 | 3.2357 | 7.1420 |
| Duration Neutrophil count decreased | 17.0000 | 11.0015 | 24.2829 |
| Duration White blood cell count decreased | 40.0000 | 25.8859 | 57.1362 |
| Duration Hypoxia | 2.0000 | 1.2943 | 2.8568 |
| Duration Hypophosphataemia | 5.0000 | 3.2357 | 7.1420 |
| Duration Neutropenia | 47.0000 | 30.4159 | 67.1350 |
| Duration Hyponatraemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Alanine aminotransferase increased | 7.0000 | 4.5300 | 9.9988 |
| Duration Encephalopathy | 9.0000 | 5.8243 | 12.8556 |
| Duration Hypokalaemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Hypocalcaemia | 7.0000 | 4.5300 | 9.9988 |
| Duration Thrombocytopenia | 63.0000 | 40.7703 | 89.9894 |
| Duration Aspartate aminotransferase increased | 7.0000 | 4.5300 | 9.9988 |
| Duration Confusional state | 7.0000 | 4.5300 | 9.9988 |
| Duration Hypertension | 5.0000 | 3.2357 | 7.1420 |
| Duration Acute Kidney Injury | 7.0000 | 4.5300 | 9.9988 |
| Duration Leukopenia | 21.0000 | 13.5901 | 29.9965 |
| Duration Lymphocyte count decreased | 64.0000 | 41.4174 | 91.4178 |
| Duration Pneumonia | 7.0000 | 4.5300 | 9.9988 |
| Duration Respiratory Failure | 7.0000 | 4.5300 | 9.9988 |
| Duration Sepsis | 7.0000 | 4.5300 | 9.9988 |
| AE cost: Cytokine release syndrome | CAD 18,366.9647 | CAD 11,886.1311 | CAD 26,235.4440 |
| Parameter | Value | SE | Distribution |
|---|---|---|---|
| Patient Characteristics | |||
| Bodyweight | 81.8 kg | 1.95296 | Normal |
| BSA | 1.978 m2 | 0.026997 | Normal |
| Resource Use | |||
| Pre-Progression Resource Use: Full blood count | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: X-ray | 0.17 | 0.033333 | Gamma |
| Pre-Progression Resource Use: Blood glucose | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: Lactate dehydrogenase | 0.33 | 0.066667 | Gamma |
| Pre-Progression Resource Use: CT Scan | 0.17 | 0.033333 | Gamma |
| Pre-Progression Resource Use: Office visit | 0.17 | 0.033333 | Gamma |
| Post-Progression Resource Use: Full blood count | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: X-ray | 0.33 | 0.066667 | Gamma |
| Post-Progression Resource Use: Blood glucose | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: Lactate dehydrogenase | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: Office visit | 1.00 | 0.2 | Gamma |
| Post-Progression Resource Use: CT Scan | 0.33 | 0.066667 | Gamma |
| Post-Progression Resource Use: Hospitalization | 0.08 | 0.016667 | Gamma |
| End-of-life Palliative care (one-off) | 1 | 0.20 | Gamma |
| Apheresis: One-Time cost | CAD 1392.37 | CAD 278.47 | Gamma |
| Tecartus Proportion ICU Stay | 0.22 | 0.04 | Beta |
| Hospital days, proportion ICU | 18 | 22.5 | Normal |
| Hospital days, proportion non-ICU | 16.50 | 10.75 | Normal |
| Proportion requiring bridging therapy | 0.37 | 0.03 | Beta |
| Cost: Initial Hospitalization: Intensive Care Unit Day | CAD 8343.73 | CAD 1668.75 | Gamma |
| Cost: Initial Hospitalization: Inpatient Day (Non-ICU) | CAD 1580.53 | CAD 316.11 | Gamma |
| Cost: Stem cell transplant | CAD 166,855.53 | CAD 33,371.11 | Gamma |
| Cost: Office visit | CAD 174.64 | CAD 34.93 | Gamma |
| Cost: Palliative care (one-off) | CAD 35,262.48 | CAD 7052.50 | Gamma |
| Cost: Full blood count | CAD 4.12 | CAD 0.82 | Gamma |
| Cost: X-ray | CAD 23.15 | CAD 4.63 | Gamma |
| Cost: Blood glucose | CAD 1.33 | CAD 0.27 | Gamma |
| Cost: Lactate dehydrogenase | CAD 1.33 | CAD 0.27 | Gamma |
| Cost: Inpatient stay | CAD 1580.53 | CAD 316.11 | Gamma |
| Cost: CT Scan | CAD 195.00 | CAD 39.00 | Gamma |
| Cost: Hospitalization | CAD 13,215.84 | CAD 2643.17 | Gamma |
| Utility | |||
| Utility: Pre-progression (up to 60 months) | 0.78 | 0.01 | Beta |
| Utility: Pre-progression, cured (beyond 60 months) | 0.7851841 | 0.01 | Beta |
| Utility: Post-progression | 0.68 | 0.024 | Beta |
| Adverse Events | |||
| Brexucabtagene autoleucel AE incidence: Cytokine release syndrome (CRS) | 62% | 0.059 | Beta |
