Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study
Abstract
:1. Introduction
2. Materials and Methods
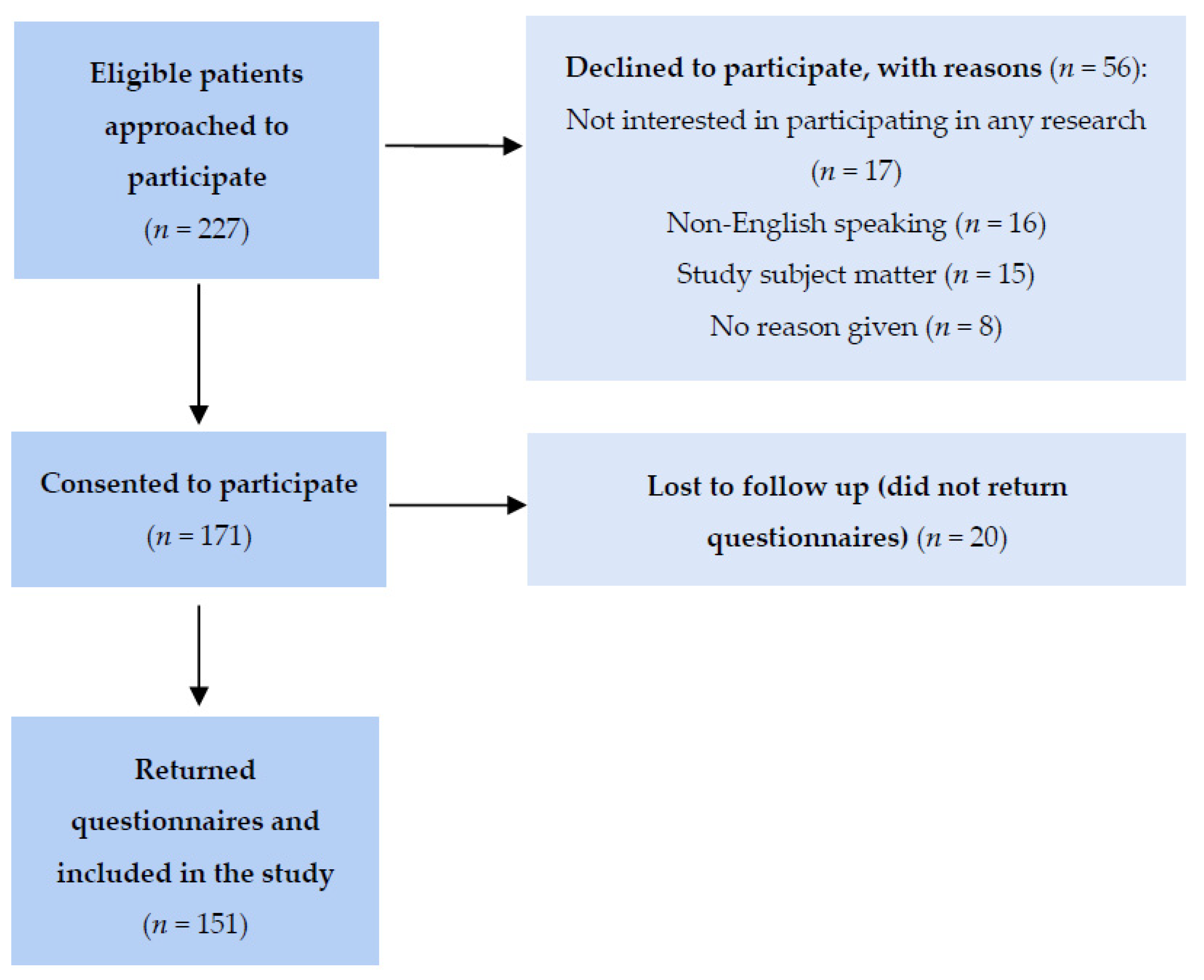
2.1. Participant Recruitment
2.2. Measures and Data Collection
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Occurrence and Frequency of GS
3.3. Patient-Provider Communication and Patient Needs around GS
3.4. Sexual Function
3.5. QoL
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Treatment Experience Questionnaire
- 1.
- How old were you when you were diagnosed with breast cancer?______________________
- 2.
- At the time of diagnosis, what endocrine therapy did your doctor prescribe to you?
- a.
- Tamoxifen
- b.
- Aromatase Inhibitors (anastrozole/letrozole/exemestane)
- c.
