Quantitative Evaluation of Proliferative Potential Using Flow Cytometry Reveals Intratumoral Heterogeneity and Its Relevance to Tumor Characteristics in Vestibular Schwannomas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Population and Tumor Characteristics
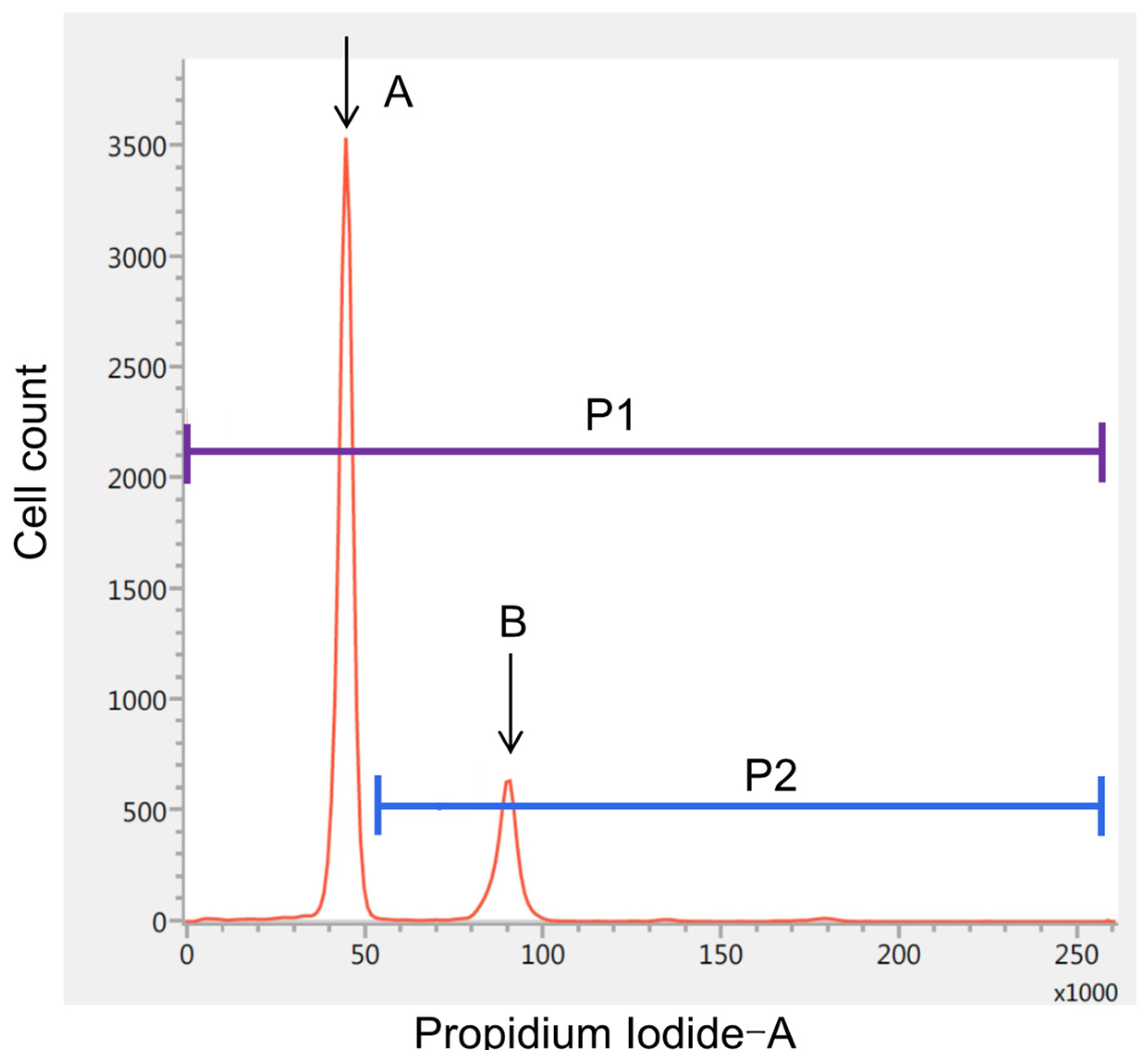
2.2. iFC and Histological Analysis
2.3. Statistical Analysis
3. Results
3.1. Patient Characteristics
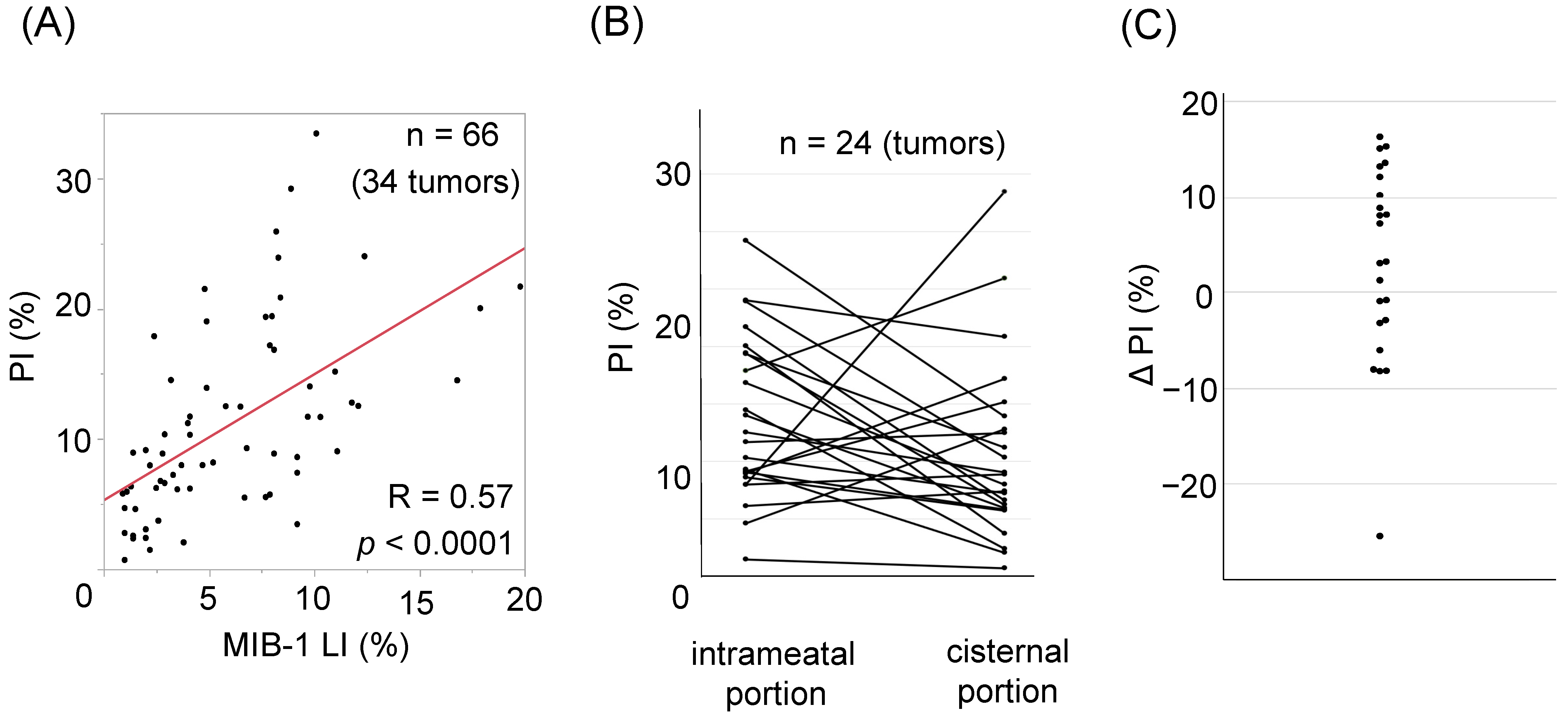
3.2. Correlation between MIB-1 and PI and Relevant Clinical Factors
3.3. Intratumoral Heterogeneity of PI
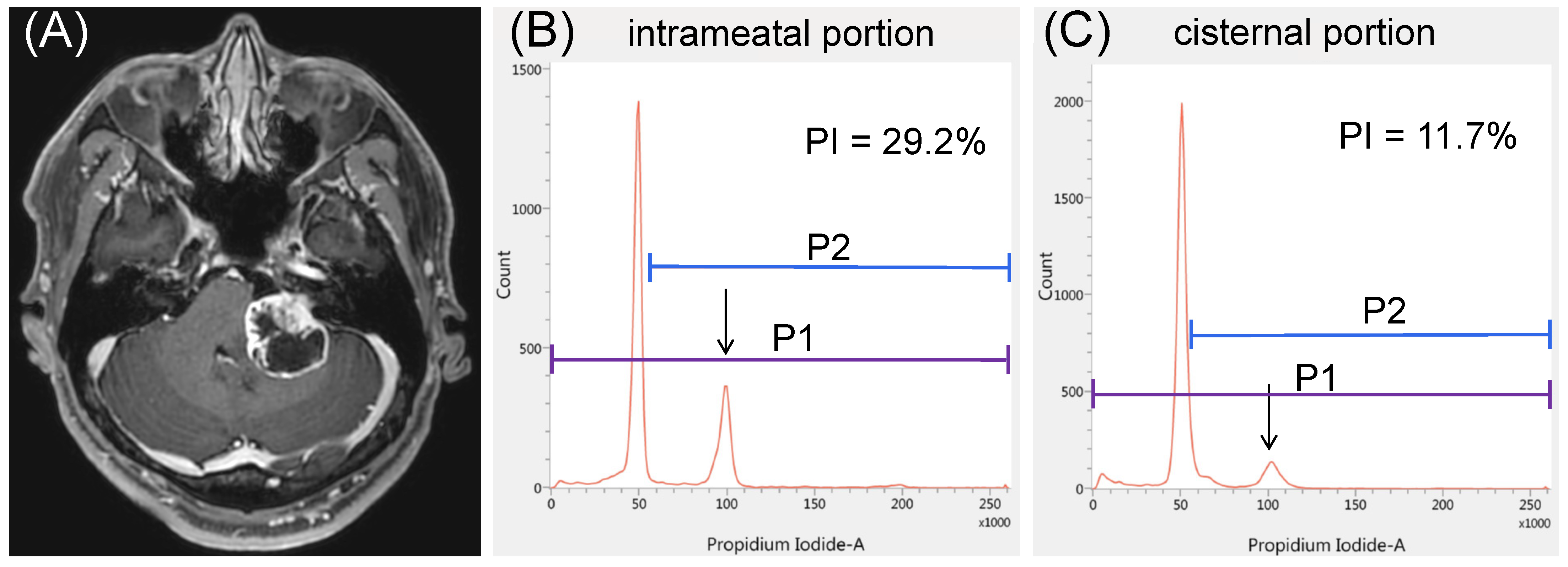
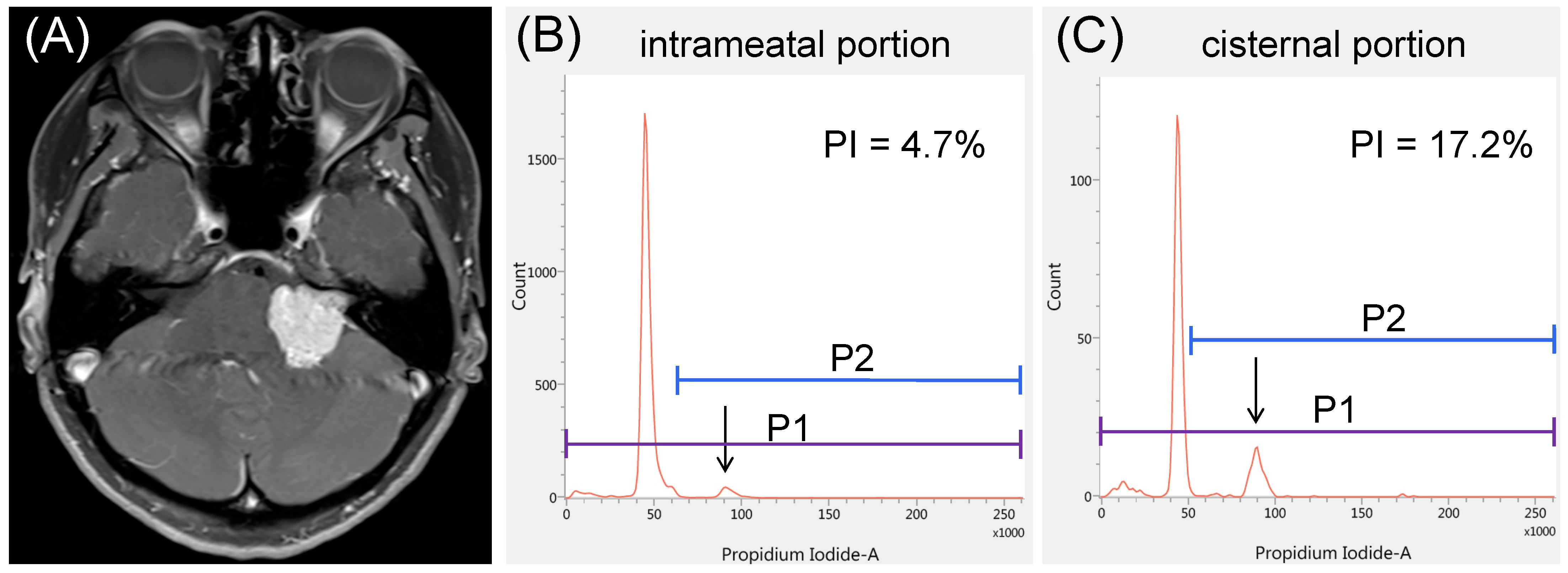
3.4. Illustrative Cases
3.4.1. Case 1
3.4.2. Case 2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seferis, C.; Torrens, M.; Paraskevopoulou, C.; Psichidis, G. Malignant Transformation in Vestibular Schwannoma: Report of a Single Case, Literature Search, and Debate. J. Neurosurg. 2014, 121, 160–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasegawa, T.; Kida, Y.; Kato, T.; Iizuka, H.; Kuramitsu, S.; Yamamoto, T. Long-Term Safety and Efficacy of Stereotactic Radiosurgery for Vestibular Schwannomas: Evaluation of 440 Patients More Than 10 Years after Treatment with Gamma Knife Surgery. J. Neurosurg. 2013, 118, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Kondziolka, D.; Mousavi, S.H.; Kano, H.; Flickinger, J.C.; Lunsford, L.D. The Newly Diagnosed Vestibular Schwannoma: Radiosurgery, Resection, or Observation? Neurosurg. Focus 2012, 33, E8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunsford, L.D.; Niranjan, A.; Flickinger, J.C.; Maitz, A.; Kondziolka, D. Radiosurgery of Vestibular Schwannomas: Summary of Experience in 829 Cases. J. Neurosurg. 2005, 102, 195–199. [Google Scholar] [CrossRef]