| Brexucabtagene autoleucel AE incidence: Pyrexia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Anaemia | 51% | 0.061 | Beta |
| Brexucabtagene autoleucel AE incidence: Platelet Count decreased | 38% | 0.059 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypotension | 22% | 0.050 | Beta |
| Brexucabtagene autoleucel AE incidence: Neutrophil count decreased | 53% | 0.061 | Beta |
| Brexucabtagene autoleucel AE incidence: White blood cell count decreased | 41% | 0.060 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypoxia | 21% | 0.049 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypophosphataemia | 22% | 0.050 | Beta |
| Brexucabtagene autoleucel AE incidence: Neutropenia | 34% | 0.057 | Beta |
| Brexucabtagene autoleucel AE incidence: Hyponatraemia | 10% | 0.037 | Beta |
| Brexucabtagene autoleucel AE incidence: Alanine aminotransferase increased | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Encephalopathy | 18% | 0.046 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypokalaemia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypocalcaemia | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Thrombocytopenia | 16% | 0.045 | Beta |
| Brexucabtagene autoleucel AE incidence: Aspartate aminotransferase increased | 10% | 0.037 | Beta |
| Brexucabtagene autoleucel AE incidence: Confusional state | 12% | 0.039 | Beta |
| Brexucabtagene autoleucel AE incidence: Hypertension | 13% | 0.041 | Beta |
| Brexucabtagene autoleucel AE incidence: Acute Kidney Injury | 7% | 0.032 | Beta |
| Brexucabtagene autoleucel AE incidence: Leukopenia | 15% | 0.043 | Beta |
| Brexucabtagene autoleucel AE incidence: Lymphocyte count decreased | 9% | 0.034 | Beta |
| Brexucabtagene autoleucel AE incidence: Pneumonia | 13% | 0.041 | Beta |
| Brexucabtagene autoleucel AE incidence: Respiratory Failure | 6% | 0.029 | Beta |
| Brexucabtagene autoleucel AE incidence: Sepsis | 6% | 0.029 | Beta |
| Disutility Cytokine release syndrome | 0.78 | 0.156 | Beta |
| Disutility Pyrexia | 0.11 | 0.022 | Beta |
| Disutility Anaemia | 0.12 | 0.024 | Beta |
| Disutility Platelet Count decreased | 0.11 | 0.022 | Beta |
| Disutility Hypotension | 0.15 | 0.03 | Beta |
| Disutility Neutrophil count decreased | 0.15 | 0.03 | Beta |
| Disutility White blood cell count decreased | 0.15 | 0.03 | Beta |
| Disutility Hypoxia | 0.11 | 0.022 | Beta |
| Disutility Hypophosphataemia | 0.15 | 0.03 | Beta |
| Disutility Neutropenia | 0.09 | 0.018 | Beta |
| Disutility Hyponatraemia | 0.15 | 0.03 | Beta |
| Disutility Alanine aminotransferase increased | 0.15 | 0.03 | Beta |
| Disutility Encephalopathy | 0.15 | 0.03 | Beta |
| Disutility Hypokalaemia | 0.15 | 0.03 | Beta |
| Disutility Hypocalcaemia | 0.15 | 0.03 | Beta |
| Disutility Thrombocytopenia | 0.11 | 0.022 | Beta |
| Disutility Aspartate aminotransferase increased | 0.15 | 0.03 | Beta |
| Disutility Confusional state | 0.15 | 0.03 | Beta |
| Disutility Hypertension | 0.15 | 0.03 | Beta |
| Disutility Acute Kidney Injury | 0.15 | 0.03 | Beta |
| Disutility Leukopenia | 0.15 | 0.03 | Beta |
| Disutility Lymphocyte count decreased | 0.15 | 0.03 | Beta |
| Disutility Pneumonia | 0.15 | 0.03 | Beta |
| Disutility Respiratory Failure | 0.15 | 0.03 | Beta |
| Disutility Sepsis | 0.15 | 0.03 | Beta |
| Duration Cytokine release syndrome | 4 | 0.8 | Gamma |
| Duration Pyrexia | 2 | 0.4 | Gamma |
| Duration Anaemia | 14 | 2.8 | Gamma |
| Duration Platelet Count decreased | 50 | 10 | Gamma |
| Duration Hypotension | 5 | 1 | Gamma |
| Duration Neutrophil count decreased | 17 | 3.4 | Gamma |
| Duration White blood cell count decreased | 40 | 8 | Gamma |
| Duration Hypoxia | 2 | 0.4 | Gamma |
| Duration Hypophosphataemia | 5 | 1 | Gamma |
| Duration Neutropenia | 47 | 9.4 | Gamma |
| Duration Hyponatraemia | 7 | 1.4 | Gamma |
| Duration Alanine aminotransferase increased | 7 | 1.4 | Gamma |
| Duration Encephalopathy | 9 | 1.8 | Gamma |
| Duration Hypokalaemia | 7 | 1.4 | Gamma |