- Ovarian function suppression (goserelin acetate/oophorectomy)
- d.
- I am not sure
- 3.
- Has your endocrine therapy changed since then?
- a.
- Yes
- b.
- No
- c.
- I am not sure
- 4.
- If YES, why did the treatment change?
- a.
- Your oncologist recommended the change
- b.
- You experienced side effects
- c.
- Other (please be specific) ______________________
- 5.
- At diagnosis, were you (please circle one):
- a.
- Pre-menopausal, peri-menopausal, post-menopausal, I am not sure.
- 6.
- Did your period stop after starting cancer treatment?
- a.
- Yes
- b.
- No
- c.
- I am not sure
- 7.
- To the best of your memory, when was your last period (month and year)?______________________
- 8.
- With regards to your menstrual history:
- a.
- When did you get your first period (age in years)? ______________________
- b.
- What was the average length of your menstrual cycle (in days)?______________________
- c.
- How many days did your period usually last, on average?______________________
- 9.
- With regards to your pregnancy history (if not applicable, skip to question 10):
- a.
- How many times have you been pregnant?______________________
- b.
- How many children have you delivered? ______________________
- c.
- How many were delivered vaginally? ______________________
- d.
- How many of them were delivered via C-section? ______________________
- e.
- How many pregnancies have you lost (i.e., miscarriage, abortion) if applicable? ______________________
- 10.
- In the past year, how often have you experienced the following?
| All the Time | Often (at Least Once a Week) | Rarely (a Few Times) | Never | |
| Vaginal dryness | ||||
| Vaginal discharge | ||||
| Vaginal bleeding | ||||
| Vaginal itchiness | ||||
| Urinary tract infections (UTIs) (please write the number of UTIs you’ve had in the past year) | ||||
| Yeast infections/vaginitis (please write the number of yeast infections/vaginitis you’ve had in the past year) | ||||
| Hot flashes/insomnia | ||||
| Decreased sex drive | ||||
| Feel depressed |
- 11.
- Are you currently sexually active?
- a.
- Yes
- b.
- No
- 12.
- Do you have a sexual partner?
- a.
- Yes
- b.
- No
- 13.
- Do you experience pain during intercourse or masturbation?
- a.
- Yes
- b.
- No
- 14.
- Has your oncologist asked you about any of the previously mentioned side effects? (i.e., sexual and/or vaginal health)?
- a.
- Yes (continue to the next question)
- b.
- No (skip to question 17)
- 15.
- If YES (i.e., you selected option A for question 14), what happened next (check all that apply)?
- a.
- Your doctor told you about different treatment options that could be offered to you
- b.
- Your doctor advised you against local hormone therapy (i.e., vaginal estrogen creams)
- c.
- Your doctor referred you to a specialist/gynecologist
- d.
- Your symptoms are mild and you did not want to take action
- e.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
- 16.
- If you were referred to a specialist/gynecologist (i.e., you selected option C for question 15), what happened next (check all that apply)?
- a.
- It was recommended that you stop endocrine therapy (stopped Tamoxifen or aromatase inhibitors)
- b.
- It was recommended that you change cancer treatment
- c.
- You were advised against local hormone therapy
- d.
- You received supportive medication for your side effects (e.g., vaginal creams, local hormone therapy)
- e.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
- 17.
- If your oncologist did NOT ask you about these side effects (i.e., you selected option B for question 14), did you bring it up with them?
- a.
- Yes
- b.
- No
- 18.
- If YES (i.e., you selected option A for question 17), what happened next?
- a.
- Your doctor told you about different treatment options that could be offered to you
- b.
- Your doctor advised you against local hormone therapy (i.e., vaginal estrogen creams)
- c.
- Your doctor referred you to a specialist/gynecologist
- d.
- Your symptoms are mild and you did not want to take action
- e.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
- 19.
- If NO (i.e., you selected option B for question 17), why didn’t you bring it up?
- a.
- Embarrassment (you find it difficult/awkward talking about your sexual and/or vaginal health)
- b.
- There was no opportunity to bring it up
- c.
- The side effects are not severe enough for you to want treatment
- d.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
- 20.
- If you were referred to a specialist/gynecologist (i.e., you selected option C for question 18), what happened next (check all that apply)?
- a.
- It was recommended that you stop endocrine therapy (stopped Tamoxifen or aromatase inhibitors)
- b.
- It was recommended that you change cancer treatment
- c.