- Schwartz, M.S.; Kari, E.; Strickland, B.M.; Berliner, K.; Brackmann, D.E.; House, J.W.; Friedman, R.A. Evaluation of the Increased Use of Partial Resection of Large Vestibular Schwannomas: Facial Nerve Outcomes and Recurrence/Regrowth Rates. Otol. Neurotol. 2013, 34, 1456–1464. [Google Scholar] [CrossRef]
- Sughrue, M.E.; Kaur, R.; Rutkowski, M.J.; Kane, A.J.; Kaur, G.; Yang, I.; Pitts, L.H.; Parsa, A.T. Extent of Resection and the Long-Term Durability of Vestibular Schwannoma Surgery. J. Neurosurg. 2011, 114, 1218–1223. [Google Scholar] [CrossRef] [Green Version]
- Van de Langenberg, R.; Hanssens, P.E.J.; van Overbeeke, J.J.; Verheul, J.B.; Nelemans, P.J.; de Bondt, B.J.; Stokroos, R.J. Management of Large Vestibular Schwannoma. Part I. Planned Subtotal Resection Followed by Gamma Knife Surgery: Radiological and Clinical Aspects. J. Neurosurg. 2011, 115, 875–884. [Google Scholar] [CrossRef] [Green Version]
- Fukuda, M.; Oishi, M.; Hiraishi, T.; Natsumeda, M.; Fujii, Y. Clinicopathological Factors Related to Regrowth of Vestibular Schwannoma after Incomplete Resection. J. Neurosurg. 2011, 114, 1224–1231. [Google Scholar] [CrossRef]
- Nakatomi, H.; Jacob, J.T.; Carlson, M.L.; Tanaka, S.; Tanaka, M.; Saito, N.; Lohse, C.M.; Driscoll, C.L.W.; Link, M.J. Long-Term Risk of Recurrence and Regrowth after Gross-Total and Subtotal Resection of Sporadic Vestibular Schwannoma. J. Neurosurg. 2017, 133, 1052–1058. [Google Scholar] [CrossRef] [Green Version]
- Breshears, J.D.; Morshed, R.A.; Molinaro, A.M.; McDermott, M.W.; Cheung, S.W.; Theodosopoulos, P.V. Residual Tumor Volume and Location Predict Progression after Primary Subtotal Resection of Sporadic Vestibular Schwannomas: A Retrospective Volumetric Study. Neurosurgery 2020, 86, 410–416. [Google Scholar] [CrossRef]