| Duration Hypocalcaemia | 7 | 1.4 | Gamma |
| Duration Thrombocytopenia | 63 | 12.6 | Gamma |
| Duration Aspartate aminotransferase increased | 7 | 1.4 | Gamma |
| Duration Confusional state | 7 | 1.4 | Gamma |
| Duration Hypertension | 5 | 1 | Gamma |
| Duration Acute Kidney Injury | 7 | 1.4 | Gamma |
| Duration Leukopenia | 21 | 4.2 | Gamma |
| Duration Lymphocyte count decreased | 64 | 12.8 | Gamma |
| Duration Pneumonia | 7 | 1.4 | Gamma |
| Duration Respiratory Failure | 7 | 1.4 | Gamma |
| Duration Sepsis | 7 | 1.4 | Gamma |
| AE cost: Cytokine release syndrome | CAD 18,366.96 | CAD 3673.39 | Gamma |
References
- Klener, P. Advances in Molecular Biology and Targeted Therapy of Mantle Cell Lymphoma. Int. J. Mol. Sci. 2019, 20, 4417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inamdar, A.A.; Goy, A.; Ayoub, N.M.; Attia, C.; Oton, L.; Taruvai, V.; Costales, M.; Lin, Y.-T.; Pecora, A.; Suh, K.S. Mantle cell lymphoma in the era of precision medicine-diagnosis, biomarkers and therapeutic agents. Oncotarget 2016, 7, 48692–48731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hamadani, M.; Habermann, T.M.; Cerhan, J.R.; Macon, W.R.; Maurer, M.J.; Go, R.S. Non-Hodgkin lymphoma subtype distribution, geodemographic patterns, and survival in the US: A longitudinal analysis of the National Cancer Data Base from 1998 to 2011. Am. J. Hematol. 2015, 90, 790–795. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Mahmud, S.; Skrabek, P.; Lix, L.; Johnston, J.B. Long-term time trends in incidence, survival and mortality of lymphomas by subtype among adults in Manitoba, Canada: A population-based study using cancer registry data. Br. Med. J. Open 2017, 7, e015106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan-Canadian Oncology Drug Review. Pan-Canadian Oncology Drug Review. Final Economic Guidance Report-Ibrutinib for Mantle Cell Lymphoma; Pan-Canadian Oncology Drug Review: Toronto, ON, Canada, 2016. [Google Scholar]
- Smith, A.; Crouch, S.; Lax, S.; Li, J.; Painter, D.; Howell, D.; Patmore, R.; Jack, A.; Roman, E. Lymphoma incidence, survival and prevalence 2004–2014: Sub-type analyses from the UK’s Haematological Malignancy Research Network. Br. J. Cancer 2015, 112, 1575–1584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jain, P.; Wang, M. Mantle cell lymphoma: 2019 update on the diagnosis, pathogenesis, prognostication, and management. Am. J. Hematol. 2019, 94, 710–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Issa, D.E.; van de Schans, S.A.; Chamuleau, M.E.; Karim-Kos, H.E.; Wondergem, M.; Huijgens, P.C.; Coebergh, J.W.W.; Zweegman, S.; Visser, O. Trends in incidence, treatment and survival of aggressive B-cell lymphoma in The Netherlands 1989–2010. Haematologica 2015, 100, 525–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, P.; Maddocks, K.; Leonard, J.P.; Ruan, J.; Goy, A.; Wagner-Johnston, N.; Rule, S.; Advani, R.; Iberri, D.; Phillips, T.; et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood 2016, 127, 1559–1563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCulloch, R.; Visco, C.; Eyre, T.A.; Frewin, R.; Phillips, N.; Tucker, D.L.; Quaglia, F.M.; McMillan, A.; Lambert, J.; Crosbie, N.; et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br. J. Haematol. 2020, 189, 684–688. [Google Scholar] [CrossRef]
- Cheah, C.Y.; Chihara, D.; Romaguera, J.E.; Fowler, N.H.; Seymour, J.F.; Hagemeister, F.B.; Champlin, R.E.; Wang, M.L. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann. Oncol. 2015, 26, 1175–1179. [Google Scholar] [CrossRef]