- You were advised against local hormone therapy
- d.
- You received supportive medication for your side effects (e.g., vaginal creams, local hormone therapy)
- e.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
- 21.
- If applicable, did your side effects improve with the change in endocrine therapy or additional supportive medication?
- a.
- Yes
- b.
- No
- 22.
- If NO (i.e., you selected option B for question 21), what happened next?__________________________________________________________________________________________________________________________________________________________________________________
- 23.
- Are you comfortable talking about your sexual health and/or vaginal health with your oncologist?
- a.
- Yes
- b.
- No
- 24.
- If NO (i.e., you selected option B for question 23), why are you uncomfortable speaking to your oncologist about this topic?__________________________________________________________________________________________________________________________________________________________________________________
- 25.
- With your current side effects in mind, check all that apply:
- a.
- I am interested in seeing a physician specialist concerning my side effects
- b.
- I am open to potential treatment for my side effects
- c.
- I want to know what my options are regarding how to best address my side effects
- d.
- My side effects aren’t severe enough for me to want treatment
- e.
- Other (please specify)__________________________________________________________________________________________________________________________________________________________________________________
References
- Burstein, H.J.; Temin, S.; Anderson, H.; Buchholz, T.A.; Davidson, N.E.; Gelmon, K.E.; Giordano, S.H.; Hudis, C.A.; Rowden, D.; Solky, A.J.; et al. Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update. J. Clin. Oncol. 2014, 32, 2255–2269. [Google Scholar] [CrossRef] [PubMed]
- Mourits, M.J.; De Vries, E.G.; Willemse, P.H.; Ten Hoor, K.A.; Hollema, H.; Van der Zee, A.G. Tamoxifen Treatment and Gynecologic Side Effects: A Review. Obstet. Gynecol. 2001, 97, 855–866. [Google Scholar] [CrossRef] [PubMed]
- Falk, S.J.; Bober, S. Vaginal Health During Breast Cancer Treatment. Curr. Oncol. Rep. 2016, 18, 32. [Google Scholar] [CrossRef]
- Cella, D.; Fallowfield, L.J. Recognition and Management of Treatment-Related Side Effects for Breast Cancer Patients Receiving Adjuvant Endocrine Therapy. Breast Cancer Res. Treat. 2008, 107, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Makubate, B.; Donnan, P.T.; Dewar, J.A.; Thompson, A.M.; McCowan, C. Cohort Study of Adherence to Adjuvant Endocrine Therapy, Breast Cancer Recurrence and Mortality. Br. J. Cancer 2013, 108, 1515–1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awan, A.; Esfahani, K. Endocrine Therapy for Breast Cancer in the Primary Care Setting. Curr. Oncol. 2018, 25, 285–291. [Google Scholar] [CrossRef] [Green Version]
- Fallowfield, L.; Cella, D.; Cuzick, J.; Francis, S.; Locker, G.; Howell, A. Quality of Life of Postmenopausal Women in the Arimidex, Tamoxifen, Alone or in Combination (ATAC) Adjuvant Breast Cancer Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 4261–4271. [Google Scholar] [CrossRef]
- Derzko, C.; Elliott, S.; Lam, W. Management of Sexual Dysfunction in Postmenopausal Breast Cancer Patients Taking Adjuvant Aromatase Inhibitor Therapy. Curr. Oncol. 2007, 14, S20–S40. [Google Scholar] [CrossRef] [Green Version]