- Oya, S.; Yoshida, S.; Tsuchiya, T.; Fujisawa, N.; Mukasa, A.; Nakatomi, H.; Saito, N.; Matsui, T. Intraoperative Quantification of Meningioma Cell Proliferation Potential Using Rapid Flow Cytometry Reveals Intratumoral Heterogeneity. Cancer Med. 2019, 8, 2793–2801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shioyama, T.; Muragaki, Y.; Maruyama, T.; Komori, T.; Iseki, H. Intraoperative Flow Cytometry Analysis of Glioma Tissue for Rapid Determination of Tumor Presence and Its Histopathological Grade: Clinical Article. J. Neurosurg. 2013, 118, 1232–1238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, G.; Eguchi, S.; Anami, H.; Ishikawa, T.; Yamaguchi, K.; Nitta, M.; Muragaki, Y.; Kawamata, T. Ultrarapid Evaluation of Meningioma Malignancy by Intraoperative Flow Cytometry. World Neurosurg. 2018, 120, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Muragaki, Y.; Shioyama, T.; Komori, T.; Maruyama, T.; Nitta, M.; Yasuda, T.; Hosono, J.; Okamoto, S.; Kawamata, T. Malignancy Index Using Intraoperative Flow Cytometry Is a Valuable Prognostic Factor for Glioblastoma Treated with Radiotherapy and Concomitant Temozolomide. Neurosurgery 2019, 84, 662–672. [Google Scholar] [CrossRef]
- Koriyama, S.; Nitta, M.; Shioyama, T.; Komori, T.; Maruyama, T.; Kawamata, T.; Muragaki, Y. Intraoperative Flow Cytometry Enables the Differentiation of Primary Central Nervous System Lymphoma from Glioblastoma. World Neurosurg. 2018, 112, e261–e268. [Google Scholar] [CrossRef]
- Gardner, G.; Robertson, J.H. Hearing Preservation in Unilateral Acoustic Neuroma Surgery. Ann. Otol. Rhinol. Laryngol. 1988, 97, 55–66. [Google Scholar] [CrossRef]