- Epperla, N.; Hamadani, M.; Cashen, A.F.; Ahn, K.W.; Oak, E.; Kanate, A.S.; Calzada, O.; Cohen, J.B.; Farmer, L.; Ghosh, N.; et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol. Oncol. 2017, 35, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; Kanagal-Shamanna, R.; Zhang, S.; Ahmed, M.; Ghorab, A.; Zhang, L.; Ok, C.Y.; Li, S.; Hagemeister, F.; Zeng, D.; et al. Long-Term Outcomes and Mutation Profiling of Patients with Mantle Cell Lymphoma (MCL) Who Discontinued Ibrutinib. Br. J. Haematol. 2018, 183, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Schuster, S.J.; Phillips, T.; Lossos, I.S.; Goy, A.; Rule, S.; Hamadani, M.; Ghosh, N.; Reeder, C.B.; Barnett, E.; et al. Observational Study of Lenalidomide in Patients with Mantle Cell Lymphoma Who Relapsed/Progressed After or Were Refractory/Intolerant to Ibrutinib (MCL-004). J. Hematol. Oncol. 2017, 10, 171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dreyling, M.; Campo, E.; Hermine, O.; Jerkeman, M.; Le Gouill, S.; Rule, S.; Shpilberg, O.; Walewski, J.; Ladetto, M.; ESMO Guidelines Committee. Newly diagnosed and relapsed mantle cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2017, 28, iv62–iv71. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Munoz, J.; Goy, A.; Locke, F.L.; Jacobsen, C.A.; Hill, B.T.; Timmerman, J.M.; Holmes, H.; Jaglowski, S.; Flinn, I.W.; et al. KTE-X19 CAR T-Cell Therapy in Relapsed or Refractory Mantle-Cell Lymphoma. N. Engl. J. Med. 2020, 382, 1331–1342. [Google Scholar] [CrossRef] [PubMed]
- U.S. Food & Drug Administration. Supplement Approval. 2021. Available online: https://www.fda.gov/media/146253/download (accessed on 23 July 2021).
- Health Canada Notice of Compliance (NOC) Online Query. 2021. Available online: https://health-products.canada.ca/noc-ac/info.do?lang=en&no=26027 (accessed on 16 August 2021).
- Canadian Agency for Drugs and Technologies in Health. Brexucabtagene Autoleucel. 2020. Available online: https://www.cadth.ca/brexucabtagene-autoleucel (accessed on 22 April 2021).
- Final Appraisal Document Autologous Anti-CD19-Transduced CD3+ Cells for Treating Relapsed or Refractory Mantle Cell Lymphoma. 2021. Available online: https://www.nice.org.uk/guidance/ta677/documents/final-appraisal-determination-document (accessed on 23 August 2021).
- Canadian Agency for Drugs and Technologies in Health (CADTH). Reimbursement Recommendation: Brexucabtagene Autoleucel (Tecartus); Canadian Agency for Drugs and Technologies in Health: Toronto, ON, Canada, 2021. [Google Scholar]
- Lambert, P.C. Modeling of the cure fraction in survival studies. Stata J. 2007, 7, 351–375. [Google Scholar] [CrossRef] [Green Version]
- Roth, J.A.; Sullivan, S.D.; Lin, V.W.; Bansal, A.; Purdum, A.G.; Navale, L.; Cheng, P.; Ramsey, S.D. Cost-effectiveness of axicabtagene ciloleucel for adult patients with relapsed or refractory large B-cell lymphoma in the United States. J. Med. Econ. 2018, 21, 1238–1245. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.K.; Muffly, L.S.; Spinner, M.A.; Barnes, J.I.; Owens, D.K.; Goldhaber-Fiebert, J.D. Cost Effectiveness of Chimeric Antigen Receptor T-Cell Therapy in Multiply Relapsed or Refractory Adult Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 2105–2119. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Oluwole, O.O.; Diakite, I.; Botteman, M.F.; Snider, J.T.; Locke, F.L. Cost effectiveness of axicabtagene ciloleucel versus tisagenlecleucel for adult patients with relapsed or refractory large B-cell lymphoma after two or more lines of systemic therapy in the United States. J. Med. Econ. 2021, 24, 458–468. [Google Scholar] [CrossRef]
- Ball, G.; Kuruvilla, J.; Boodoo, C.; Jain, M.D. PCN108 Cost-Effectiveness of Axicabtagene Ciloleucel (AXI-CEL) and Tisagenlecleucel (TISA-CEL) in Adult Patients with Relapsed or Refractory (R/R) Large B-Cell Lymphoma (LBCL) in Canada. Value Health 2021, 24, S39. [Google Scholar] [CrossRef]
- CADTH. Guidelines for the Economic Evaluation of Health Technologies: Canada, 4th ed.; CADTH: Ottawa, ON, Canada, 2018. [Google Scholar]
- Guidelines for the Economic Evaluation of Health Technologies: Canada. 2015. Available online: https://www.cadth.ca/about-cadth/how-we-do-it/methods-and-guidelines/guidelines-for-the-economic-evaluation-of-health-technologies-canada (accessed on 12 August 2021).