- Chin, S.N.; Trinkaus, M.; Simmons, C.; Flynn, C.; Dranitsaris, G.; Bolivar, R.; Clemons, M. Prevalence and Severity of Urogenital Symptoms in Postmenopausal Women Receiving Endocrine Therapy for Breast Cancer. Clin. Breast Cancer 2009, 9, 108–117. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, C.; Li, W.; Jin, F.; Wang, A. Incidence and Severity of Sexual Dysfunction among Women with Breast Cancer: A Meta-Analysis Based on Female Sexual Function Index. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2019, 27, 1171–1180. [Google Scholar] [CrossRef]
- Stabile, C.; Goldfarb, S.; Baser, R.E.; Goldfrank, D.J.; Abu-Rustum, N.R.; Barakat, R.R.; Dickler, M.N.; Carter, J. Sexual Health Needs and Educational Intervention Preferences for Women with Cancer. Breast Cancer Res. Treat. 2017, 165, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Carter, J.; Lacchetti, C.; Andersen, B.L.; Barton, D.L.; Bolte, S.; Damast, S.; Diefenbach, M.A.; DuHamel, K.; Florendo, J.; Ganz, P.A.; et al. Interventions to Address Sexual Problems in People With Cancer: American Society of Clinical Oncology Clinical Practice Guideline Adaptation of Cancer Care Ontario Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2018, 36, 492–511. [Google Scholar] [CrossRef] [PubMed]
- Reese, J.B.; Sorice, K.; Beach, M.C.; Porter, L.S.; Tulsky, J.A.; Daly, M.B.; Lepore, S.J. Patient-Provider Communication about Sexual Concerns in Cancer: A Systematic Review. J. Cancer Surviv. 2017, 11, 175–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.J.; Chuang, J.; Bickell, N.A.; Wisnivesky, J.P. Patient-Provider Communication and Hormonal Therapy Side Effects in Breast Cancer Survivors. Women Health 2017, 57, 976–989. [Google Scholar] [CrossRef] [PubMed]
- Baumgart, J.; Nilsson, K.; Stavreus-Evers, A.; Kask, K.; Villman, K.; Lindman, H.; Kallak, T.; Sundström-Poromaa, I. Urogenital Disorders in Women with Adjuvant Endocrine Therapy after Early Breast Cancer. Am. J. Obstet. Gynecol. 2011, 204, 26.e1–26.e7. [Google Scholar] [CrossRef]
- Baumgart, J.; Nilsson, K.; Evers, A.S.; Kallak, T.K.; Poromaa, I.S. Sexual Dysfunction in Women on Adjuvant Endocrine Therapy after Breast Cancer. Menopause 2013, 20, 162–168. [Google Scholar] [CrossRef]
- Etikan, I. Comparison of Convenience Sampling and Purposive Sampling. Am. J. Theor. Appl. Stat. 2016, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Vaismoradi, M.; Turunen, H.; Bondas, T. Content Analysis and Thematic Analysis: Implications for Conducting a Qualitative Descriptive Study. Nurs. Health Sci. 2013, 15, 398–405. [Google Scholar] [CrossRef]
- Rosen, R.; Brown, C.; Heiman, J.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R. The Female Sexual Function Index (FSFI): A Multidimensional Self-Report Instrument for the Assessment of Female Sexual Function. J. Sex Marital Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef]
- Wiegel, M.; Meston, C.; Rosen, R. The Female Sexual Function Index (FSFI): Cross-Validation and Development of Clinical Cutoff Scores. J. Sex Marital Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef]
- Neijenhuijs, K.I.; Hooghiemstra, N.; Holtmaat, K.; Aaronson, N.K.; Groenvold, M.; Holzner, B.; Terwee, C.B.; Cuijpers, P.; Verdonck-de Leeuw, I.M. The Female Sexual Function Index (FSFI)—A Systematic Review of Measurement Properties. J. Sex. Med. 2019, 16, 640–660. [Google Scholar] [CrossRef] [PubMed]
- Baser, R.E.; Li, Y.; Carter, J. Psychometric Validation of the Female Sexual Function Index (FSFI) in Cancer Survivors. Cancer 2012, 118, 4606–4618. [Google Scholar] [CrossRef]