- Falcioni, M.; Fois, P.; Taibah, A.; Sanna, M. Facial Nerve Function after Vestibular Schwannoma Surgery. J. Neurosurg. 2011, 115, 820–826. [Google Scholar] [CrossRef] [PubMed]
- Frischer, J.M.; Gruber, E.; Schöffmann, V.; Ertl, A.; Höftberger, R.; Mallouhi, A.; Wolfsberger, S.; Arnoldner, C.; Eisner, W.; Knosp, E.; et al. Long-Term Outcome after Gamma Knife Radiosurgery for Acoustic Neuroma of All Koos Grades: A Single-Center Study. J. Neurosurg. 2018, 130, 388–397. [Google Scholar] [CrossRef] [Green Version]
- Bailo, M.; Boari, N.; Gagliardi, F.; Franzin, A.; Piloni, M.; Spina, A.; Gemma, M.; Vecchio, A.D.; Bolognesi, A.; Mortini, P. Gamma Knife Radiosurgery for Residual and Recurrent Vestibular Schwannomas after Previous Surgery: Clinical Results in a Series of 90 Patients and Review of the Literature. World Neurosurg. 2017, 98, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Tu, H.T.; Chuang, C.Y.; Chang, C.S.; Chou, H.H.; Lee, M.T.; Huang, C.F. Gamma Knife Radiosurgery for Large Vestibular Schwannomas Greater Than 3 cm in Diameter. J. Neurosurg. 2018, 128, 1380–1387. [Google Scholar] [CrossRef] [Green Version]
- Iorio-Morin, C.; AlSubaie, F.; Mathieu, D. Safety and Efficacy of Gamma Knife Radiosurgery for the Management of Koos Grade 4 Vestibular Schwannomas. Neurosurgery 2016, 78, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, S.; Yamamoto, M.; Kawabe, T.; Koiso, T.; Aiyama, H.; Kasuya, H.; Barfod, B.E. Long-Term Follow-Up Results of Stereotactic Radiosurgery for Vestibular Schwannomas Larger than 8 cc. Acta Neurochir. 2019, 161, 1457–1465. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Kano, H.; Awan, N.R.; Lunsford, L.D.; Niranjan, A.; Flickinger, J.C.; Novotny, J.; Bhatnagar, J.P.; Kondziolka, D. Gamma Knife Radiosurgery for Larger-Volume Vestibular Schwannomas. J. Neurosurg. 