- NICE DSU Technical Support Document 19: Partitioned Survival Analysis for Decision Modelling in Health Care: A Critical Review. 2017. Available online: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2017/06/Partitioned-Survival-Analysis-final-report.pdf (accessed on 2 January 2018).
- Hettle, R.; Corbett, M.; Hinde, S.; Hodgson, R.; Jones-Diette, J.; Woolacott, N.; Palmer, S. The assessment and appraisal of regenerative medicines and cell therapy products: An exploration of methods for review, economic evaluation and appraisal. Health Technol. Assess. 2017, 21, 1–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuster, S.J.; Bishop, M.R.; Tam, C.S.; Waller, E.K.; Borchmann, P.; McGuirk, J.P.; Jäger, U.; Jaglowski, S.; Andreadis, C.; Westin, J.R.; et al. Tisagenlecleucel in Adult Relapsed or Refractory Diffuse Large B-Cell Lymphoma. N. Engl. J. Med. 2018, 380, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Nastoupil, L.J.; Jain, M.D.; Feng, L.; Spiegel, J.Y.; Ghobadi, A.; Lin, Y.; Dahiya, S.; Lunning, M.; Lekakis, L.; Reagan, P.; et al. Standard-of-Care Axicabtagene Ciloleucel for Relapsed or Refractory Large B-Cell Lymphoma: Results from the US Lymphoma CAR T Consortium. J. Clin. Oncol. 2020, 38, 3119–3128. [Google Scholar] [CrossRef] [PubMed]
- Pasquini, M.C.; Hu, Z.H.; Curran, K.; Laetsch, T.; Locke, F.; Rouce, R.; Pulsipher, M.A.; Phillips, C.L.; Keating, A.; Frigault, M.J.; et al. Real-world evidence of tisagenlecleucel for pediatric acute lymphoblastic leukemia and non-Hodgkin lymphoma. Blood Adv. 2020, 4, 5414–5424. [Google Scholar] [CrossRef] [PubMed]
- Neelapu, S.S.; Locke, F.L.; Bartlett, N.L.; Lekakis, L.J.; Reagan, P.M.; Miklos, D.B.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; et al. A Comparison of Two-Year Outcomes in ZUMA-1 (Axicabtagene Ciloleucel) and SCHOLAR-1 in Patients with Refractory Large B Cell Lymphoma. Blood 2019, 134, 4095. [Google Scholar] [CrossRef]
- Statistics Canada. Life Tables, Canada, Provinces and Territories 1980/1982 to 2016/2018; Statistics Canada: Ottawa, ON, Canada, 2020.
- Maurer, M.J.; Ghesquières, H.; Jais, J.P.; Witzig, T.E.; Haioun, C.; Thompson, C.A.; Delarue, R.; Micallef, I.N.; Peyrade, F.; Macon, W.R.; et al. Event-free survival at 24 months is a robust end point for disease-related outcome in diffuse large B-cell lymphoma treated with immunochemotherapy. J. Clin. Oncol. 2014, 32, 1066–1073. [Google Scholar] [CrossRef] [PubMed]
- NICE DSU Technical Support Document 14: Survival Analysis for Economic Evaluations alongside Clinical Trials-Extrapolation with Patient-Level Data. 2011. Available online: http://scharr.dept.shef.ac.uk/nicedsu/wp-content/uploads/sites/7/2016/03/NICE-DSU-TSD-Survival-analysis.updated-March-2013.v2.pdf (accessed on 19 December 2017).