- Bartula, I.; Sherman, K.A. The Female Sexual Functioning Index (FSFI): Evaluation of Acceptability, Reliability, and Validity in Women with Breast Cancer. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2015, 23, 2633–2641. [Google Scholar] [CrossRef] [PubMed]
- Hilditch, J.R.; Lewis, J.; Peter, A.; van Maris, B.; Ross, A.; Franssen, E.; Guyatt, G.H.; Norton, P.G.; Dunn, E. A Menopause-Specific Quality of Life Questionnaire: Development and Psychometric Properties. Maturitas 1996, 24, 161–175. [Google Scholar] [CrossRef]
- Dolye, C.; Adams, L.; McAndrew, A.; Burlein-Hall, S.; DasGupta, T.; Blake, J.; Fitch, M. Validation of the MENQOL for Use with Women Who Have Been Treated for Gynecologic or Breast Cancer. Can. Oncol. Nurs. J. 2018, 28, 228–233. [Google Scholar]
- Ribi, K.; Luo, W.; Walley, B.A.; Burstein, H.J.; Chirgwin, J.; Ansari, R.H.; Salim, M.; van der Westhuizen, A.; Abdi, E.; Francis, P.A.; et al. Treatment-Induced Symptoms, Depression and Age as Predictors of Sexual Problems in Premenopausal Women with Early Breast Cancer Receiving Adjuvant Endocrine Therapy. Breast Cancer Res. Treat. 2020, 181, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Canzona, M.R.; Fisher, C.L.; Wright, K.B.; Ledford, C.J.W. Talking about Sexual Health during Survivorship: Understanding What Shapes Breast Cancer Survivors’ Willingness to Communicate with Providers. J. Cancer Surviv. 2019, 13, 932–942. [Google Scholar] [CrossRef]
- Flynn, K.E.; Reese, J.B.; Jeffery, D.D.; Abernethy, A.P.; Lin, L.; Shelby, R.A.; Porter, L.S.; Dombeck, C.B.; Weinfurt, K.P. Patient Experiences with Communication about Sex during and after Treatment for Cancer. Psychooncology 2012, 21, 594–601. [Google Scholar] [CrossRef] [Green Version]
- van Londen, G.J.; Donovan, H.S.; Beckjord, E.B.; Cardy, A.L.; Bovbjerg, D.H.; Davidson, N.E.; Morse, J.Q.; Switzer, G.E.; Verdonck-de Leeuw, I.M.; Dew, M.A. Perspectives of Postmenopausal Breast Cancer Survivors on Adjuvant Endocrine Therapy-Related Symptoms. Oncol. Nurs. Forum 2014, 41, 660–668. [Google Scholar] [CrossRef] [Green Version]
- Sousa, M.; Peate, M.; Lewis, C.; Jarvis, S.; Willis, A.; Hickey, M.; Friedlander, M. Exploring Knowledge, Attitudes and Experience of Genitourinary Symptoms in Women with Early Breast Cancer on Adjuvant Endocrine Therapy. Eur. J. Cancer Care (Engl.) 2018, 27, e12820. [Google Scholar] [CrossRef]
- Sousa, M.S.; Peate, M.; Jarvis, S.; Hickey, M.; Friedlander, M. A Clinical Guide to the Management of Genitourinary Symptoms in Breast Cancer Survivors on Endocrine Therapy. Ther. Adv. Med. Oncol. 2017, 9, 269–285. [Google Scholar] [CrossRef] [PubMed]
- Fogh, M.; Højgaard, A.; Rotbøl, C.B.; Jensen, A.B. The Majority of Danish Breast Cancer Survivors on Adjuvant Endocrine Therapy Have Clinically Relevant Sexual Dysfunction: A Cross-Sectional Study. Acta Oncol. 2021, 60, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Milata, J.L.; Otte, J.L.; Carpenter, J.S. Oral Endocrine Therapy Non-Adherence, Side Effects, Decisional Support, and Decisional Needs in Women with Breast Cancer. Cancer Nurs. 2018, 41, E9–E18. [Google Scholar] [CrossRef] [PubMed]
- Font, R.; Espinas, J.A.; Gil-Gil, M.; Barnadas, A.; Ojeda, B.; Tusquets, I.; Segui, M.A.; Margelí, M.; Arcusa, A.; Prat, A.; et al. Prescription Refill, Patient Self-Report and Physician Report in Assessing Adherence to Oral Endocrine Therapy in Early Breast Cancer Patients: A Retrospective Cohort Study in Catalonia, Spain. Br. J. Cancer 2012, 107, 1249–1256. [Google Scholar] [CrossRef] [Green Version]
| All Patients | |
|---|---|
| n = 151 | |
| Age at diagnosis, years (mean ± SD) | 52.2 ± 10.2 |
| <50 | 66 (43.7%) |