2011, 114, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Panigrahi, M.; Kumar, D.; Vooturi, S.; Madigubba, S. MIB Index as Predictor of Recurrence in Sporadic Vestibular Schwannomas. World Neurosurg. 2018, 120, e1203–e1207. [Google Scholar] [CrossRef]
- Freeman, S.R.M.; Ramsden, R.T.; Saeed, S.R.; Alzoubi, F.Q.; Simo, R.; Rutherford, S.A.; King, A.T. Revision Surgery for Residual or Recurrent Vestibular Schwannoma. Otol. Neurotol. 2007, 28, 1076–1082. [Google Scholar] [CrossRef]
- Bailo, M.; Boari, N.; Franzin, A.; Gagliardi, F.; Spina, A.; Del Vecchio, A.; Gemma, M.; Bolognesi, A.; Mortini, P. Gamma Knife Radiosurgery as Primary Treatment for Large Vestibular Schwannomas: Clinical Results at Long-Term Follow-Up in a Series of 59 Patients. World Neurosurg. 2016, 95, 487–501. [Google Scholar] [CrossRef]
- Huang, M.J.; Kano, H.; Mousavi, S.H.; Niranjan, A.; Monaco, E.A.; Arai, Y.; Flickinger, J.C.; Lunsford, L.D. Stereotactic Radiosurgery for Recurrent Vestibular Schwannoma after Previous Resection. J. Neurosurg. 2017, 126, 1506–1513. [Google Scholar] [CrossRef] [Green Version]
- Yokoyama, M.; Matsuda, M.; Nakasu, S.; Nakajima, M.; Handa, J. Clinical Significance of Ki-67 Staining Index in Acoustic Neurinoma. Neurol. Med. Chir. 1996, 36, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Wach, J.; Brandecker, S.; Güresir, A.; Schuss, P.; Vatter, H.; Güresir, E. The Impact of the MIB-1 Index on Facial Nerve Outcomes in Vestibular Schwannoma Surgery. Acta Neurochir. 2020, 162, 1205–1213. [Google Scholar] [CrossRef] [Green Version]
- Kasbekar, A.V.; Adan, G.H.; Beacall, A.; Youssef, A.M.; Gilkes, C.E.; Lesser, T.H. Growth Patterns of Residual Tumor in Preoperatively Growing Vestibular Schwannomas. J. Neurol. Surg. B Skull Base 2018, 79, 319–324. [Google Scholar] [CrossRef]