- Precision HEOR KP. Meta-Analysis and Indirect Comparison of Interventions for Relapsed or Refractory Mantle Cell Lymphoma Previously Treated with Bruton Tyrosine Kinase Inhibitors; Precision HEOR: Bethesda, MD, USA, 2020. [Google Scholar]
- Guyot, P.; Ades, A.E.; Ouwens, M.J.; Welton, N.J. Enhanced secondary analysis of survival data: Reconstructing the data from published Kaplan-Meier survival curves. BMC Med. Res. Methodol. 2012, 12, 9. [Google Scholar] [CrossRef] [Green Version]
- Bank of Canada. @bankofcanada. 2020. Available online: https://www.bankofcanada.ca/ (accessed on 10 December 2021).
- Walker, H.; Anderson, M.; Farahati, F.; Howell, D.; Librach, S.L.; Husain, A.; Sussman, J.; Viola, R.; Sutradhar, R.; Barbera, L. Resource use and costs of end-of-Life/palliative care: Ontario adult cancer patients dying during 2002 and 2003. J. Palliat. Care 2011, 27, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Kite Pharma Inc. Clinical Study Report, Primary Analysis: A Phase 2 Multicenter Study Evaluating the Efficacy of KTE-C19 in Subjects with Relapsed/Refractory Mantle Cell Lymphoma (ZUMA-2); Kite Pharma Inc.: Los Angeles, CA, USA, 2019. [Google Scholar]
- Ministry of Health and Long Term Care. Schedule of Benefits: Physician Services under the Health Insurance Act; Ministry of Health and Long Term Care: Toronto, ON, Canada, 2020.
- Holbro, A.; Ahmad, I.; Cohen, S.; Roy, J.; Lachance, S.; Chagnon, M.; LeBlanc, R.; Bernard, L.; Busque, L.; Roy, D.C.; et al. Safety and Cost-Effectiveness of Outpatient Autologous Stem Cell Transplantation in Patients with Multiple Myeloma. Biol. Blood Marrow Transplant. 2013, 19, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Canadian Institute for Health Information. Patient Cost Estimator; Canadian Institute for Health Information: Ottawa, ON, Canada, 2020. [Google Scholar]
- Zheng, B.; Reardon, P.M.; Fernando, S.M.; Webber, C.; Thavorn, K.; Thompson, L.H.; Tanuseputro, P.; Munshi, L.; Kyeremanteng, K. Costs and Outcomes of Patients Admitted to the Intensive Care Unit with Cancer. J. Intensive Care Med. 2020, 36, 203–210. [Google Scholar] [CrossRef]
- Ministry of Health and Long Term Care. Exceptional Access Program; Ministry of Health and Long Term Care: Toronto, ON, Canada, 2019.
- Pan-Canadian Oncology Drug Review. Pan-Canadian Oncology Drug Review. Final Economic Guidance Report-Bendamustine; Pan-Canadian Oncology Drug Review: Toronto, ON, Canada, 2013. [Google Scholar]
- Final Economic Guidance Report Daratumumab (Darzalex) + VMP for Multiple Myeloma. Available online: https://www.cadth.ca/sites/default/files/pcodr/pcodr_daratumumab_darzalex_mm_fn_rec.pdf (accessed on 21 May 2020).
- Pan-Canadian Oncology Drug Review. Pan-Canadian Oncology Drug Review. Final Economic Guidance Report Pertuzumab-Trastuzumab for Early Breast Cancer; Pan-Canadian Oncology Drug Review: Toronto, ON, Canada, 2018. [Google Scholar]
- Hambley, B.; Caimi, P.F.; William, B.M. Bortezomib for the treatment of mantle cell lymphoma: An update. Ther. Adv. Hematol. 2016, 7, 196–208. [Google Scholar] [CrossRef] [PubMed]
- Anthracyclines. 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK538187/ (accessed on 14 September 2021).
- Desai, M.; Newberry, K.; Ou, Z.; Wang, M.; Zhang, L. Lenalidomide in relapsed or refractory mantle cell lymphoma: Overview and perspective. Ther. Adv. Hematol. 2014, 5, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Visco, C.; Zambello, R.; Paolini, R.; Finotto, S.; Zanotti, R.; Zaja, F.; Nadali, G.; Trentin, L.; Rodella, E.; Lissandrini, L.; et al. Rituximab, Bendamustine and Cytarabine (R-BAC) Is a Very Active Regimen in Patients with Mantle Cell Lymphoma Not Eligible for Intensive Chemotherapy or Autologous Transplant. Blood 2011, 118, 2677. [Google Scholar] [CrossRef]
- Ministry of Health and Long Term Care. Schedule of Benefits for Laboratory Services; Ministry of Health and Long Term Care: Toronto, ON, Canada, 2020.