| ≥50 | 85 (56.3%) |
| Tumor characteristics | |
| HR+, HER2-negative | 123 (81.5%) |
| HR+, HER2-positive | 28 (18.5%) |
| Menopausal status at diagnosis | |
| Pre-menopausal | 68 (45.0%) |
| Peri-menopausal | 11 (7.3%) |
| Post-menopausal | 72 (47.7%) |
| History of chemotherapy | 87 (57.6%) |
| Breast cancer surgery | |
| Lumpectomy | 96 (63.6%) |
| Mastectomy | 48 (31.8%) |
| None | 7 (4.6%) |
| History of breast reconstruction | 38 (25.2%) |
| Prophylactic oophorectomy to initiate ET | 17 (11.3%) |
| Use of hormone replacement therapy prior to breast cancer diagnosis | 19 (12.6%) |
| Age at time of survey, years (mean ± SD) | 56.2 ± 10.3 |
| <50 | 38 (25.2%) |
| ≥50 | 113 (74.8%) |
| Metastatic cancer at time of survey | 18 (11.9%) |
| Current ET at time of survey | |
| Tamoxifen | 77 (51.0%) |
| Anastrozole | 33 (21.9%) |
| Letrozole | 23 (15.2%) |
| Exemestane | 18 (11.9%) |
| Concurrent ovarian function suppression therapy with ET at time of survey | 9 (6.0%) |
| Lapse in ET adherence ever noted in EMR | 17 (11.3%) |
| Symptom | n (%) 1 | All the Time | Often 2 | Rarely 3 | Never |
|---|---|---|---|---|---|
| Vaginal dryness | 142 | 42 (29.6) | 14 (9.9) | 40 (28.2) | 46 (32.4) |
| Vaginal discharge | 141 | 17 (12.1) | 27 (19.2) | 31 (22.0) | 66 (46.8) |
| Vaginal bleeding | 137 | 2 (1.5) | 1 (0.7) | 21 (15.3) | 113 (82.5) |
| Vaginal itchiness | 137 | 5 (3.7) | 16 (11.7) | 45 (32.9) | 71 (51.8) |
| Hot flashes/insomnia | 141 | 63 (44.7) | 32 (22.7) | 30 (21.3) | 16 (11.4) |
| Decreased sex drive | 130 | 49 (37.7) | 32 (24.6) | 25 (19.2) | 24 (18.5) |
| Feel depressed | 132 | 14 (10.6) | 35 (26.5) | 38 (28.8) | 45 (34.1) |
| 1 to 2 | 3 to 4 | 5 or more | None | ||
| Urinary tract infection (UTI) | 135 | 12 (8.9) | 4 (3.0) | 3 (2.2) | 116 (85.9) |
| Yeast infection/vaginitis | 130 | 14 (10.8) | 5 (3.9) | 1 (0.8) | 110 (84.6) |
| FSFI Domain | n 1 | Score (Mean ± SD) |
|---|---|---|
| Desire | 55 | 2.9 ± 1.1 |
| Arousal | 54 | 3.8 ± 1.2 |
| Lubrication | 53 | 4.4 ± 1.4 |
| Orgasm | 55 | 4.4 ± 1.3 |
| Satisfaction | 52 | 4.5 ± 1.3 |
| Pain | 52 | 4.5 ± 1.6 |
| Total score | 49 | 24.2 ± 6.3 |
| MENQOL Domain | n 1 | Score (Mean ± SD) |
|---|---|---|
| Vasomotor | 129 | 4.1 ± 2.3 |
| Psychosocial | 122 | 3.6 ± 1.8 |
| Physical | 114 | 3.7 ± 1.6 |
| Sexual | 119 | 3.9 ± 2.4 |
| Total score | 98 | 3.9 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moskalewicz, A.; Di Tomaso, A.; Kachura, J.J.; Scime, S.; Nisenbaum, R.; Lee, R.; Haq, R.; Derzko, C.; Brezden-Masley, C. Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study. Curr. Oncol. 2022, 29, 1813-1827. https://doi.org/10.3390/curroncol29030149
Moskalewicz A, Di Tomaso A, Kachura JJ, Scime S, Nisenbaum R, Lee R, Haq R, Derzko C, Brezden-Masley C. Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study. Current Oncology. 2022; 29(3):1813-1827. https://doi.org/10.3390/curroncol29030149
Chicago/Turabian StyleMoskalewicz, Alexandra, Amy Di Tomaso, Jacob J. Kachura, Samantha Scime, Rosane Nisenbaum, Ronita Lee, Rashida Haq, Christine Derzko, and Christine Brezden-Masley. 2022. "Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study" Current Oncology 29, no. 3: 1813-1827. https://doi.org/10.3390/curroncol29030149
APA StyleMoskalewicz, A., Di Tomaso, A., Kachura, J. J., Scime, S., Nisenbaum, R., Lee, R., Haq, R., Derzko, C., & Brezden-Masley, C. (2022). Gynecologic Symptoms among Hormone Receptor-Positive Breast Cancer Patients on Oral Endocrine Therapy: A Cross-Sectional Study. Current Oncology, 29(3), 1813-1827. https://doi.org/10.3390/curroncol29030149