- Bloch, D.C.; Oghalai, J.S.; Jackler, R.K.; Osofsky, M.; Pitts, L.H. The Fate of the Tumor Remnant after Less-than-Complete Acoustic Neuroma Resection. Otolaryngol. Head Neck Surg. 2004, 130, 104–112. [Google Scholar] [CrossRef]
| Factor | Value |
|---|---|
| No. of patients | 34 |
| No. of tumors | 34 |
| No. of specimens | 66 |
| Age (year), mean (range) | 57.8 (16–78) |
| Sex | |
| Male (%) | 13 (38.2) |
| Female (%) | 21 (61.8) |
| Tumor size (mm), mean (range) | 32.3 (14–55) |
| Tumor type | |
| Solid (%) | 21 (61.8) |
| Cystic (%) | 13 (38.2) |
| Size of the internal auditory meatus (mm), mean (range) | 9.3 (5–20) |
| Preoperative hearing function (G-R hearing scale) * | |
| Grade I | 6 |
| Grade II | 3 |
| Grade III | 4 |
| Grade IV | 0 |
| Grade V | 20 |
| MIB-1 labeling index, mean (range) † | 5.9 (0.9–19.8) |
| PI, mean (range) † | 11.06 (0.71–33.45) |
| Resection rate | |
| GTR (%) | 10 (29.4) |
| NTR (%) | 8 (23.5) |
| STR (%) | 16 (47.1) |
| Postoperative facial nerve function ‡ | |
| Good (%) | 30 (93.8) |
| Poor (%) | 2 (6.3) |
| Recurrence (%) | 5 (14.7) |
| Factor | Recurrence (n = 5) | No Recurrence (n = 29) | p-Value |
|---|---|---|---|
| Age (year), mean | 58.0 | 57.8 | 0.98 |
| Male sex, n (%) | 1 (20.0) | 12 (41.4) | 0.63 |
| Tumor size (mm), mean | 36.0 | 31.9 | 0.56 |
| Cystic tumor, n (%) | 1 (20) | 12 (41.4) | 0.63 |
| Poor preoperative hearing function, n (%) * | 4 (100) | 20 (69.0) | 0.55 |
| MIB-1 labeling index, mean | 7.7 | 8.4 | 0.77 |
| PI, mean † | 8.1 | 14.5 | 0.13 |
| STR | 5 | 0 | 0.02 |
| Factor | imPI/cPI Ratio > 1 (n = 15) | imPI/cPI Ratio < 1 (n = 9) | p-Value |
|---|---|---|---|
| Age (year), mean | 56.1 | 58.7 | 0.77 |