- Ibrutinib for Treating Relapsed or Refractory Mantle Cell Lymphoma. Technology Appraisal Guidance [TA502]-Committee Papers. 2016. Available online: https://www.nice.org.uk/guidance/ta502/documents/committee-papers (accessed on 15 August 2019).
- Ara, R.; Brazier, J.E. Populating an economic model with health state utility values: Moving toward better practice. Value Health 2010, 13, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cameron, D.; Ubels, J.; Norström, F. On what basis are medical cost-effectiveness thresholds set? Clashing opinions and an absence of data: A systematic review. Glob. Health Action 2018, 11, 1447828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assouline, S.; Li, S.; Gisselbrecht, C.; Fogarty, P.; Hay, A.; van den Neste, E.; Shepherd, L.E.; Schmitz, N.; Baetz, T.; Keating, A.; et al. The conditional survival analysis of relapsed DLBCL after autologous transplant: A subgroup analysis of LY.12 and CORAL. Blood Adv. 2020, 4, 2011–2017. [Google Scholar] [CrossRef] [PubMed]
- (INESSS) Indeesess. Avis au Ministre-TECARTUS pour le Traitement du Lymphome à Cellules du Manteau. 2021. Available online: https://www.inesss.qc.ca/fileadmin/doc/INESSS/Inscription_medicaments/Avis_au_ministre/Juillet_2021/Tecartus__06.pdf (accessed on 27 November 2021).
- Simons, C.L.; Malone, D.; Wang, M.; Maglinte, G.A.; Inocencio, T.; Wade, S.W.; Bennison, C.; Shah, B. Cost-effectiveness for KTE-X19 CAR T therapy for adult patients with relapsed/refractory mantle cell lymphoma in the United States. J. Med. Econ. 2021, 24, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.; Nastoupil, L.J. Real-World Experiences of CAR T-Cell Therapy for Large B-Cell Lymphoma: How Similar Are They to the Prospective Studies. J. Immunother. Precis. Oncol. 2021, 4, 150–159. [Google Scholar] [CrossRef]
- Jain, P.; Nastoupil, L.; Westin, J.; Lee, H.J.; Navsaria, L.; Steiner, R.E.; Ahmed, S.; Moghrabi, O.; Oriabure, O.; Chen, W.; et al. Outcomes and management of patients with mantle cell lymphoma after progression on brexucabtagene autoleucel therapy. Br. J. Haematol. 2021, 192, e38–e42. [Google Scholar] [CrossRef] [PubMed]
- Locke, F.L.; Ghobadi, A.; Jacobson, C.A.; Miklos, D.B.; Lekakis, L.J.; Oluwole, O.O.; Lin, Y.; Braunschweig, I.; Hill, B.T.; Timmerman, J.M.; et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): A single-arm, multicentre, phase 1–2 trial. Lancet Oncol. 2019, 20, 31–42. [Google Scholar] [CrossRef]
- Bansal, A.; Sullivan, S.D.; Lin, V.W.; Purdum, A.G.; Navale, L.; Cheng, P.; Ramsey, S.D. Estimating Long-Term Survival for Patients with Relapsed or Refractory Large B-Cell Lymphoma Treated with Chimeric Antigen Receptor Therapy: A Comparison of Standard and Mixture Cure Models. Med. Decis. Mak. 2019, 39, 294–298. [Google Scholar] [CrossRef] [PubMed]
- Ontario Drug Benefit Formulary/Comparative Drug Index 2021. Available online: https://www.formulary.health.gov.on.ca/formulary/ (accessed on 27 November 2021).



| Elements | Description |
|---|---|
| Target population | ZUMA-2 trial population (R/R mantle cell lymphoma following treatment with a BTKi |
| Treatments | Brexucabtagene autoleucel vs. BSC |
| Model design | Partitioned survival mixture cure model for brexucabtagene autoleucel Partitioned survival model for BSC |
| Model inputs | Efficacy (PFS and OS), safety Utility values Treatment-related costs, disease-related costs, end-of-life costs |
| Outcomes of interest | Costs by category LYs and QALYs Incremental costs, incremental LYs, incremental QALYs Incremental cost/LY and cost/QALY gained |
| Perspective | Canadian healthcare system perspective |
| Health states | Pre-progression survival Post-progression survival Death |
| Time horizon | Canadian healthcare system perspective |
| Discount | 1.5% per year for both costs and outcomes |
| Cycle length | 1 month |
| Year of cost and currency | 2021 Canadian dollar |
| Sensitivity analysis | One-way deterministic sensitivity analyses Probabilistic sensitivity analyses Scenario analyses |
| Programming software | Microsoft Excel 365 |
| Treatment | Proportion of Patients on Intervention in Base Case (%) |
|---|---|
| Rituximab | 68.2% |
| Bendamustine | 57.4% |
| Bortezomib | 5.5% |
| Anthracycline-based | 7.3% |
| Total | 138.5% |
| Health States | Value | Standard Error | Reference |
|---|---|---|---|
| Pre-progression | 0.780 | 0.010 | NICE ibrutinib, 2016 [56] |
| Pre-progression for long-term survivors | 0.812 | 0.010 | Calculated from Ara and Brazier, 2010 [57] |
| Post-progression | 0.680 | 0.024 | NICE ibrutinib, 2016 [56] |
| Brexucabtagene Autoleucel | Literature-Based Meta-Analysis | Incremental | |
|---|---|---|---|
| Median survival (years) | 12.71 | 0.88 | 11.83 |
| Total undiscounted years | 13.22 | 1.76 | 11.46 |
| Pre-progression | 9.30 | 1.68 | 7.63 |
| Post-progression | 3.92 | 0.09 | 3.83 |
| Total discounted years | 11.26 | 1.70 | 9.56 |
| Pre-progression | 7.95 | 1.63 | 6.33 |
| Post-progression | 3.31 | 0.08 | 3.23 |
| Total discounted QALYs | 8.34 | 1.31 | 7.03 |
| Pre-progression | 6.23 | 1.27 | 4.97 |
| Pre-Progression, pre-cure point | 1.95 | 1.11 | 0.85 |
| Pre-Progression, post-cure point | 4.28 | 0.16 | 4.12 |
| Post-progression | 2.14 | 0.05 | 2.09 |
| Adverse events | −0.04 | −0.01 | −0.03 |
| Total discounted costs | CAD 688,040 | CAD 66,108 | CAD 621,933 |
| Total treatment-related costs | CAD 589,375 | CAD 27,946 | CAD 561,429 |
| Total drug acquisition | CAD 533,523 | CAD 27,221 | CAD 506,302 |
| Total apheresis | CAD 1392 | CAD 0 | CAD 1392 |
| Total drug administration | CAD 211 | CAD 726 | CAD −515 |
| Total lymphodepletion chemotherapy | CAD 646 | CAD 0 | CAD 646 |
| Total bridging therapy | CAD 220 | CAD 0 | CAD 220 |
| Total hospitalization | CAD 53,383 | CAD 0 | CAD 3383 |
| Total disease management | CAD 57,739 | CAD 2490 | CAD 55,249 |
| Pre-progression | CAD 3939 | CAD 1225 | CAD 2714 |
| Post-progression | CAD 53,800 | CAD 1264 | CAD 52,535 |
| Other costs | CAD 40,926 | CAD 35,671 | CAD 5255 |
| End of life care | CAD 29,582 | CAD 34,589 | CAD −5007 |
| Adverse events | CAD 11,344 | CAD 1082 | CAD 10,262 |
| Cost/QALY | CAD 88,503 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ball, G.; Lemieux, C.; Cameron, D.; Seftel, M.D. Cost-Effectiveness of Brexucabtagene Autoleucel versus Best Supportive Care for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma following Treatment with a Bruton’s Tyrosine Kinase Inhibitor in Canada. Curr. Oncol. 2022, 29, 2021-2045. https://doi.org/10.3390/curroncol29030164
Ball G, Lemieux C, Cameron D, Seftel MD. Cost-Effectiveness of Brexucabtagene Autoleucel versus Best Supportive Care for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma following Treatment with a Bruton’s Tyrosine Kinase Inhibitor in Canada. Current Oncology. 2022; 29(3):2021-2045. https://doi.org/10.3390/curroncol29030164
Chicago/Turabian StyleBall, Graeme, Christopher Lemieux, David Cameron, and Matthew D. Seftel. 2022. "Cost-Effectiveness of Brexucabtagene Autoleucel versus Best Supportive Care for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma following Treatment with a Bruton’s Tyrosine Kinase Inhibitor in Canada" Current Oncology 29, no. 3: 2021-2045. https://doi.org/10.3390/curroncol29030164
APA StyleBall, G., Lemieux, C., Cameron, D., & Seftel, M. D. (2022). Cost-Effectiveness of Brexucabtagene Autoleucel versus Best Supportive Care for the Treatment of Relapsed/Refractory Mantle Cell Lymphoma following Treatment with a Bruton’s Tyrosine Kinase Inhibitor in Canada. Current Oncology, 29(3), 2021-2045. https://doi.org/10.3390/curroncol29030164