| Male sex, n (%) | 8 (53.3) | 3 (33.3) | 0.42 |
| Tumor size | 35.4 | 28.4 | 0.03 |
| Cystic tumor | 4 (46.7) | 2 (22.2) | 0.39 |
| Size of the internal auditory meatus | 8.5 | 9.7 | 0.65 |
| Preoperative facial nerve paresis, n (%) * | 1 (6.7) | 3 (33.0) | 0.13 |
| Poor preoperative hearing, n (%) † | 13 (86.7) | 4 (44.4) | 0.06 |
| Good postoperative facial nerve function, n (%) | 14/14 (100) | 6/8 (75) | 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oya, S.; Yoshida, S.; Hanakita, S.; Inoue, M. Quantitative Evaluation of Proliferative Potential Using Flow Cytometry Reveals Intratumoral Heterogeneity and Its Relevance to Tumor Characteristics in Vestibular Schwannomas. Curr. Oncol. 2022, 29, 1594-1604. https://doi.org/10.3390/curroncol29030134
Oya S, Yoshida S, Hanakita S, Inoue M. Quantitative Evaluation of Proliferative Potential Using Flow Cytometry Reveals Intratumoral Heterogeneity and Its Relevance to Tumor Characteristics in Vestibular Schwannomas. Current Oncology. 2022; 29(3):1594-1604. https://doi.org/10.3390/curroncol29030134
Chicago/Turabian StyleOya, Soichi, Shinsuke Yoshida, Shunya Hanakita, and Mizuho Inoue. 2022. "Quantitative Evaluation of Proliferative Potential Using Flow Cytometry Reveals Intratumoral Heterogeneity and Its Relevance to Tumor Characteristics in Vestibular Schwannomas" Current Oncology 29, no. 3: 1594-1604. https://doi.org/10.3390/curroncol29030134
APA StyleOya, S., Yoshida, S., Hanakita, S., & Inoue, M. (2022). Quantitative Evaluation of Proliferative Potential Using Flow Cytometry Reveals Intratumoral Heterogeneity and Its Relevance to Tumor Characteristics in Vestibular Schwannomas. Current Oncology, 29(3), 1594-1604. https://doi.org/10.3390/curroncol29030134






